Abstract
A glycinate derivative of α-methylprednisolone (MP) was prepared and conjugated to a linear cyclodextrin polymer (CDP) with a loading of 12.4% w/w. The polymer conjugate (CDP-MP) self-assembled into nanoparticles with a size of 27 nm. Release kinetics of MP from the polymer conjugate showed a half-life (t1/2) of 50 h in phosphate buffer solution (PBS) and 19 h in human plasma. In vitro, the proliferation of human lymphocytes was suppressed to a similar extent but with a delayed effect when CDP-MP was compared with free MP. In vivo, CDP-MP was administered intravenously to mice with collagen-induced arthritis and compared with free MP. CDP-MP was administered weekly for six weeks (0.07, 0.7, and 7 mg/kg/week) and MP was administered daily for six weeks (0.01, 0.1, and 1 mg/kg/day). Body weight changes were minimal in all animals. After 28 days, a significant decrease in arthritis score was observed in animals treated weekly with an intermediate or high dose of CDP-MP. Additionally, dorsoplantar swelling was reduced to baseline in animals treated with CDP-MP at the intermediate and high dose level. Histological evaluation showed a reduction in synovitis, pannus formation and disruption of architecture at the highest dose level of CDP-MP. MP administered daily at equivalent cumulative doses showed minimal efficacy in this model. This study demonstrates that conjugation of MP to a cyclodextrin-polymer may improve its efficacy, leading to lower doses and less frequent administration for a safer and more convenient management of rheumatoid arthritis.
Introduction
Rheumatoid arthritis (RA) is a chronic disease that causes inflammation on the synovial membrane that protects and lubricates the joints (CitationBodman and Roitt 1994). In advanced stages of disease, it can cause deformity and loss of joint function with severe pain. More than two million Americans are affected by RA (CitationLawrence et al 1998). There is no known cure for this disease. RA patients are treated with three general classes of drugs such as disease-modifying antirheumatic drugs (DMARDs), nonsteroidal anti-inflammatory agents (NSAIDs), and corticosteroids to reduce joint inflammation and pain, maximize joint function, and prevent joint destruction and deformity (CitationByron and Mowat 1985; CitationHyrich et al 2006). Methylprednisolone (MP), a corticosteroid (GC), was chosen as a candidate for our study. In the classic mode of action, GCs diffuse into the cytoplasm and bind to the ubiquitous glucocorticoid receptor (cGCR). The cGCR is a multiprotein complex that sheds HSP90 and other chaperones after GC binding, followed by nuclear translocation of the complex. In the nucleus, the activated cGCR leads to induction or inhibition of transcription of specific regulator proteins. In addition to these genomic effects, rapid nongenomic actions also occur. Nongenomic effects are less well defined but are proposed to involve membrane-bound glucocorticoid receptors, nonnuclear cytosolic effects, and effects mediated through nonspecific membrane binding of GC at high doses (CitationFarrell and Kelleher 2003; CitationButtgereit et al 2004). Downstream effects of GCs include the following: Inhibition of inflammatory mediators (IL-1, TNF-α, GM-CSF, IL-3, IL-4, IL-5, IL-8, prostaglandins, nitric oxide), inhibition of lymphocyte proliferation, inhibition of inflammatory cell migration, and induction of apoptosis in thymocytes.
MP is a synthetic glucocorticoid drug taken orally or administered intravenously. MP is commonly used in arthritis therapy and other acute and chronic inflammatory diseases (CitationHayball et al 1992; CitationOkada 2005). Short term i.v. pulses of MP are useful as bridge therapy in patients refractory to other therapies (CitationLaan et al 1999) and play a role in combination therapy with newer biological DMARDs with slow onset of activity (CitationKonttinen et al 2005). Intravenous doses of methylprednisolone, however, have high rate of clearance. Due to inadequate distribution of MP to site of inflammation, it requires large and frequent dosing (>7.5 mg/day when given on a daily oral schedule, up to 1000 mg every other day when given intravenously) to achieve an appropriate therapeutic effect (CitationSnell 1976; CitationLaan et al 1999). Although MP is known to be very effective in inflammatory diseases, it has serious side effects upon chronic exposure, including weight gain, glaucoma, osteoporosis, and psychosis (CitationSaag 2002). One approach to reduce dosing amounts, frequency of administration, and adverse side effects while maintaining the drug efficiency, is the development of new drug delivery systems with inflammatory site targeting and long circulating time.
Liposomes or polymer conjugates for inflammatory diseases are currently under active study by many research groups (CitationChandrasekar et al 2007; CitationKhoury et al 2006). Polymer conjugates or liposomes are able to stabilize drugs and prolong their plasma half-life. Polymer conjugates are different from liposome systems in that drugs are bound to the carrier instead of being trapped in the cavity of the particles. Polymer conjugates of drugs have gained interest for pharmaceutical applications over the last two decades for their excellent ability to reduce toxicities while maintaining or increasing efficacy (CitationDuncan 2003; CitationVicent 2007). Polymer conjugates can extend release pharmacokinetics and result in improved biodistribution characteristics through the so-called enhanced permeability and retention (EPR) effect (CitationMatsumura and Maeda 1986). The EPR effect has been characterized as the ability of long-circulating macromolecules to extravagate through the abnormally leaky vasculature in tissues with deregulated neovascularization and inadequate lymphatic drainage, such as tumors, leading to drug accumulation.
The linear cyclodextrin polymer (CDP) is composed of β-cyclodextrin and poly ethylene glycol (). CDP is water-soluble, biocompatible, nontoxic, and nonimmunogenic (CitationCheng et al 2003; CitationSchluep et al 2006a, Citation2006b). CDP conjugates of hydrophobic small molecule drugs self-assemble into nanoparticles with diameters between 30–50 nm. Preclinically, nanoparticulate CDP conjugates have been shown to accumulate in tumor tissue through the EPR effect (CitationSchluep et al 2006b). Rheumatoid arthritis is also known to cause deregulated formation of new blood vessels in the inflamed joints. Liposomes and polymer conjugates have been shown to accumulate at the sites of inflammation due to locally enhanced capillary permeability (CitationPaleolog and Fava 1998). In fact radioactively labeled liposomes with a sub-100-nm size have been used successfully to image and detect sites of inflammation in both animal models of arthritis as well as in humans (CitationBoerman et al 1997; CitationDams et al 1999; CitationLaverman et al 1999).
Figure 1 Schematic representation of the structure of CDP-MP, a conjugate of a glycinate derivative of α-methylprednisolone (MP) and a cyclodextrin polymer (CDP). m Number of ethylene glycol repeating units (average m = 77 for PEG with Mw 3,400); n number of repeating units of CDP-MP (average n = 24 ± 5 for parent polymer Mw of 117 kDa).
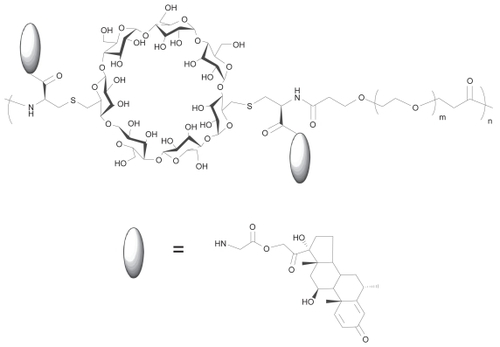
Here, we investigate a nanoparticulate CDP conjugate of MP, in which the drug is conjugated to the polymer through a glycinate ester (). An ester linker was used because most ester prodrugs show acceptable stability in vitro while allowing suitable drug release in vivo (CitationMørk and Bundgaard 1992; CitationSriram et al 2004). Ester prodrugs can be activated by pH-dependent hydrolysis and enzyme-induced cleavage. Elevated esterase levels have been detected in the synovial fluid of rheumatoid arthritic patients providing a mechanism for site-specific release of ester-linked prodrugs (CitationKar et al 1976). CDP-MP nanoparticles were studied in vitro for their ability to inhibit the proliferation of human lymphocytes and in vivo in order to determine their efficacy in a collagen-induced arthritis model after intravenous delivery.
Materials and methods
General
Unless otherwise specified, chemicals were purchased from Aldrich (St. Louis, MO) and used without further purification. Cyclodextrin polymer (CDP) was prepared according to the previous procedure (CitationCheng et al 2003). α-methylprednisolone was purchased from Steraloids, Inc (Newport, RI). Lyophilized human plasma was purchased from Sigma (St. Louis, MO) and reconstituted with PBS. NMR spectra were recorded on a Varian AMX 500 MHz or a Varian 300 MHz spectrometer. Mass spectral (MS) analysis was performed using either an electrospray mass spectrometer equipped with LCQ ion trap (Thermo Finnigan, Waltham, MA) and fitted with an electrospray ionization source or a MALDI-TOF mass spectrometer (Voyager DE-PRO, Applied Biosystems, Foster City, CA). MWs of the polymer samples were analyzed on a GPC system equipped with Agilent 1100 Series HPLC system, a Wyatt DAWN Heleos, Wyatt Optical rex, and two gel permeation columns in series (PL-aquagel-OH-40 8 μm 300 × 7.5 mm and PL-aquagel-OH-50 8 μm 300 × 7.5 mm, Polymer Laboratories, Amherst, MA) calibrated using poly(ethyleneoxide) standard and eluted using PBS (1X) at a concentration of 6 mg/mL and at a 1.0 mL/min flow rate at 30 °C. Release kinetics and polymer drug-loading of the MP derivative were analyzed with a C-18 reverse phase column on a Agilent 1100 HPLC system equipped with an UV detector at 250 nm using an isocratic mobile phase of potassium phosphate buffer (pH 4.1) and acetonitrile. Particle size of CDP-MP was measured on Zeta PALS, Brookhaven Instrument Corporation (Holtsville, NY).
Synthesis of 21-O-t-Boc-glycinate ester of α-methylprednisolone (MP) and 21-O-trifluo glycinate ester of MP
t-Boc-glycine (0.42 g, 2.4 mmol) was dissolved in 50 mL of anhydrous tetrahydrofuran at room temperature, and to this solution were added N,N’-Diisopropylcarbodiimide (0.30 g, 2.4 mmol), 4-(Dimethylamino)pyridine (33 mg, 0.27 mmol), and MP (1.0 g, 2.77 mmol) at 0 °C. The reaction mixture was stirred for ½ h at 0 °C and then stirred at room temperature for additional 15 h. Tetrahydrofuran was removed under reduced pressure to yield a white solid. The solid was redissolved in diethyl ether (50 mL) and washed with 0.1N HCl (10 mL) twice. It and reduced under vacuum was then dried over MgSO4 to yield white solid. It was purified by flash column chromatography using methylenechloride:acetone (9:1) mixture to yield white solid (0.88 g, 62%). 1H NMR δ7.31 (d), 6.26 (q), 6.00 (s), 5.02 (m), 4.47 (CDCl3) (m), 4.04 (m), 2.98 (s), 2.68 (m), 2.22 (m), 2.11 (m), 1.82–1.60 (m), 1.40 (m), 1.10 (m), 1.03 (m), 0.94 (s). ESI/MS (m/z) expected 531.28; Found 554.1 (M + Na).
21-O-t-Boc-glycinate ester of α-methylprednisolone (110 mg, 0.21 mmol) was dissolved in a mixture of methylenechloride (10 mL) and trifluoroacetic acid (TFA) (10 mL) and stirred at room temperature for 1 h. TFA glycinate MP was precipitated in diethyl ether (100 mL), washed with diethyl ether (20 mL) twice and the solids were dried under vacuum to yield white solid (38 mg, 34% Yield). 1 δ 7.36 (d), 6.17 (q), 5.88 (s), 5.04 H NMR (D2O) (m), 4.28 (m), 3.93 (s), 2.56–2.00 (m), 1.97–1.75 (m), 1.58 (m), 1.48–1.27 (m), 1.23 (s), 0.94 (m), 0.82 (m), 0.67 (s). ESI/MS (m/z) expected 431.23; Found 431.9 [M]+.
Synthesis of CDP-MP
CDP (1.0 g, 0.21 mmol) was dissolved in dry N,N-dimeth-ylformamide (20 mL). The mixture was stirred for 20 min. 21-O-trifluoro glycinate ester of MP (250 mg, 0.46 mmol), N,N-diisopropylethylamine (0.080 mL, 0.46 mmol), N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride (120 mg, 0.62 mmol), and N-hydroxysuccinimide (52 mg, 0.46 mmol) were added to the polymer solution and stirred for 4 h. The polymer was precipitated with ethylacetate (100 mL). The precipitate was dissolved in pH3 water (100 mL) which was prepared by acidification with HCl. The solution was dialyzed using 25,000 MWCO membrane (Spectra/Por 7) for 24 h in pH3 water. It was filtered through 0.2 μm filters (Nalgene) and lyophilized to yield white solid (630 mg, 50%). Loading of MP was determined to be total of 12.4% with 0.08% free MP by HPLC.
Release of MP from conjugates
Release of MP in PBS
CDP-MP was dissolved in PBS (1x, pH 7.4) at a concentration of 1 mg/mL and incubated at 37 °C. Samples were at selected time point quenched by addition of 8.5% H3PO4 and stored at −80 °C until the analysis. The released MP was measured by HPLC at 250 nm and compared to a standard curve of MP.
Release of MP in human plasma
CDP-MP was dissolved in PBS (1x, pH 7.4) at a concentration of 1 mg/mL. Human plasma was prepared at a concentration of 1 mg/mL. 250 μL of each solution, CDP-MP and human plasma was aliquoted and combined to make the total volume of 500 μL. Samples were then incubated at 37 °C, quenched at selected time point by addition of 8.5% H3PO4 and stored at −80 °C until the analysis. It was then loaded on a preconditioned solid phase column (Oasis HLB 1 cm3 cartridge from Waters, Milford, MA) and eluted with 5 mL with acetonitrile. Spike recovery was greater than 99%. The released MP was measured by HPLC at 250 nm and compared to a standard curve.
In vitro evaluation
Isolation and culture of leukocytes for analysis
Peripheral blood mononuclear cells (PBMC) were used as a source of lymphocytes/monocytes from a human source. Human PBMC were isolated from whole blood by Ficoll-Hypaque gradient centrifugation. The cells were cultured at 1 × 106 cells/ml (2 × 105 cells/well) in RPMI 1640 supplemented with 25 mM HEPES, 4 mM l-glutamine, 100 U/ml penicillin/streptomycin, and 10% heat inactivated FBS. The cells were exposed either to MP or CDP-MP preparations in the presence of phytohemagglutinin or concanavilin A (mitogens for T cells).
Lymphocyte proliferation (Mitogen)
For the evaluation of lymphocyte proliferation in response to mitogen, cultures were set up in microtiter plates. Cells were exposed either to MP or CDP-MP preparations (1, 0.3, 0.1, 0.03, 0.01, 0.003, 0.001, 0.0003, 0.0001, 0.00003, 0.00001, and 0.000003 μg/mL) in the presence of phytohemagglutinin L (PHA, 10 μg/ml) or concanavilin A (Con A, 1.25 μg/mL) (mitogens for T cells). Controls included stimulated cultures and cultures with no stimulus. At 2 or 4 days after initiation of culture, tritiated thymidine was added to the cultures to be incorporated in the DNA undergoing synthesis. Cells were cultivated without replenishing the media for the duration of the study. At 3 or 5 days after initiation of culture, the cells were harvested via a multiwell harvester and the amount of thymidine incorporated (as a measure of proliferation) was assessed by scintillation counter.
Analysis
After counting of thymidine incorporation, the data were analyzed for stimulation index (the level of thymidine incorporation in stimulated cultures over thymidine incorporation in unstimulated cultures). Further the level of decrease in proliferation caused by MP or CDP-MP compared with untreated, stimulated cultures was assessed.
In vivo study in immune mediated arthritis model
Animal care
All animals received humane care as defined by the National Research Council’s criteria for humane care. The study protocols were approved by the Institutional Animal Care and Use Committee before initiation of the studies.
Sample preparation
A stock of 5 mg/mL MP was made in absolute ethanol for the nonconjugated formulation. This stock was then diluted to 200 μg/ml (1 volume of stock + 24 volumes of saline) for the top dose. Ten fold dilutions were prepared in saline for the lower doses. CDP-MP was diluted in 5% aqueous dextrose solution (D5W). As control groups, methyl prednisolone diluent injection and CDP (polymer alone control) in D5W were compared with drug-dosed groups.
Arthritis induction and assessment
Arthritis was induced in mice by immunization with bovine collagen type II (CII) in adjuvant in the skin at the base of the tail, and assessed regularly (CitationCannetti et al 2003). Bovine CII was dissolved in 10 mM acetic acid at a concentration of 4 mg/mL. Emulsification of antigen in Freund’s complete adjuvant (CFA; Sigma Aldrich) was carried out by passing between two locking hub syringes with the apparatus at 4 °C to ensure stability of the collagen. DBA/1 mice (Harlan, Livermore, CA) were injected with 100 μg of CII in CFA at the base of the tail on day zero. On day 21 mice received a booster injection of 50 μg of CII in incomplete FA (IFA; Sigma Aldrich) to improve the incidence and synchronization of arthritis onset. On day 29 animals were scored and divided equally into groups with equivalent severity of arthritis. They were then treated with MP, CDP-MP or placebo according to . Typically >80% of animals develop arthritis. Fifty animals were immunized and 40 were carried on for further treatment. Assessments were conducted by an independent operator. After the start of therapy, the mice were weighed twice weekly.
Table 1 In vivo assay of CDP-MPTable Footnotea
Arthritis score
Joint swelling was scored (arthritis score) by a standardized method by an experienced observer. In brief, a score of 0–4 is assigned as follows: 0 – no evidence of hyperemia and/or inflammation; 1 – hyperemia with little or no paw swelling; 2 – swelling confined predominantly to the ankle region with modest hyperemia; 3 – increased paw swelling and hyperemia of the ankle and metatarsal regions; 4 – maximal paw swelling and hyperemia involving the ankle, metatarsal and tarsal regions. For final analysis scores were summed for all paws, thus the maximum possible score was 16.
Dorsoplantar swelling
Paw thickness was measured with 0–10-mm calipers. Dorsoplantar swelling was measured twice weekly after initiation of treatment until the onset of necropsy.
Histological evaluation
Animals were sacrificed on day 42. Front and rear paw sections were removed and bisected following sacrifice, and the paw was fixed in 10% neutral buffered formalin for at least 5 days before decalcification for 18 days in 10% formic acid. Paws were then dehydrated and embedded in paraffin blocks. Sections (5 μm thickness) were cut along a longitudinal axis, mounted, and stained with hematoxylin and eosin (H+E). Specimens were cut approximately to the mid line, and then sagittal central samples mounted for evaluation. The sections were stained with H+E, and Masson’s trichrome stain (CitationWong et al 2006). Images at a 100x magnification were captured using a Nikon E600 microscope equipped with a digital camera. Histological analysis was determined by a blinded pathologist using a validated scoring system for cellular infiltrate, synovial hypertrophy, and bone destruction. Histological analysis was conducted to determine the extent of joint damage. A minimum of three separate sections per specimen were evaluated in a blinded fashion. On the front paws, wrist and metacarpal joints were scored and on the rear paws, ankle, and metatarsal joints were scored. Slides were evaluated for the presence of synovitis, pannus formation, marginal erosions, architectural changes (mostly subluxation), and destruction. Synovitis was judged by thickness of the synovial membrane and scored from 1 (less than 3 cells thick) to 5 (beyond 30 cells thick); pannus was scored as 0 (no erosions visible) to 5 (loss of visible cartilage and major bone loss due to erosion); architectural changes scored from 0 (normal joint architecture) to 5 (complete fibrosis and collagen bridging). An overall score based on these collective points ranging from 0 (classical normal joint appearance) to 5 (destructive erosive arthritis with major bone remodeling), was then assigned to each section. The loss of bone and cartilage components was assessed. Masson’s trichrome histological staining was used to quantify the bone collagen content as a parameter of bone osteolysis.
Statistical evaluation
Statistical significance between the different treatment groups and untreated controls was evaluated using a Student’s two-sample, two-tailed t-Test with unequal variance (hetroscedastic) in Microsoft Excel (Microsoft Corp., Redmond, WA).
Results
Synthesis of CDP-MP
An MP glycinate derivative was prepared by attaching glycine to the primary alcohol group of MP through an ester linkage. Glycinate MP was then covalently attached to CDP (MW 117 kDa). The conjugate was purified by dialysis and lyophilized to yield a white solid. The amount of MP bound to CDP was determined to be 12.4% (w/w) with 0.08% of free MP by HPLC. CDP conjugation of MP changed the water solubility of MP from insoluble (0.12 mg/mL) to highly soluble (>than 200 mg of CDP-MP/mL). The particle size of the polymer conjugate in deionized water was measured to be 27 nm by dynamic light scattering (DLS; ). Particle size of the conjugate (10 mg/mL) was also evaluated in physiological conditions (PBS, pH 7.4) at both 25 and 37 °C and found to be 26 and 30 nm, respectively (data not shown). The stability of nanoparticles was monitored by incubation of the CDP-MP conjugate (10 mg/mL) in PBS (pH 7.4) or water for up to 45 hours at 37 °C. No significant change in particle size was observed in either solvent over that time period (data not shown).
Table 2 Comparison in particle size of CDP after MP loading and drug releaseTable Footnotea (n = 5)
In vitro evaluation
Release of MP from conjugates
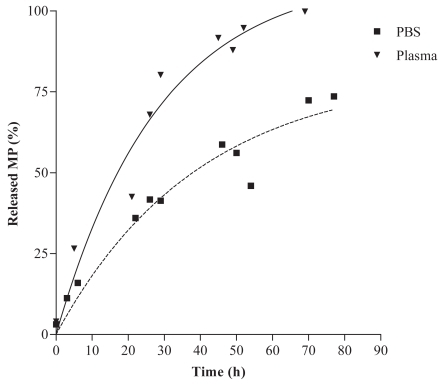
Release kinetics of MP from the conjugate were investigated in PBS (pH 7.4) and human plasma (pH 7.4) at 37 °C over 3 days (). In PBS, the half-life (t1/2) of MP released from the polymer was 50h. In human plasma, the half-life (t 1/2) of MP released from the polymer was 19 h. The half-life of MP in human plasma was expected to be shorter due to the presence of esterases (CitationMørk et al 1992; CitationSriram et al 2004).
Figure 2 Release kinetics of MP from the conjugates in PBS (pH 7.4) and human plasma (pH 7.4) at 37 °C over 3 days.
Release of MP from the conjugates also resulted in the disassembly of the nanoparticles to single polymer strands. While CDP-MP conjugates had particle size of 27 nm by DLS, treatment of CDP-MP with base resulted in a complete release of drug from the conjugate and a concomitant reduction in particle size to 9.7 nm, corresponding to the single strand hydrodynamic diameter of the parent polymer ().
Inhibition of proliferation of human lymphocytes
The effect of MP and CDP-MP on the proliferation of human lymphocytes was evaluated at day 3 and day 5 after initiation of culture (). Proliferation of human lymphocytes was induced by exposing them to concanavilin A (Con A) or phytohemagglutinin L (PHA), two potent mitogens. The stimulation of human cells with Con A and PHA did result in increased proliferation of the control cells. Concomitant incubation of stimulated cells with MP and CDP-MP lead to a concentration dependent inhibition of lymphocyte proliferation. A difference in the concentration of drug needed to cause a 50% reduction in cell prolifera- of the stimulation index) was observed between tion (IC50 the different treatment groups. In the 3 day assay and for of free MP was determined Con A as a stimulus, the IC50 to be 0.0069 μg/mL where that of CDP-MP was four times higher at 0.025 μg/mL. For PHA as a stimulus, the 3 day IC50 of free MP was 0.029 μg/mL and the of CDP-MP IC50 was 0.67 μg/mL. The Con A stimulated cells were more sensitive to treatment with the steroid formulations than the PHA stimulated cells.
Table 3 Calculated IC50 values for the stimulation index in human lymphocytes for MP and conjugated MP (μg/ml in MP equivalents)Table Footnotea (n = 3)
In the 5 day assay the Con A stimulated cells were also more sensitive to treatment with the steroid formulations than the PHA stimulated cells. For Con A as a stimulus, the IC50 of free MP was 0.00088 μg/mL whereas the IC50 of CDP-MP was 0.000024 μg/mL. The conjugate was 36 times more potent than free MP. For PHA as a stimulus, the IC50 of free MP was 0.021 μg/mL and the IC50 of CDP-MP was 0.096 μg/mL. Free MP was approximately four times as potent as CDP-MP in that case.
In vivo evaluation
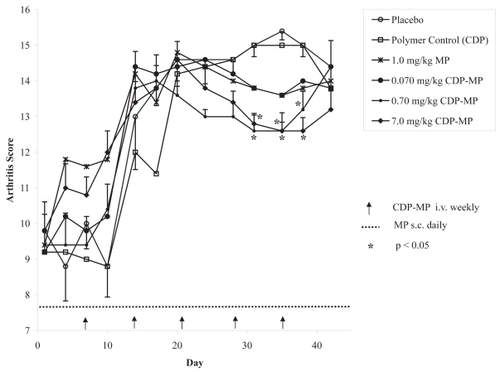
The effect of MP and CDP-MP on a murine model of collagen-induced arthritis was evaluated for 42 days after 28 days of collagen induction time (). Free MP was subcutaneously dosed at dosages of 0.01, 0.1, and 1.0 mg/kg/day for the period of the study while CDP-MP was intravenously dosed at dosages of 0.07, 0.7, and 7.0 mg/kg/week, resulting in equivalent cumulative weekly doses in the low, medium and high dose groups for both agents. There were sporadic changes in body weight over the course of the study in all the treatment groups, however these changes were not consistent over time (data not shown). The major changes seen in the study were in symptoms of arthritis. Arthritis score is a gross composite measure of swelling and the extent of arthritic changes observed. The animals were randomized at baseline to have approximately equivalent arthritic scores. As time went on, the scores became worse. Polymer control (CDP) and free MP at the two lower dose levels showed responses similar to placebo treated animals. The highest dose of free MP (1 mg/kg/day) and CDP-MP at 0.07 mg/kg/week showed a similar reduction in arthritis scores after day 28, however the effect was not statistically significant (p > 0.16). In contrast, a significant reduction in arthritis score after day 28 was seen in groups of animals treated with the two highest doses of CDP-MP at 0.7 and 7.0 mg/kg/week (p < 0.006).
Figure 3 Arthritis score by a standardized method (ranges 0 to 4). After combining scores for all paws, the maximum possible score is 16. Mean arthritis score (n = 5) is shown except for low doses of MP alone (0.01 and 0.1 mg/kg/day). Arthritis scores for these groups were similar to control animals (data not shown). Standard error of the mean (SEM) of arthritis scores is shown only for control vs. treatments that were significantly effective (p < 0.05).
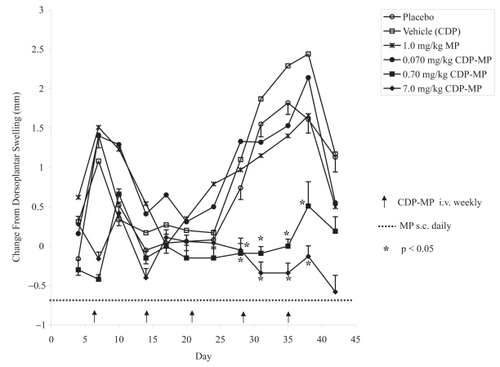
The second parameter evaluated was dorsoplantar swelling, a quantitative measurement of arthritic symptoms (). In the mouse model of collagen-induced arthritis disease progression results in a temporal rise and subsequent decrease of this parameter. Daily doses of all free MP at 0.01, 0.1, and 1.0 mg/kg/day showed similar results to weekly doses of CDP-MP at 0.07 mg/kg/week with no significant reductions in dorsoplantar swelling compared to untreated animals (p > 0.33). However, after day 28, the two highest doses of CDP-MP at 0.7 and 7.0 mg/kg/week significantly reduced dorsoplantar swelling (p < 0.03).
Figure 4 Change in dorsoplantar swelling. CDP-MP at 0.7 and 7 mg/kg show significant reduction in dorsoplantar swelling. Mean dorsoplantar swelling (n = 5) is shown except for low doses of MP alone (0.01 and 0.1 mg/kg/day). Dorsoplantar swelling for these groups was similar to control animals (data not shown). SEM of dorsoplantar swelling is shown only for control vs. treatments that were significantly effective (p < 0.05).
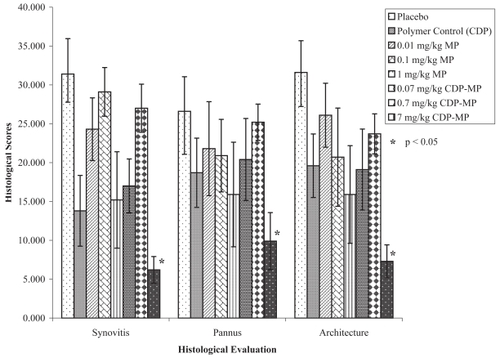
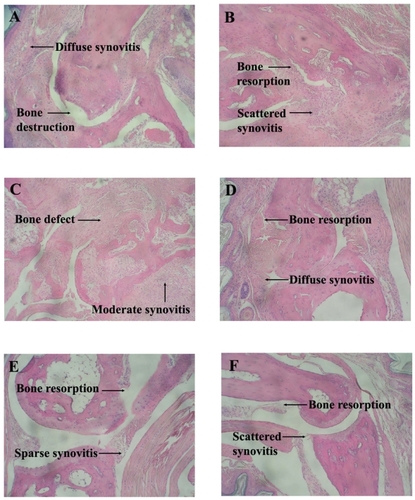
Histological evaluation was performed to determine the extent of joint damage at day 42. An overall score based on collective points from synovitis, pannus formation (thickened layers of granulation tissue) and architectural changes was assessed. Histological evidence of reduced collective points consistent with the dorsaplantar swelling data was observed ( and ). Vehicle treated control mice showed arthritis with diffuse synovitis and bone destruction. CDP treated mice showed scattered synovitis and bone resorption. Free MP-treated mice at 0.01 and 0.1 mg/kg/day showed minimal inhibition of inflammation. Mice treated with free MP at 0.01 mg/kg/day showed moderate synovitis and full thickness bone defect whereas mice treated at 0.1 mg/kg/day showed diffuse synovitis and bone resorption. CDP-MP at 0.7 mg/kg/week resulted in sparse synovitis with only small area of bone resorption. Mice treated with CDP-MP at 7.0 mg/kg/week showed scattered synovitis with small area of bone resorption. CDP-MP at 7.0 mg/kg resulted in a significant reduction in synovitis, pannus, and bone destruction compared to control animals (p < 0.04). Free MP at all dose levels and CDP-MP at 0.07 and 0.7 mg/kg did not show significant reduction in histological scores.
Figure 5 Histology photomicrographs of Hematoxylin and Eosin stained joints of mice with collagen induced arthritis. A. Vehicle control with diffuse synovitis and bone destruction. B. CDP with scattered synovitis and bone resorption. C. Free MP (0.01 mg/kg/day) with moderate synovitis and full thickness bone defect. D. Free MP (0.1 mg/kg/day) with diffuse synovitis and bone resorption. E. CDP-MP (0.7 mg/kg/week) with sparse synovitis and small area of bone resorption F. CDP-MP (7 mg/kg/week) with scattered synovitis and small area of bone resorption.
Discussion
The investigation of a CDP based nanoparticle prodrug containing MP for the treatment of RA was based on the following main considerations: First, corticosteroids are effective in the management of RA when administered systemically (orally or through i.m. or i.v. injection) or locally through direct intra-articular injections (CitationArmstrong et al 1981; CitationNeumann et al 1985). However, the rapid clearance and unfavorable biodistribution of MP requires frequent and high doses to achieve the desired therapeutic effect. This in turn can lead to a number of side effects, making it desirable to develop a parenteral formulation that would allow for lower doses, less systemic exposure and a reduction in dosing frequency. Second, nanoparticle formulations have been shown to exhibit inflammatory site targeting in various autoinflammatory diseases, including RA (CitationBoerman et al 1997; CitationDams et al 2000). Third, CDP based prodrugs of poorly water soluble drugs self-assemble into stabilized nanoparticles with a size of 30–50 nm (CitationCheng et al 2003; CitationSchluep et al 2006a, Citation2006b).
The purpose of the current study was to investigate if a conjugate of CDP with MP could improve the efficacy and reduce the dosing frequency compared to the small molecule drug in an animal model of RA. To that end we prepared a CDP-MP conjugate by covalently attaching a glycinate derivative of MP to a high molecular weight CDP (117 kD) through an ester linkage. The resulting polymer conjugate was highly water soluble (>200 mg/mL) and self-assembled into nanoparticles with an average diameter of 27 nm. This is consistent with our previous observations with this cyclodextrin-based polymer system. For example, a glycinate ester conjugate of camptothecin with the same parent polymer (IT-101) resulted in nanoparticles in a similar size range. This self-assembly requires covalent attachment of the small molecule drug to the polymer. Parent polymer by itself and conjugate from which all the drug was released showed a particle size of 9.7 nm by DLS, consistent with the expected hydrodynamic diameter of single strand polymer.
Release kinetics
Release kinetics of MP from CDP-MP were studied in vitro in PBS and human plasma. The half-life in PBS and plasma was 50 h and 19 h, respectively. Glycinate ester prodrugs can be activated by base catalyzed hydrolysis as well as enzymatically by esterases. The increased release in plasma is consistent with esterase catalyzed cleavage of the glycinate ester. However, the half-life of the CDP-MP prodrug in plasma is still considerably longer than what is typically observed with small molecule ester prodrugs, which normally have half lives of 1 to 3 hours (CitationMørk and Bundgaard 1992; CitationSriram et al 2004). This resistance to esterase cleavage is most likely due to steric hindrance by the polymer or inaccessibility of the ester groups within the nanoparticle. Appropriate linker stability in plasma allows for a long circulating pool of prodrug with extended release kinetics, reducing the need for frequent dosing. While we have not studied the pharmacokinetics of the present compound in vivo, based on our previous investigations of similar CDP conjugates, one can expect substantial improvements in the pharmacokinetics of the nanoparticle compared to the small molecule analog (CitationSchluep et al 2006b). With camptothecin as a model compound, the plasma half-life of the small molecule in rats was less than 2 hours whereas the CDP conjugate showed a plasma half-life of close to 20 hours.
Efficacy in vitro
The polymeric conjugates are expected to be inactive in their prodrug form and must release the drug from the delivery system to provide their anti-inflammatory effect. We therefore studied the ability of MP and CDP-MP to inhibit the proliferation of human lymphocytes in a 3- and 5-day assay in vitro. In order to compare the ability to inhibit proliferation between free MP and CDP-MP, the cultures were exposed to different concentrations of the drugs in the presence of phytohemagglutinin (PHA) or concanavilin A (Con A) as mitogens. Stimulation of isolated human lymphocytes with Con A and PHA, two lectin mitogens, resulted in proliferation of the control cells. MP acts at the G1 phase by inhibiting cytokine gene transcription in antigen presenting cells (eg, IL-1) and T lymphocytes (eg, IL-2). The Con A stimulated cells were more sensitive to treatment with both MP or CDP-MP compared to the PHA stimulated cells. The reason for this difference is unknown at this time, however, the general response to treatment was with MP and CDP-MP was similar in both cases. At an early timepoint (3 days) CDP-MP was less potent than free MP by a factor of 4 in the case of Con A and by a factor of 23 in the case of PHA as stimulus. While the potency of MP remained about the same in the 5 day assay, the potency of CDP-MP was increased considerably. In fact, CDP-MP was about 36-fold more potent than free MP at day 5 in the Con A stimulated cells and only about 4-fold less potent than free MP in the PHA stimulated cells. This delayed effect is consistent with a slow release of the drug over time. These data also show that release of the drug from the polymer conjugate is necessary for a biologic effect to occur.
Rationale of methylprednisolone dosage
Studies in rat and murine CIA models indicate that daily doses of 1–4 mg/kg prednisolone or methylprednisolone orally, or by subcutaneous or intraperitoneal administration were effective in reducing paw edema, arthritis score, joint destruction and pro-inflammatory cytokine levels (CitationGeiger 1994; CitationSmith and Sly 1996; CitationKamada et al 1997; CitationPaul-Clark et al 2002; CitationRioja 2004; CitationMancini 2007). The low end of this range was chosen as the maximum daily dose for free MP whereas CDP-MP was dosed at equivalent doses but on a weekly schedule. Because daily intravenous injections in mice are difficult to execute for prolonged periods of time due to tail vein scarring, the more reproducible and convenient subcutaneous route was chosen for the MP control animals. This is supported by studies in rats indicating that subcutaneous and intravenous injections of MP are expected to result in equivalent pharmacodynamics and activity (CitationFrenkel and Havenhill 1963; CitationHazra 2007).
Efficacy in vivo
The efficacy of CDP-MP was studied in vivo in a collagen induced arthritis model. In this model, arthritis is induced in mice by subcutaneous injection of bovine collagen type II. Animals develop the typical signs of arthritis such as joint swelling and histological changes such as cellular infiltrates, synovial hypertrophy and bone destruction. Many of these changes happen in a distinct temporal manner, therefore treated and untreated animals are monitored over a period of 42 days. All treatments were well tolerated with no significant body weight changes observed in any of the treatment groups. Joint swelling was scored by arthritis score which is a standardized method to measure swelling and hyperemia. At earlier time points, both free MP and CDP-MP did not show significant difference in the reduction of inflammation. However, after 28 days, significant decreases in arthritis score were shown in animals treated with weekly doses of CDP-MP at 0.7 and 7.0 mg/kg by intravenous injection. Free MP at the highest dose (1.0 mg/kg daily, 7.0 mg/kg weekly) resulted in a reduction in arthritis score that was similar to the one observed with 0.07 mg/kg CDP-MP, however these effects were not statistically significant compared to control animals. A second parameter analyzed was dorsoplantar swelling, a quantitative measurement of arthritic symptoms. Dorsoplantar swelling was reduced to baseline in animals treated weekly with 0.7 and 7.0 mg/kg of CDP-MP. In contrast free MP given daily at equivalent cumulative doses had a non-significant effect on dorsoplantar swelling. This result was confirmed by histological evaluation of the inflamed joints, where only the high dose group of, CDP-MP at 7.0 mg/kg showed a significant reduction in bone resorption, pannus formation, and synovitis. In summary, weekly CDP-MP was effective in reducing the symptoms of collagen induced arthritis in mice at doses up to 100-fold lower using weekly rather than daily injections compared with MP alone.
In vitro studies presented here and in vivo pharmaco-kinetics and biodistribution studies with a similar polymer nanoparticle prodrug (CitationSchluep et al 2006b) show that this may be achieved through three specific mechanisms: (A) Increased circulation half-life and slow release of drug from the nanoparticle prodrug. We have seen plasma half-lives of close to 20 hours in rats dosed with a similar nanoparticle containing camptothecin. Combined with the slow release kinetics of MP in plasma shown in this study, this allows for a reduction in dosing frequency compared to the small molecule analog MP with a reported plasma half-life of less than two hours. (B) Increase in biodistribution to target organs. We and others have shown that long circulating nanoparticles accumulate in organs of the reticuloendothelial system such as spleen and liver (CitationStorm 1995; CitationGaur et al 2000; CitationZhang and Mehvar 2001a, Citation2001b; CitationBrigger et al 2002; CitationSchluep et al 2006b). Additionally, multiple publications have shown that nanoparticles accumulate in sites of inflammation, such as arthritic joints (CitationBoerman 1997; CitationDams 2000; CitationMetselaar et al 2003). (C) Multiple reports show increased enzymatic activity including esterase activity at sites of inflammation (CitationMørk and Bundgaard 1992; CitationSriram 2004). This provides a site specific release mechanism of the MP from its polymer carrier.
In an attempt to reduce the toxicity and increase the effectiveness of immunosuppressants, local immunosuppression at the site of inflammation has been used for the treatment of arthritis (CitationBliddal 2006; CitationBouysset 2006; CitationNeustadt 2006). This strategy is based on evidence indicating that in addition to the inhibition of the systemic immune system (such as inhibition of splenic lymphocytes), the inhibition of immune events at the site of inflammation provides increased patient benefit. Here we present data that CDP conjugates of MP showed improved efficacy when compared to free MP in a collagen induced arthritis model. CDP conjugation may be an attractive strategy for targeting MP and other immunosuppressive agents to their sites of action.
Disclosure
The authors report no conflicts of interest in this work.
References
- ArmstrongRDEnglishJGibsonT1981Serum methylprednisolone levels following intra-articular injection of methylprednisolone acetateAnn Rheum Dis4057147332377
- BliddalHTerslevLQvistgaardE2006Safety of intra-articular injection of etanercept in small-joint arthritis: an uncontrolled, pilot-study with independent imaging assessmentJoint Bone Spine737141717064943
- BodmanKBRoittIM1994The pathophysiology of rheumatoid arthritisFund Am Clin Immunol27381
- BoermanOCOyenWJStormG1997Technetium-99m labeled liposomes to image experimental arthritisAnn Rheum Dis56369739227166
- BouyssetMHuguenyPGintzB2006Corticosteroid injections and synoviortheses of the foot and ankle in rheumatoid arthritisFoot and ankle in rheumatoid arthritisParisSpringer-Verlag12030
- BriggerIDubernetCCouvreurP2002Nanoparticles in cancer therapy and diagnosisAdv Drug Deliv Rev546315112204596
- ButtgereitFStraubRHWehlingM2004Glucocorticoids in the treatment of rheumatic diseasesArthritis Rheum5034081715529366
- ByronMAMowatAG1985Corticosteroid prescribing in rheumatoid arthritis-the fiction and the factRheumatology241646
- CannettiCALeungBPCulshawS2003IL-18 enhances collagen-induced arthritis by recruiting neutrophils via TNF-α and leukotriene B4J Immunol17110091512847274
- ChandrasekarDSistlaRAhmadFJ2007Folate coupled poly(ethyleneglycol) conjugates of anionic poly(amidoamine) dendrimer for inflammatory tissue specific drug deliveryJ Biomed Mater Res A829210317269145
- ChengJKhinKTJensenGS2003Synthesis of linear, α-cyclodextrin-based polymers and their camptothecin conjugatesBioconjugate Chem14100717
- DamsETReijnenMMOyenWJ1999Imaging experimental intraabdominal abscesses with 99mTc-PEG liposomes and 99mTc-HYNIC IgGAnn Surg229551710203089
- DamsETMOyenWJGBoermanOC200099mTc-PEG liposomes for the scintigraphic detection of infection and inflammation: Clincal evaluationJ Nuc Med4162230
- DuncanR2003The drawing era of polymer therapeuticsNature Rev Drug Discov23476012750738
- FarrelRJKelleherD2003Glucocorticoid resistance in inflammatory bowel diseaseJ Endocrinol1783394612967327
- FrenkelJKHavenhillMA1963The corticoid sensitivity of golden hamsters, rats and miceLab Invest1212042014098351
- GaurUSahooSKDeTK2000Biodistribution of fluoresceinated dextran using novel nanoparticles evading reticuloendothelial systemInt J Pharm20211010915921
- GeigerTRordorfCCosenti-VargasA1994CGP 47969A: Effect on collagen induced arthritis in DBA/1 miceJ Rheumatol21199277869300
- HazraAPyszczynskiNDuBoisDC2007Pharmacokinetics of methylprednisolone after intravenous and intramuscular administration in ratsBiopharm Drug Dispos282637317569107
- HayballPJCoshDGAhernMJ1992High dose oral methylprednisolone in patients with rheumatoid arthritis: pharmacokinetics and clinical responseEur J Clin Pharm42858
- HyrichKSymmonsDWatsonK2006Baseline comorbidity levels in biologic and standard DMARD treated patients with rheumatoid arthritis: results from a national patient registerAnn Rheum Dis65895816339291
- KamadaHGotoMMatsuuraS1997Immunopharmacological studies on collagen-induced arthritis in Dark Agouti (DA) ratsJpn J Pharmacol74313229307327
- KarNCCracchioloAMirraJ1976Acid, neutral, and alkaline hydrolases in arthritic synoviumAm J Clin Pathol6522083109
- KhouryMLouis-PlencePEscriouV2006Efficient new cationic liposome formulation for systemic delivery of small interfering RNA silencing tumor necrosis factor α in experimental arthritisArthritis Rheum5418677716729293
- KonttinenYTSeitsaloSLehtoM2005Current management: Management of rheumatic diseases in the era of biological anti-rheumatic drugsActa Orthop76614916263606
- LaanRFJMJansenTLTAvan RielPLCM1999Glucocorticosteroids in the management of rheumatoid arthritisRheumatology3861210334676
- LavermanPDamsETOyenWJ1999A novel method to label liposomes with 99mTc by the hydrazino nicotinyl derivativeJ Nucl Med4019279935076
- LawrenceRCHelmickCGArnettFC1998Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United StatesArthritis Rheum41778999588729
- ManciniLPaul-ClarkMJRosignoliG2007Calcitonin and prednisolone display antagonistic actions on bone and have synergistic effects in experimental arthritisAm J Pathol17010182717322385
- MatsumuraYMaedaHA1986New concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent SMANCSCancer Res66387922946403
- MetselaarJMWaubenMHMWagenaar-HilbersJPA2003Complete remission of experimental arthritis by joint targeting of glucocorticoids with long-circulating lipososmesArthritis Rheum4820596612847701
- MørkNBundgaardH1992Stereoselective enzymatic hydrolysis of various ester prodrugs of ibuprofen and flurbiprofen in human plasmaPharm Res949261495894
- NeumannVHopkinsRDixonJ1985Combination therapy with pulsed methylprednisolone in rheumatoid arthritisAnn Rheum Dis44747512865930
- NeustadtDH2006Intra-articular injections for osteoarthritis of the kneeCleveland Clin J Med73897911
- OkadaAA2005Immunomodulatory therapy for ocular inflammatory disease: A basic manual and review of the literatureOcul Immunol Inflamm133355116419419
- PaleologEMFavaRA1998Angiogenesis in rheumatoid arthritis: implications for future therapeutic strategicsSpringer Semin Immunopathol2073949836370
- Paul-ClarkMJManciniLDel SoldatoP2002Potent antiarthritic properties of a glucocorticoid derivative, NCX-1015, in an experimental model of arthritisProc Natl Acad Sci USA9916778211805287
- RiojaIBushKABucktonJB2004Joint cytokine quantification in two rodent arthritis models: kinetics of expression, correlation of mRNA and protein levels and response to prednisolone treatmentClin Exp Immunol137657315196245
- SaagKG2002Glucocorticoid use in rheumatoid arthritisCur Rheumatol Rep421825
- SchluepTHwangJChengJ2006aPreclinical efficacy of the camptothecin-polymer conjugate IT-101 in multiple cancer modelsClin Cancer Res1216061416533788
- SchluepTChengJKhinKT2006bPharmacokinetics and biodistribution of the camptothecin – polymer conjugate IT-101 in rats and tumor-bearing miceCancer Chemother Pharmacol576546216133526
- SmithRJSlyRM1996Type II collagen-induced arthritis in the diabetic-resistant biobreeding rat: inflammatory and histopathological features of joint pathology and effects of anti-inflammatory and antirheumatic drugs on this chronic arthritic processJ Pharmacol Exp Ther2771801138667252
- SnellES1976The pharmacological properties of corticosteroids in relation to clinical efficacyBr J Demartol941523
- SriramDYogeeswariPSricharkravarthyN2004Synthesis of stavudine amino acid ester prodrugs with broad-spectrum chemotherapeutic properties for the effective treatment of HIV/AIDSBioorg Med Chem Lett141085714980640
- StormGBelliotSODaemenT1995Surface modification of nanoparticles to oppose uptake by the mononuclear phagocyte systemAdv Drug Deliv Rev173148
- VicentMJ2007Polymer-drug conjugates as modulators of cellular apoptosisAAPS J2E200717907762
- WilliamsBDO’SullivanMMSagguGS1987Synovial accumulation of technetium labeled liposomes in rheumatoid arthritisAnn Rheum Dis4631483592788
- WongJBennettWFergusonMW2006Microscopic and histological examination of the mouse hindpaw digit and flexor tendon arrangement with 3D reconstructionJ Anat2095334517005025
- ZhangXMehvarR2001Dextran-methylprednisolone succinate as a prodrug of methylprednisolone: plasma and tissue dispositionJ Pharm Sci9020788711745766
- ZhangXMehvarR2001Dextran-methylprednisolone succinate as a prodrug of methylprednisolone: dose-dependent pharmacokinetics in ratsInt J Pharm2291738211604270