Abstract
We studied the effects of a C60 water suspension at 4 μg/mL (nC60) and the water soluble fullerenol C60(OH)24 at final concentrations of 1–100 μg/mL on human umbilical vein endothelial cells (HUVECs) in culture. We found that a 24 hr treatment of HUVECs with C60(OH)24 at 100 μg/mL significantly increased cell surface expression of ICAM-1(CD54) (67 ± 4% CD54+ cells vs. 19 ± 2 % CD54+ cells in control; p < 0.001). In addition, this treatment induced the expression of tissue factor (CD142) on HUVECs (54 ± 20% CD142+ cells vs 4 ± 2% CD142+ cells in control; p = 0.008) and increased exposure of phosphatidylserine (PS) (29 ± 2% PS+ cells vs. 12 ± 5% PS+ cells in control; p < 0.001). Analysis of cell cycle and DNA fragmentation (TUNEL) showed that both nC60 and C60(OH)24 caused G1 arrest of HUVECs and C60(OH)24 induced significant apoptosis (21 ± 2% TUNEL+ cells at 100 μg/mL of C60(OH)24 vs. 4 ± 2% TUNEL+ cells in control; p < 0.001). We also demonstrated that both nC60 and C60(OH)24 induced a rapid concentration dependent elevation of intracellular calcium [Ca2+]i. This could be inhibited by EGTA, suggesting that the source of [Ca2+]i in fullerene stimulated calcium flux is predominantly from the extracellular environment. In conclusion, fullerenol C60(OH)24 had both pro-inflammatory and pro-apoptotic effects on HUVECs, indicating possible adverse effects of fullerenes on the endothelium.
Introduction
Carbon fullerenes have enormous practical potential in the field of biomedical nano-technology. Fullerenes and carbon nanotubes have already had a profound impact on the development of diagnostic biosensors, drug delivery nanosystems, and imaging nanoprobes for intravascular use. In addition, fullerenes can be used as components in a variety of plastics, including filtration membranes (CitationBosi et al 2003; CitationEndo et al 2004; CitationBianco et al 2005; CitationSinha and Yeow 2005; CitationDjordjevic et al 2006; CitationLacerda et al 2006; CitationPolizu et al 2006; CitationHarrison and Atala 2007). It has been reported that hydroxylated fullerene derivatives have potent antioxidant properties (CitationDugan et al 1996; CitationTsai et al 1997; CitationHuang et al 1998; CitationStraface et al 1999; CitationWang et al 1999; CitationJin et al 2000). Furthermore, the cytoprotective effects of fullerene derivatives in oxidative stress, mostly by free radical scavenging, have been demonstrated in different cell lines in vitro, and also on animal models, in vivo (CitationLin et al 1999; CitationTsao et al 1999; CitationLai et al 2000; CitationGharbi et al 2005; CitationDaroczi et al 2006). It has been shown that fullerene derivatives have anti-viral and anti-bacterial properties. Also, their photodynamic cytotoxicity has been employed in experimental anti-tumor therapy and pathogen inactivation (CitationKasermann and Kempf 1997; CitationTabata et al 1997). However, several studies are raising safety concerns by showing possible cytotoxic effects of fullerenes and their derivatives (CitationSera et al 1996; CitationChen et al 1998; CitationKamat et al 1998; CitationWolff et al 2000; CitationOberdorster 2004; CitationSayes et al 2005; CitationIsakovic et al 2006; CitationZhu et al 2006). Although, it has been demonstrated that residual tetrahydrofuran (THF) used for the C60 fullerene solubilization substantially potentiates the toxicity of C60, the recent studies using the THF free water suspensions of fullerenes or water soluble polyhydroxylated fullerenols indicate their cytotoxic effects (CitationIsakovic et al 2006; CitationZhu et al 2006). Despite the fact that many of the fullerene applications are based on intravascular administration of these compounds, very little is known about the effects of fullerenes on endothelial cells (CitationYamawaki and Iwai 2006). Here we present a study investigating pro-apoptotic and pro-inflammatory effects of water soluble fullerenol C60(OH)24 and a C60 fullerene water suspension (nC60) on cultured human umbilical vein endothelial cells.
Materials and methods
Fullerenes
The water soluble poly-hydroxylated fullerene derivative fullerenol C60(OH)24 (at final concentrations of 1–100 μg/mL) from MER Corp., Tuscon, AZ was used for all experiments. In addition, the water nanocrystalline suspensions of insoluble C60 (nC60) (C60 of 99.5% purity from SES Res., Houston, TX, USA) was prepared by continuous stirring 400 mg of C60 powder in 400 mL of Milli-Q water for 2 weeks at room temperature. After stirring, the suspension was centrifuged twice at 4000 g for 20 minutes to remove larger aggregates of undissolved fullerene. The final supernatant which contained 21 μg/mL of nC60 was collected, and stored at 4 °C. The concentration of nC60 was determined by extracting the C60 from the suspension by toluene and analyzing the absorbance at 336 nm, as described by CitationLyon et al (2006). In tissue culture experiments, the supernatant was diluted with the culture medium to a final concentration of 4 μg/mL. As a control, the fullerene vehicle (Millli-Q H2O) was prepared in parallel by continuous stirring for 2 weeks at room temperature and manipulated the same way as nC60 suspension.
Analysis of hydrodynamic size distribution of fullerene particles using Dynamic Light Scattering (DLS)
The Malvern Zetasizer Nano ZS instrument (Southborough, MA) with a back scattering detector was used for measuring the hydrodynamic size (diameter) in batch mode (no fractionation) at 25 °C in a low volume quartz cuvette. The relative particle volume is used as the size class for histograms of the size distribution (the diameter is plotted on the abscissa and the particle volume on the ordinate). The average diameter (weighted by particle volume) of the largest peak in the volume distribution is reported as the hydrodynamic size, along with its standard deviation and the percentage of the total volume contained in the largest peak. The nC60 sample concentration was 21 μg/mL as described in the stock preparation (see above). The hydroxylated C60 derivative (C60(OH)24) was weighed and dissolved in 10 mM NaCl to give a final concentration of 2 mg/mL and filtered through a 0.02μm filter (Anotop 10 Plus, Whatman International Ltd., Maidstone, England). A minimum of twelve measurements were made per sample.
Human umbilical vein endothelial cells (HUVECs)
HUVECs (2nd passage) from Cambrex (Walkersville, MD), were cultured in either 6 or 24 well plates (Becton Dickinson Labware, Franklin Lakes, NJ) with EGM-2 media containing 2% fetal bovine serum and supplements (Cambrex). Cells were used for experiments when they reached 80%–90% confluence (~48 hrs in culture). HUVECs were washed and incubated for 24 hrs at 37 °C with fullerenol C60(OH)24 or fullerene nC60 in water diluted one to five with the EGM-2 medium. After incubation, cells were harvested with 20 mM HEPES buffer with 100 mM NaCl, 0.5% BSA and 10 mM EDTA, pH 7.4. After harvesting, the cells were centrifuged (300 g for 5 min), washed with Hanks’ balanced salt solution with 0.35% bovine serum albumin (HBSS/BSA) and used for analysis.
Antibodies
Cy-Chrome conjugated anti-human monoclonal antibody (mAb) to CD54 (ICAM-1, clone HA58), phycoerythrin-conjugated (PE) anti-human mAb to CD142 (tissue factor, clone HTF-1), isotype matched controls, annexin V (FITC-conjugated), and an APO-BRDU kit were purchased from BD Pharmingen (San Diego, CA). All antibodies were titrated to ensure saturating concentrations.
Labeling of endothelial cells
Approximately 105 cells were resuspended in 50 μL of HBSS/BSA and incubated with saturating concentration of the mAbs or annexin V. Non-labeled cells and cells incubated with either relevant isotype controls or with annexin V in the presence of 20 mM EDTA were prepared as controls. After 20 min incubation at room temperature, the suspension of labeled cells was diluted with 2 mL of HBSS/BSA and centrifuged (300 × g for 5 min). Sedimented cells were resuspended in 0.5 mL of HBSS/BSA and analyzed using flow cytometry.
Flow cytometry of endothelial cells
Cell samples were analyzed as described previously (CitationSimak et al 2002; CitationSimak, Holada, and Vostal et al 2002). Briefly, a FACSCalibur flow cytometer (Becton Dickinson, San Diego, CA, USA) equipped with CELLQuest software, with forward scatter (FSC) and side scatter (SSC) in linear mode was used. Populations of intact cells were gated according to their light-scattering characteristics in order to exclude debris, and 10,000 gated cells were analyzed for each sample. The total percentage (%) of CD54+, CD142+, and PS+ (annexin V-binding) cells was evaluated. Apoptosis (TUNEL) was analyzed using the APO-BRDU kit following the manufacturer’s instructions (BD Pharmingen, San Diego, CA). In addition, cell cycle analysis was performed using ModFit software (Verity Software House, Topsham, ME).
Intracellular free Ca2+ assay
The acute effect of fullerenes on intracellular free Ca2+ concentration was studied in HUVEC loaded with a Ca2+- sensitive probe (FURA-2AM). Changes in fluorescence in individual cells (n = 100) were monitored at 340 nm and 380 nm excitation (the rate of data capture was 170/min) using a Nikon inverted epi-fluorescence/phase microscope equipped with a low-light level integrating CCD camera with a microphotometer assembly (InCyt I/P-2 TM Imaging and Photometry System, Intracellular Imaging Inc., Cincinnati, OH). Intracellular [Ca2+]i in real time was calculated from the ratio of emission detected at 510 nm at two excitation wavelengths (340 nm and 380 nm) and by comparison to a standard curve established for these settings using buffers of known free [Ca2+] with the InCyt Im2 software.
Statistical analyses
Results are presented as means ± standard deviations (SD). Kruskal-Wallis One Way Analysis of Variance on Ranks and multiple comparisons using Student-Newman-Keuls method or Dunett’s method was used where appropriate (SPSS 12; SPSS, Chicago, IL). A value of p < 0.05 was considered statistically significant.
Results
Hydrodynamic size distribution of fullerene particles in nC60 and C60(OH)24 preparations
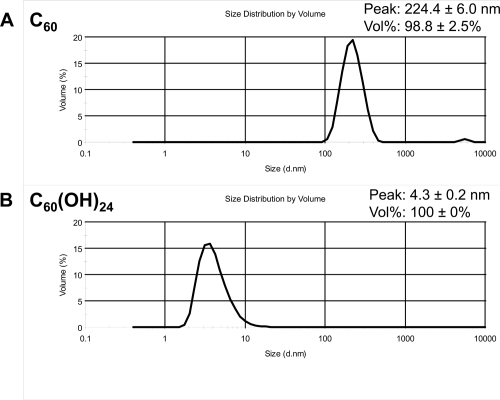
The mean size distribution by volume and the mean hydrodynamic diameter of nC60 and C60(OH)24 are shown in . Based on the size distributions of particles present in the nC60 water suspension (), the major water soluble component is about 224 nm in size. The small peak at about 5000 nm corresponds to approximately 2% of the total particle volume, and may be due to aggregates. Since unmodified nC60 is not immediately water soluble, we stirred C60 in water for two weeks, which resulted in a nanocrystalline suspension of stable aggregates (nC60). nC60 refers to an unknown number ‘n’ of C60 molecules agglomerating to form the suspension. The formed clusters contain unmodified C60 in their centers, surrounded by partially hydroxylated C60 on the outside (CitationFortner et al 2005). In contrast, the fullerenol C60(OH)24 is very soluble in water. Based on its size distribution by volume (), only one peak at 4.3 nm was observed, as expected for the hydroxylated C60 derivative.
High dose fullerenol C60(OH)24 induced elevated HUVEC surface expression of ICAM-1 (CD54), tissue factor (CD142), and phosphatidylserine (PS)
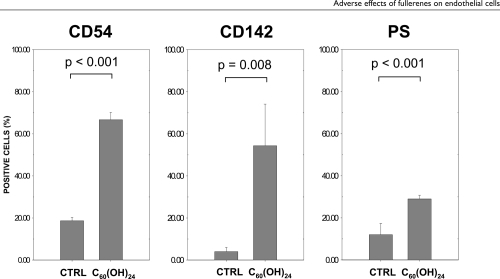
HUVECs were cultured for 24 hrs with different concentrations of fullerenol C60(OH)24, (1, 10, 50, and 100 μg/mL), fullerene nC60 at 4 μg/mL, or with the vehicle. Flow cytometry was used to analyze the difference in expression of several surface markers. shows that the 24 hr treatment of HUVECs with 100 μg/mL C60(OH)24 significantly increased cell surface expression of ICAM-1(CD54) (67 ± 4% CD54+ cells vs 19 ± 2% CD54+ cells in control; p < 0.001). This treatment also induced expression of tissue factor (CD142) on HUVECs (54 ± 20% CD142+ cells vs 4 ± 2% CD142+ cells in control; p = 0.008). In addition, an increase in exposed phosphatidylserine (PS, detected by Annexin V) (29 ± 2% PS+ cells vs 12 ± 5% PS+ cells in control; p < 0.001) was identified. However, treating the HUVECs with lower concentrations of fullerenol C60(OH)24 or nC60 at 4 μg/mL did not induce a significant increase of surface expression of CD54, CD142 or PS.
Both fullerene C60 and fullerenol C60(OH)24 induced G1 arrest and a high dose of fullerenol induced significant apoptosis (TUNEL) in HUVECs
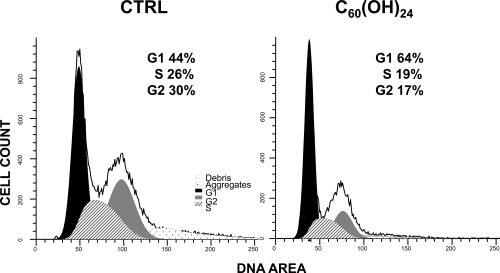
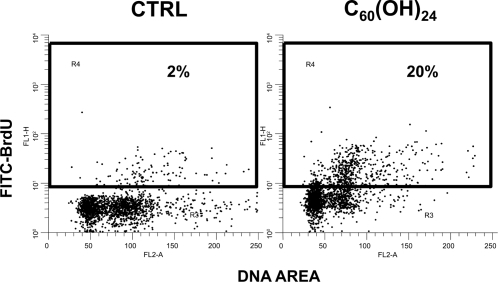
Analysis of the cell cycle by flow cytometry showed that both nC60 at 4 μg/mL and C60(OH)24 at concentrations 10 - 100 μg/mL caused significant G1 arrest in HUVECs after 24 hours of treatment (). In addition, fullerenol C60(OH)24 at 100 μg/mL induced significant apoptosis after 24 hours treatment of HUVECs, as compared to control cells treated with the vehicle (21 ± 2% TUNEL+ cells at 100 μg/mL of C60(OH)24 vs. 4 ± 2% TUNEL+ cells in control; p < 0.001) (). Treating the HUVECs with lower concentrations of fullerenol C60(OH)24 or nC60 at 4 μg/mL did not induce significant increase of TUNEL+ cells.
Both fullerene nC60 and fullerenol C60(OH)24 induced acute Ca2+ influx in HUVECs
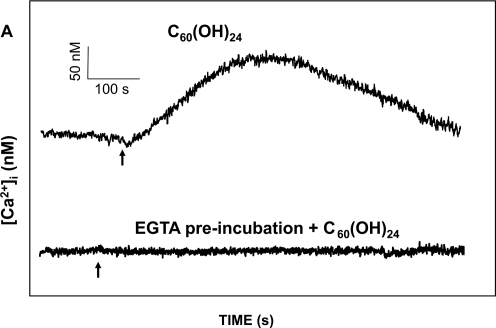
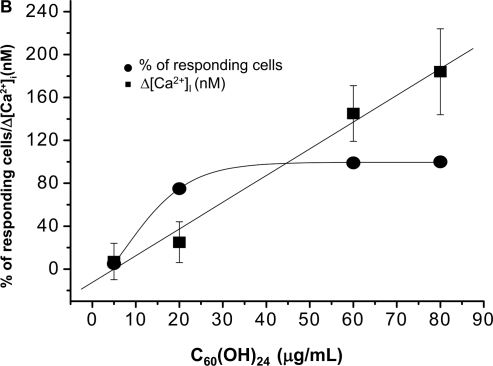
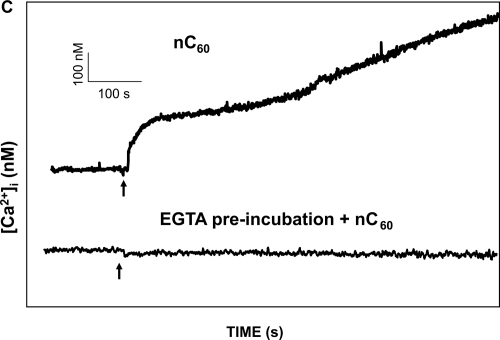
The acute effect of fullerenes on intracellular free Ca2+ concentration [Ca2+]i was studied, using a ratio fluorometry in HUVECs. Cells were loaded with a Ca2+-sensitive probe FURA-2AM. We demonstrated that both nC60 and C60(OH)24 induced a rapid concentration dependent elevation of [Ca2+]i. nC60 caused a continuous increase in [Ca2+]i and was about ten times more potent compared to C60(OH)24 (). This activity of both C60(OH)24 and nC60 could be inhibited by EGTA, suggesting that the source of [Ca2+]i in fullerene stimulated calcium flux is predominantly from the extracellular environment.
Figure 5 Analysis of C60(OH)24 and nC60 induced intracellular Ca2+ increase (Δ[Ca2+]i) in HUVECs: (A) The upper tracing shows the time course of C60(OH)24-induced Δ[Ca2+]i in a representative cell. The lower tracing shows a time course in a representative cell in a Ca2+ free solution (containing 1 mM EGTA). Arrows indicate when C60(OH)24 (final conc. 80 μg/mL) was added. There was no response observed when the fullerenol vehicle was added (not shown). (B) A dose-response curves for the effect of C60(OH)24 on Δ[Ca2+]i and the percentage of cells responded. Mean values of 30 cells ± SD are shown. (C) The upper tracing shows the time course of nC60-induced Δ[Ca2+]i in a representative cell. The lower tracing shows a time course in a representative cell in a Ca2+ free solution (containing 1 mM EGTA). Arrows indicate when nC60 (final conc. 10 μg/mL) was added. There was no response observed when the nC60 vehicle was added (not shown). (D) A dose-response curves for the effect of nC60 on Δ[Ca2+]i and the percentage of cells responded. Mean values of 30 cells ± SD are shown.
![Figure 5 Analysis of C60(OH)24 and nC60 induced intracellular Ca2+ increase (Δ[Ca2+]i) in HUVECs: (A) The upper tracing shows the time course of C60(OH)24-induced Δ[Ca2+]i in a representative cell. The lower tracing shows a time course in a representative cell in a Ca2+ free solution (containing 1 mM EGTA). Arrows indicate when C60(OH)24 (final conc. 80 μg/mL) was added. There was no response observed when the fullerenol vehicle was added (not shown). (B) A dose-response curves for the effect of C60(OH)24 on Δ[Ca2+]i and the percentage of cells responded. Mean values of 30 cells ± SD are shown. (C) The upper tracing shows the time course of nC60-induced Δ[Ca2+]i in a representative cell. The lower tracing shows a time course in a representative cell in a Ca2+ free solution (containing 1 mM EGTA). Arrows indicate when nC60 (final conc. 10 μg/mL) was added. There was no response observed when the nC60 vehicle was added (not shown). (D) A dose-response curves for the effect of nC60 on Δ[Ca2+]i and the percentage of cells responded. Mean values of 30 cells ± SD are shown.](/cms/asset/77ff7368-e9d7-4074-a7b3-94daf7958be4/dijn_a_1680_f0005_b.jpg)
Discussion
Our study showed that fullerenol induced pro-inflammatory activation of cultured HUVECs, which was indicated by the increase of CD54 (ICAM-1) and CD142 (TF) surface expression. The intercellular adhesive molecule 1 (ICAM-1), also referred to as CD54, is an adhesive receptor of the immunoglobulin gene superfamily. It is constitutively expressed at low levels on most types of vascular endothelial cells, and is highly upregulated on pro-inflammatory cytokine activated endothelium (CitationMutin et al 1997a). Tissue factor (TF), also referred to as CD142, is a cellular receptor and cofactor of activated factor VIIa. Tissue factor initiates activation of the plasma coagulation system, which leads to the generation of thrombin activity (CitationSteffel et al 2006). Although functional TF is not exposed in significant levels on resting HUVECs, its expression is greatly increased after stimulation with pro-inflammatory cytokines and some other stimuli, similarly to ICAM-1 (CitationMutin et al 1997b). In addition, the observed increased exposure of phosphatidylserine (PS) on the plasma membrane of HUVECs indicates a disturbance of the membrane asymmetry, likely due to pro-apoptotic stimulation or other types of cell activation. Although the exposure of PS on cells incubated with the fullerenol vehicle (the Milli-Q water) was higher than expected, the difference in PS-surface exposure between vehicle- treated and fullerenol-treated cells was highly significant (P < 0.001). PS is an essential cofactor for activation complexes in the plasma coagulation system. Also, it is a signaling molecule for phagocytes (CitationZwaal et al 2005). Thus, our results show that the phenotype of endothelial cells treated with fullerenol become pro-inflammatory and pro-coagulant. It is important to further investigate whether fullerenes exhibit similar effects on the endothelium in vivo in animal models.
Our cell cycle analysis showed that both nC60 and C60(OH)24 induced G1 arrest in HUVECs. Polyhydroxylated fullerenol-1 was reported to inhibit the proliferative responses in a variety of cells, including smooth muscle cells and human lymphocytes, in a concentration dependent manner. The tested concentration range was of 10−6 to 10−2 M, which corresponds to approximately 1 μg/mL to 10 mg/mL. It was also shown that fullerenol inhibited cytosolic protein kinase C activity. Therefore, it was suggested that the anti-proliferative effect of fullerenol-1 on vascular smooth muscle cells may partly be mediated through the inhibition of protein tyrosine kinase (CitationLu et al 1998).
We found that C60(OH)24 induced apoptosis in HUVECs. This was clearly detected by TUNEL assay, which is a common method for detecting DNA fragmentation. In addition, the observed increase in PS exposure of fullerenol treated cells can be considered as supporting evidence of pro-apoptotic activation of HUVECs. Our finding contradicts the results of a recent study by Yamawaki and Iwai (CitationYamawaki and Iwai 2006). These authors concluded that fullerenol did not cause apoptosis based on the failure to demonstrate cleavage of caspase 3 and PARP. However, their study showed autophagic cell death in fullerenol treated HUVECs. We may therefore speculate that both apoptotic and autophagic cell death may occur in fullerenol treated endothelium.
To investigate a possible mechanism of fullerene action on endothelium, we demonstrated that both nC60 and C60(OH)24 induced influx of extracellular Ca2+ in HUVECs. This suggests that fullerenes may either activate some of the Ca2+ channels on the cell membrane, or that they may create new channels/pores to facilitate Ca2+ influx, or both. In either case, it results in an increase in intracellular Ca2+, which may induce activation and/or apoptotic changes of the cells.
In conclusion, the results of this pilot study show possible adverse effects of fullerenes on the endothelium. The hydroxyfullerene C60(OH)24, had both pro-inflammatory and pro-apoptotic effects on endothelial cells. In addition, cell cycle arrest and increase of intracellular Ca2+ was observed in cells treated with hydroxyfullerene C60(OH)24 and also with a low concentration of nC60. With the wide potential applications of fullerenes in mind, the safety hazards of these materials need to be thoroughly evaluated. We suggest that additional in depth studies should be performed to investigate possible adverse effects of fullerenes on the cardiovascular system in exposed populations.
Notes
The findings and conclusions in this article have not been formally disseminated by the Food and Drug Administration and should not be construed to represent any Agency determination or policy.
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
References
- BiancoAKostarelosKPratoMApplications of carbon nanotubes in drug deliveryCurr Opin Chem Biol20059674916233988
- BosiSDa RosTSpallutoGFullerene derivatives: an attractive tool for biological applicationsEur J Med Chem2003389132314642323
- ChenHHYuCUengTHAcute and subacute toxicity study of water-soluble polyalkylsulfonated C60 in ratsToxicol Pathol199826143519502397
- DarocziBKariGMcAleerMFIn vivo radioprotection by the fullerene nanoparticle DF-1 as assessed in a zebrafish modelClin Cancer Res20061270869117145832
- DjordjevicABogdanovicGDobricSFullerenes in biomedicineJ Buon20061139140417309168
- DuganLLGabrielsenJKYuSPBuckminsterfullerenol free radical scavengers reduce excitotoxic and apoptotic death of cultured cortical neuronsNeurobiol Dis19963129359173920
- EndoMHayashiTKimYAApplications of carbon nanotubes in the twenty-first centuryPhilos Transact A Math Phys Eng Sci200436222233815370479
- FortnerJDLyonDYSayesCMC60 in water: nanocrystal formation and microbial responseEnviron Sci Technol20053943071615984814
- GharbiNPressacMHadchouelM[60]fullerene is a powerful antioxidant in vivo with no acute or subacute toxicityNano Lett2005525788516351219
- HarrisonBSAtalaACarbon nanotube applications for tissue engineeringBiomaterials2007283445316934866
- HuangYLShenCKLuhTYBlockage of apoptotic signaling of transforming growth factor-beta in human hepatoma cells by carboxyfullereneEur J Biochem199825438439652391
- IsakovicAMarkovicZTodorovic-MarkovicBDistinct cytotoxic mechanisms of pristine versus hydroxylated fullereneToxicol Sci2006911738316476688
- JinHChenWQTangXWPolyhydroxylated C(60), fullerenols, as glutamate receptor antagonists and neuroprotective agentsJ Neurosci Res200062600711070504
- KamatJPDevasagayamTPPriyadarsiniKIOxidative damage induced by the fullerene C60 on photosensitization in rat liver microsomesChem Biol Interact1998114145599839628
- KasermannFKempfCPhotodynamic inactivation of enveloped viruses by buckminsterfullereneAntiviral Res19973465709107386
- LacerdaLBiancoAPratoMCarbon nanotubes as nanomedicines: from toxicology to pharmacologyAdv Drug Deliv Rev20065814607017113677
- LaiHSChenWJChiangLYFree radical scavenging activity of fullerenol on the ischemia-reperfusion intestine in dogsWorld J Surg200024450410706918
- LinAMChyiBYWangSDCarboxyfullerene prevents iron-induced oxidative stress in rat brainJ Neurochem19997216344010098871
- LuLHLeeYTChenHWThe possible mechanisms of the antiproliferative effect of fullerenol, polyhydroxylated C60, on vascular smooth muscle cellsBr J Pharmacol199812310971029559892
- LyonDYAdamsLKFalknerJCAntibacterial activity of fullerene water suspensions: effects of preparation method and particle sizeEnviron Sci Technol2006404360616903271
- MutinMDignat-GeorgeFSampolJImmunologic phenotype of cultured endothelial cells: quantitative analysis of cell surface moleculesTissue Antigens1997a50449589389318
- MutinMDignat-GeorgeFSampolJImmunologic phenotype of cultured endothelial cells: quantitative analysis of cell surface moleculesTissue Antigens1997b50449589389318
- OberdorsterEManufactured nanomaterials (fullerenes, C60) induce oxidative stress in the brain of juvenile largemouth bassEnviron Health Perspect200411210586215238277
- PolizuSSavadogoOPoulinPApplications of carbon nanotubes-based biomaterials in biomedical nanotechnologyJ Nanosci Nanotechnol20066188390417025102
- SayesCMGobinAMAusmanKDNano-C60 cytotoxicity is due to lipid peroxidationBiomaterials20052675879516005959
- SeraNTokiwaHMiyataNMutagenicity of the fullerene C60-generated singlet oxygen dependent formation of lipid peroxidesCarcinogenesis199617216398895484
- SimakJHoladaKD’AgnilloFCellular prion protein is expressed on endothelial cells and is released during apoptosis on membrane microparticles found in human plasmaTransfusion2002423344211961239
- SimakJHoladaKVostalJGRelease of annexin V-binding membrane microparticles from cultured human umbilical vein endothelial cells after treatment with camptothecinBMC Cell Biol200231112052248
- SinhaNYeowJTCarbon nanotubes for biomedical applicationsIEEE Trans Nanobioscience200541809516117026
- SteffelJLuscherTFTannerFCTissue factor in cardiovascular diseases: molecular mechanisms and clinical implicationsCirculation20061137223116461845
- StrafaceENataliniBMontiDC3-fullero-tris-methanodicarboxylic acid protects epithelial cells from radiation-induced anoikia by influencing cell adhesion abilityFEBS Lett19994543354010431834
- TabataYMurakamiYIkadaYPhotodynamic effect of polyethylene glycol-modified fullerene on tumorJpn J Cancer Res1997881108169439687
- TsaiMCChenYHChiangLYPolyhydroxylated C60, fullerenol, a novel free-radical trapper, prevented hydrogen peroxide- and cumene hydroperoxide-elicited changes in rat hippocampus in-vitroJ Pharm Pharmacol199749438459232545
- TsaoNKanakammaPPLuhTYInhibition of Escherichia coli-induced meningitis by carboxyfullerenceAntimicrob Agents Chemother1999432273710471578
- WangICTaiLALeeDDC(60) and water-soluble fullerene derivatives as antioxidants against radical-initiated lipid peroxidationJ Med Chem19994246142010579823
- WolffDJPapoiuADMialkowskiKInhibition of nitric oxide synthase isoforms by tris-malonyl-C(60)-fullerene adductsArch Biochem Biophys20003782162310860539
- YamawakiHIwaiNCytotoxicity of water-soluble fullerene in vascular endothelial cellsAm J Physiol Cell Physiol2006290C149550216407415
- ZhuSOberdorsterEHaaschMLToxicity of an engineered nanoparticle (fullerene, C60) in two aquatic species, Daphnia and fathead minnowMar Environ Res200662SupplS5916709433
- ZwaalRFComfuriusPBeversEMSurface exposure of phosphatidylserine in pathological cellsCell Mol Life Sci2005629718815761668