Abstract
Background:
Needlestick injuries, mostly due to unsafe needle devices, are a frequent adverse event among health care workers and patients on chronic treatment, such as hemophiliacs. To improve the safety of these procedures, a needleless reconstitution system, Bio-Set® has been implemented for the sucrose-formulated recombinant factor VIII (rFVIII-FS) Kogenate® Bayer (Bayer Healthcare, Berlin, Germany). The aim of this study was to collect patients’ satisfaction and safety data regarding the administration of rFVIII-FS with this new device.
Methods:
This was a multicenter, prospective, postmarketing surveillance study collecting data from seven Italian Haemophilia Centers within the framework of an international project involving patients from nine European countries. The patients were asked to fill out two preference questionnaires (one assessing the old method and one assessing the new method) directly after the training and two further preference questionnaries (assessing the new method) after a period of about 3 and 12 months.
Results:
A total of 44 male hemophilia A patients were included in the analysis. At the end of the 12-month observation period, physicians assessed the patients’ satisfaction with Kogenate® Bayer with Bio-Set® in 40.9% (n = 18) as “very satisfied” and in 45.5% (n = 20) as “satisfied”, whereas “not satisfied” ratings were given for 9.1% (n = 4) of patients (data missing from two patients, 4.5%). The compliance of the patients compared with the last method before switch to the Bio-Set® device was rated as “better”, “equal”, and “worse” in 72.7% (n = 32), 20.5% (n = 9), and 2.3% (n = 1) of patients, respectively. Three patients (6.8%) experienced adverse events, but only one event was related to rFVIII infusion (inhibitor development in a patient who had little prior exposure to rFVIII) itself and not to the new device per se.
Conclusions:
The great majority of Italian patients who switched from an older method of rFVIII reconstitution to rFVIII-FS with the new reconstitution method preferred the new method. The ease of use, perceived safety from needlesticks, and the speed of reconstitution were identified as main advantages by the majority of patients.
Introduction
Needlestick injuries are frequent adverse events in health care workers worldwide.Citation1–Citation4 Ten years ago, the Occupational Safety and Health Administration (OSHA) organization in the United States estimated that about 800,000 needlesticks occurred every year among American health care workers and most needlestick injuries were due to unsafe needle devices rather than due to lack of care. OSHA recommended the introduction of devices with incorporated safety features (ie, not just accessories) that would provide a barrier between hands and needle, and would remain in place at all times, ie, before disassembly and after disposal. The safety system should be simple and easy to operate with little or no training, and should not have a negative impact on the delivery of patient care.Citation5 These recommendations were endorsed by the US Food and Drug Administration.Citation6 In a study conducted in Italy during the period 1995–2004, needlestick exposure was reported by 4.9% of health care workers with acute hepatitis B and 14.3% of health care workers with acute hepatitis C.Citation7
In view of the frequency of needlesticks among skilled health care workers, it is reasonable to assume that the problem exists also among the caregivers of patients on chronic treatment with medicinal products to be administered by intravenous route, such as patients with hemophilia A.Citation8 For this reason, a needleless reconstitution system, Bio-Set® (a trademark of Biodome SAS), has been implemented for the sucrose-formulated recombinant coagulation factor VIII (rFVIII-FS) Kogenate® Bayer (Bayer Healthcare, Berlin, Germany), in which the vial with solvent is replaced by a prefilled syringe and the vial containing powder is fitted with a self-contained device with protective cap, Bio-Set®.Citation9
An international multicenter postmarketing surveillance study was carried out in nine European countries (Austria, France, Germany, Greece, Hungary, Italy, Spain, Switzerland, and the United Kingdom) to compare the level of satisfaction of patients with hemophilia A before and after switching from another existing factor VIII reconstitution method to the new system with Bio-Set®; In addition, safety and quality of life (QoL) data were collected.Citation10 This article describes the results obtained in the subset of patients recruited in Italy who were observed for a period of 12 months.
Methods
Design of the study
This was the Italian substudy of a prospective, noninterventional, noncontrolled, multicenter postmarketing surveillance study carried out at seven hematology centers in Italy. The aim was to observe approximately 50 patients with hemophilia A, who were going to switch from any other system to the reconstitution system with Bio-Set®.
Bio-Set® is a needleless reconstitution device. The patients were treated with commercially available product, according to the dosage regimen prescribed by the hematologist. The product was administered via intravenous bolus injections using the new system for reconstitution.
The main observation period for each patient started on the day they received the instructions and a training on how to use the new system and ended after accumulation of a minimum of 20 exposure days (ED) with Bio-Set® or after 3 months (whichever was sooner). The Italian patients were subsequently followed-up for another 9 months.
Upon entry into the study, demographic information and medical history data were collected using case report forms.
Outcome assessments
Patients were asked to complete a 25-item preference questionnaire twice, first assessing the old method and then assessing the new one, using a 7-point semiquantitative rating scale for each item which ranged from “strongly agree” to “strongly disagree”, directly after training with the new method and after a period of about 3 and 12 months. Two domains of the preference questionnaire were analyzed in addition to the total score: “ease/confidence” (8 items) and “worry/safety” (10 items). Patients were also asked to indicate their preferred reconstitution method (new method, old method, or no preference) for a panel of 8 domains plus their overall preference. Additional QoL questionnaires could be optionally completed. For adults, the Haemo-QoL-A questionnaire was used.Citation11 For children aged 4–7 years and 8–16 years, two versions of Haemo-QoL were used.Citation12 The Haemo-QoL for children consisted of a questionnaire to be answered by the children themselves and a second questionnaire to be answered by their parents. However, the interpretation of the results was not possible due to the low number of questionnaires completed.
At the end of the main study period as well as at the end of the follow-up period, the hematologists also analyzed exposure to rFVIII-FS, enquired about adverse events, and assessed patient satisfaction of the new system with Bio-Set® using a 3-item semiquantitative rating scale, as well as compliance compared with the old method.
Statistical analysis
All patients who received training with the new system and completed at least one preference questionnaire were included in the descriptive statistical analysis. Descriptive analyses of the data were performed using summary statistics for categorical and quantitative data. Incidence rates for specific events were calculated as the number of specific events reported divided by the number of patients at risk, where the number of specific events was defined as the number of patients reporting the specific event and the number of patients at risk was defined as total number patients exposed to rFVIII-FS during the observation period. For multiple occurrences of a specific event within one patient, the event was counted only once. Regarding patient preference, item responses were transformed into scores using 0–100 scales to calculate the scores of the domains “ease/confidence” and “worry/safety”.
The study was conducted according to Italian and European regulations related to observational postmarketing studies; the study was approved by an appropriate Ethics Committee and all the patients or their parents gave their informed consent in writing.
Results
Study population
During the period November 2005–July 2007, a total of 45 patients entered the Italian substudy and 44 were evaluable for the descriptive statistical analysis. Their main demographic and baseline characteristics are shown in . All patients were Caucasian males, except one, who was Asian, and nearly half of the patients (45.5%, n = 20) were aged up to 18 years old; except for one elderly patient (older than 65 years), all remaining patients were young or middle-aged adults.
Table 1 Demographic and baseline characteristics of the study population
For most of the patients, the diagnosis of hemophilia A was known for more than 10 years (n = 32, 72.7%). Most of the patients suffered from severe hemophilia A (<1% FVIII activity; n = 33, 75%) and had a history of extensive exposure to FVIII (>150 days; n = 35, 79.5%). Few patients had a history of inhibitors to FVIII (n = 5, 11.4%) or actually suffered from an inhibitor to FVIII at enrollment (n = 2, 4.5%).
More than one-third of the patients (n = 16, 36.4%) had a known family history of hemophilia A; the affected family members were brothers and uncles (n = 6, 37.5% each) or grandfathers (n = 4, 25.0%).
Although data were missing for one patient, 43 patients were on treatment with rFVIII-FS and treatment was self-administered by 31.8% of patients (n = 18) at study start.
Treatment
On average, 30.0 ± 9.6 IU/kg (range, 15–60 IU/kg) were given for prophylaxis to more than half of the patients (26 patients, 59.1%) either 2 or 3 times a week. On-demand treatment was reported for 36.4% of patients (n = 16), who took on average 31.7 ± 7.6 IU/kg (range, 25–40 IU/kg). Further, two patients underwent an immune tolerance induction or inhibitor adapted therapy during the study.
Patient preference
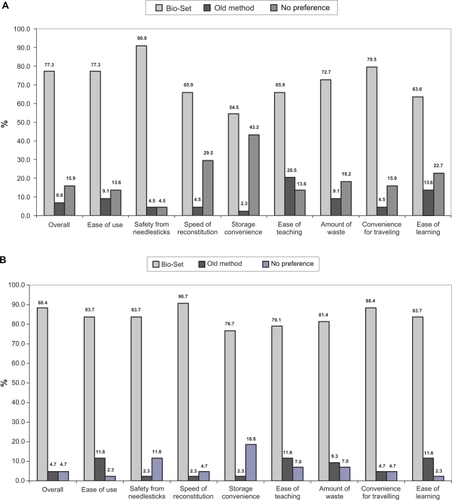
Overall, patient preference was in favor of the new system immediately after training: 77.3% (n = 34) preferred Bio-Set®, 6.8% (n = 3) preferred the old method, and 15.9% (n = 7) had no preference. After 3 months of experience with the new system (or at least 20 days of exposure), the proportion of patients increased to 85.7% (n = 36) and after 12 months up to 88.4% (n =38). Immediately after training, the main reason for the preference was safety from needlesticks (90.9%), followed by convenience for traveling (79.5%), ease of use (77.3%), and amount of waste (72.7%), whereas after 12 months of experience, the main reasons shifted to speed of reconstitution (90.7%) and convenience for traveling (88.4%), followed by safety from needlesticks (83.7%), ease of use (83.7%), and ease of learning (83.7%; and ).
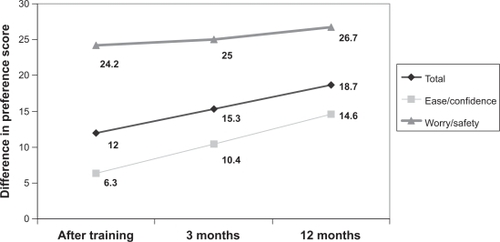
Patient preference was in favor of the new reconstitution system also in terms of mean total preference score: immediately after training, it was 61.3 ± 14.1 for the old method vs 77.0 ± 13.7 for the new method; the score increased over time to 81.5 ± 10.4 after 12 months. Domain analysis disclosed a much greater difference between the two methods in the domain “worry/safety” than in the domain “ease/confidence”: immediately after training, the mean preference score related to “worry/safety” for the old method was 50.2 ± 17.2 vs 76.4 ± 13.2 for the new one, whereas the mean perference score related to “ease/confidence” was 70.2 ± 16.4 for the old method vs 78.3 ± 15.2 for the new one. The preference scores increased slightly after gaining experience: the worry/safety score increased up to 79.1 ± 12.1 and the ease/confidence score up to 83.4 ± 12.9 after 12 months. The course of the differences in median scores is illustrated in .
Regarding individual items, the preference score was consistently in favor of the new reconstitution system with Bio-Set®.
Satisfaction and compliance
After 3 months, 88.6% of patients were at least satisfied with the new system (n = 39) and 6.8% (n = 3) were not. Information on this question was missing for two patients (4.5%). The satisfaction rate was similar after 12 months (86.4% very satisfied or satisfied, 9.1% not satisfied, information missing from two patients).
Compared with the last method before switch, compliance was rated as “better” with the new method than with the old method in 63.6% (n = 28) of patients, “equal” in 27.3% (n = 12), and “worse” in 4.5% (n = 2) at 3 months; this information was missing from two patients (4.5%). At 12 months, compared with the last method, compliance was rated as “better” than the old method in 72.7% (n = 12), “equal” in 20.5% (n = 5), and “worse” in 2.3% (n = 1; missing data 4.5%, n = 2).
Safety
Although three patients (6.8%) experienced adverse events, two of these were considered unrelated to study medication, but all three adverse events were considered not related to the new device.
A 2-year-old boy with a known diagnosis of severe hemophilia A, who had already been exposed to rFVIII-FS before the study for about 20 days, developed inhibitor antibodies (high titer, 34 BU) after about 5 months (total EDs, 12); treatment with rFVIII-FS was continued. An human immunodeficiency virus-positive 41-year-old man with concomitant hepatitis died on account of carcinoma of the liver; the death was considered not to be related to rFVIII-FS. A 30-year-old man reported musculoskeletal pain in his ankle, which resolved after 6 days; it was considered not to be related to rFVIII-FS, which was continued.
Discussion
In a previous study, Butler et alCitation8 showed that patients, caregivers, and nurses preferred the Bio-Set® method compared with the conventional 2-vial transfer needle reconstitution method with regard to worry/safety, ease/confidence, and overall preference. In addition, time savings have been associated with Bio-Set® use compared with the conventional reconstituion method in another study.Citation13
This postmarketing surveillance study, which evaluated patients’ longer-term satisfaction, suggests that enrolled patients prefer the new system with Bio-Set® to the old reconstitution method, mainly in view of its perceived safety regarding the prevention of needlesticks and its convenience in terms of speed of reconstitution and reduced medical waste. In addition, the smaller package size was also preferred because it simplifies aspects of patients’ daily lives such as traveling. This study also suggests that the new reconstitution method is safe, as the only adverse reaction reported to be related to study medication was the development of FVIII inhibitors, which is a well-known complication of first exposures to rFVIII therapy early in childhood and obviously not related to the use of the reconstitution device.
The strength of the study is that it reflects clinical reality, since it was an observational study, and the patient population was a representative sample of the patients seen in clinics in Italy, both in terms of age (all age groups were included), and in terms of the severity of the disease (most of the patients attending hemophilia centers in Italy have severe disease as was the case in this study where approximately 75% of the patients had severe hemophilia). Moreover, the sample was quite large, considering that it was a national substudy (n = 44 patients).
This study is limited by the lack of a control group and the fact that all patients but one switched from rFVIII-FS with the prior reconstitution method, not from a variety of products.
Conclusion
This postmarketing surveillance study indicates that the new system with Bio-Set® is a safe method for the reconstitution of rFVIII-FS that patients prefer to the prior one. This new reconstitution method could improve patients’ compliance with therapy, especially for those receiving long-term prophylaxis treatment.
Acknowledgements
The authors thank Margaret Musso, MD, for assistance in checking data and for the critical development of tables and figures of the manuscript. The study was supported by a grant from Bayer HealthCare.
Disclosure
The authors report no conflicts of interest in this work.
References
- ElderAPatersonCSharps injuries in UK health care: a review of injury rates, viral transmission and potential efficacy of safety devicesOccup Med (Lond)20065656657417065314
- CutterJGammonJReview of standard precautions and sharps management in the communityBr J Community Nurs200712546017363868
- BerguerRHellerPJStrategies for preventing sharps injuries in the operating roomSurg Clin North Am2005851299130516326210
- ChalupkaSMMarkkanenPGalliganCQuinnMSharps injuries and bloodborne pathogen exposures in home health careAAOHN J200856152918293597
- Crown-CyrANeedlestick injuries prompt new awarenessJSHQ199832026
- United States Department of Labor Occupational Safety and Health Administration. Needlestick/sharps injuries. 2008 Available from: http://www.osha.gov/STLC/etools/hospital/hazards/sharps/sharps.html
- TostiMEMarianoASpadaEfor Sistema Epidemiologico Integrato dell’Epatite Virale Acuta Collaborating GroupIncidence of parenterally transmitted acute viral hepatitis among healthcare workers in ItalyInfect Control Hosp Epidemiol20072862963217464931
- ButlerRLarsonPMannixSEvaluation of user preference for a needleless factor VIII delivery device for haemophilia patientsJ Outcomes Res200486378
- European Medicines Agency EPARs for authorised medicinal products for human use. 2009 Available from: http://www.emea.europa.eu/humandocs/PDFs/EPAR/Kogenatebayer/H-275-PI-en.pdf Accessed February 1, 2010.
- VidovicNMussoRKlamrothREnriquezMMAchillesKPost-marketing surveillance study of KOGENATE® Bayer with Bio-Set® in patients with haemophilia A: evaluation of patients’ satisfaction after switch to the new reconstitution systemHaemophilia20091029 [Epub ahead of print].
- RentzAFloodEAltisentCfor Members of the HAEMO-QoL-A Steering CommitteeCross-cultural development and psychometric evaluation of a patient-reported health-related quality of life questionnaire for adults with haemophiliaHaemophilia2008141023103418665853
- von MackensenSBullingerMfor Haemo-QoL GroupDevelopment and testing of an instrument to assess the quality of life of children with haemophilia in Europe (Haemo-QoL)Haemophilia200410Suppl 1172514987245
- BrewsterJMannixSButlerRLloydARentzAMLarsonPTime and cost savings with Bio-Set device in reconstituting FVIII concentrate [abstract]Blood20041045303