Abstract
There is a growing understanding of the pathophysiology of secondary hyperparathyroidism (SHPT) and a recent emergence of new agents for SHPT treatment in patients with advanced kidney disease. At the same time, appreciation that mineral metabolic derangements promote vascular calcification and contribute to excess mortality, along with recognition of potentially important “non-classical” actions of vitamin D, have prompted the nephrology community to reexamine the use of various SHPT treatments, such as activated vitamin D sterols, phosphate binders, and calcimimetics. In this review, the evidence for treatment of SHPT with calcimimetics and vitamin D analogs is evaluated, with particular consideration given to recent clinical trials that have reported encouraging findings with cinacalcet use. Additionally, several controversies in the pathogenesis and treatment of SHPT are explored. The proposition that calcitriol deficiency is a true pathological state is challenged, the relative importance of the vitamin D receptor and the calcium sensing receptor in parathyroid gland function is summarized, and the potential relevance of non-classical actions of vitamin D for patients with advanced renal disease is examined. Taken collectively, the balance of evidence now supports a treatment paradigm in which calcimimetics are the most appropriate primary treatment for SHPT in the majority of end stage renal disease patients, but which nevertheless acknowledges an important role for modest doses of activated vitamin D sterols.
Introduction
Several cardinal findings over the past decade have thrust derangements of mineral metabolism to the forefront of nephrology. Studies demonstrating the association of phosphorous with mortality in end stage renal disease (ESRD) patients,Citation1,Citation2 the prevalence of vascular calcification even in young ESRD patients,Citation3 and the association of calcium-based binders with increased vascular calcification relative to non-calcium based bindersCitation4–Citation7 have prompted a re-examination of traditional therapies for secondary hyperparathyroidism (SHPT) of advanced renal disease.
Accompanying increased awareness of the importance of mineral metabolic dysregulation have been numerous clinical and scientific advancements. The development and introduction of calcimimetics for the treatment of SHPT stands as a major clinical milestone. Calcimimetics, which are allosteric modulators of the calcium sensing receptor (CASR), have rapidly achieved widespread use in ESRD patients with SHPT. In parallel, there have also been major advancements in understanding the pathogenesis of SHPT, with a greater appreciation of the biological functions of the CASR, the vitamin D receptor (VDR), the regulation of 1,25(OH)2 D production and metabolism by the 1α-hydroxylase/24-hydroxylase (Cyp17b1/Cyp24) enzyme system, and the role of fibroblast growth factor-23 (FGF23), the principle phosphaturic factor. Additionally, there is growing interest in potential “non-classical” actions of vitamin D, ie, those actions beyond its traditional role in the maintenance of mineral homeostasis. Unfortunately, the clinician’s success in reaching targets for control of calcium (Ca), phosphate (P), calcium × phosphorous product (Ca × P), and parathyroid hormone (PTH) remains suboptimal.
In this review, we explore in detail the body of the evidence for treatment of SHPT with both calcimimetics and vitamin D analogs, also known as activated vitamin D compounds, vitamin D sterols, or calcitriol analogs, and discuss the clinical study evidence as well as the experimental basis for these treatments. We also consider several emerging controversies with direct relevance to SHPT treatment, specifically (1) whether reduced 1,25(OH)2 D in chronic kidney disease (CKD) is pathological condition requiring treatment or an adaptive response to decreased renal function, (2) whether the CASR or the VDR is relatively more important in parathyroid gland (PTG) function, and (3) whether “non-classical” actions of vitamin D have relevance for patients with advanced kidney disease. We conclude with a conceptual framework for the treatment of SHPT in end stage renal disease (ESRD).
Review criteria
A PubMed search of English-language articles from 1988 to the present was undertaken. The search strategy utilizing a first term of “calcimimetics”, “cinacalcet”, “calcium sensing receptor”, “vitamin D”, “vitamin D receptor”, “FGF23”, or “fibroblast growth factor”, coupled by the operator AND with a second term of “secondary hyperparathyroidism”, “end stage renal disease”, “chronic kidney disease”, “chronic kidney failure”, or “chronic renal failure.” Original reports as well as reviews were examined. For all reviews, references to original reports were sought. Bibliographies of each report were examined for additional relevant articles not captured by the initial search strategy. This search strategy was augmented by hand-searches for publications of interest in the authors’ personal libraries.
Discussion
What evidence exists that cinacalcet improves mineral metabolic control in SHPT?
A relatively large number of clinical studies have been undertaken to investigate the effects of cinacalcet on the control of SHPT in ESRD patients. There have been eight published randomized controlled trials (RCTs) plus the ACHIEVE trial, which is currently in submission. Initial trials were designed to test the effects of cinacalcet when added to standard-of-care therapy (consisting of either no treatment other than oral phosphorous binders or, much more commonly, vitamin D analogs), while later trials utilized designs in which cinacalcet was tested in combination with low-dose vitamin D analogs against higher-dose vitamin D “monotherapy.” More recently, five “single-treatment-arm” studies, in which cohorts of subjects were compared to themselves pre- and post-treatment with cinacalcet, have been published. While not true RCTs, these latter studies provide invaluable real-world clinical insights.
Randomized controlled trials of cinacalcet
In 2002, the first evidence that cinacalcet lowered PTH levels emerged.Citation8 When cinacalcet, then known as AMG 073, or placebo was given to 30 hemodialysis (HD) patients for 8 days, treated subjects demonstrated a statistically significant improvement in PTH levels, measured as percent change from baseline. Calcium, P and Ca × P also fell, although p-values were not reported. Soon thereafter, Quarles et alCitation9 and Lindberg et alCitation10 reported on true placebo-controlled RCTs of cinacalcet. Both studies consisted of 12-week titration phases and 6-week assessment phases. Quarles et al studied 71 HD patients, approximately two-thirds of whom were taking vitamin D analogs, and found that cinacalcet treatment resulted in robustly significant decreases in PTH, Ca, and Ca × P, when measured as percent change from baseline. Phosphorus did not change. Vitamin D analog use did not affect the results, because when subjects were divided into those who had an increase, decrease, or no change in vitamin D analog dose over the course of the study, reductions in percent change in PTH remained significant (p = 0.008). The study by Lindberg et al involving 78 HD patients found that cinacalcet-treated subjects achieved statistically-significant improvements in percent change of PTH, Ca, P, and Ca × P (p < 0.001 for all). Again, about two-thirds of subjects were using vitamin D analogs at the start of the study; these investigators used logistic regression to determine that its use was not associated with reductions in PTH.
Larger studies, with increased power to examine multiple biochemical outcomes, were subsequently conducted. Lindberg et al studied 395 HD and peritoneal dialysis (PD) patients, and found that cinacalcet was associated with statistically-significant decreases in both mean and percent change of PTH, Ca, and Ca × P, as well as the percent of subjects achieving PTH ≤ 300 pg/mL and Ca × P < 55 mgCitation2/dL2.Citation11 The largest study of cinacalcet versus placebo was that of Block et al,Citation12 which consisted of two identical parallel trials (in North America and Europe) enrolling a total of 741 HD patients. The percent of subjects reaching PTH ≤ 250 pg/mL (the primary endpoint), the percent change in PTH from baseline, and the difference in final mean PTH value were all statistically significant, as were decreases in Ca and P. In stratified analyses of sex, age, race (African versus European descent), dialysis duration, strata of initial PTH (using cutoffs of 300, 500, and 800 pg/mL), and use of vitamin D analogs, cinacalet was superior in the attainment of the primary endpoint.
All of the above studies were designed as “real world” studies in which providers had the liberty to adjust vitamin D analog doses as they saw fit, albeit according to a study-specific standardized protocols. This was done to allow the subjects to continue to receive standard-of-care therapy, which at that time included vitamin D analogs for SHPT. In each of the studies, vitamin D analog doses remained relatively constant within each arm as well as comparable across arms at the baseline and final timepoints. While this type of design allows for the isolation of the effects of cinacalcet, it does not permit a determination of whether one agent is more effective than another for control of SHPT, or yield insights as to whether one therapy (cinacalet) could replace another (vitamin D analogs) as primary therapy.
A recent metaanalysis by Strippoli et al formally examined the above studies.Citation13 These investigators concluded that, for absolute levels of PTH, Ca, P, and Ca × P, cinacalcet resulted in significantly different outcomes relative to placebo. However, several other RCTs were either not included in the above analysis, or were published subsequent to it. Martin et al randomized 410 HD patients to cinacalcet or placebo and found that cinacalcet-treated subjects had statistically-significant decreases in several biochemical endpoints, including percent change in PTH, mean concentrations of PTH, Ca, P, and Ca × P, and the proportion of subjects reaching PTH < 250 pg/mL.Citation14 The percentage of subjects receiving vitamin D analogs was no different between groups at baseline and at study completion. Fukugawa et al used lower doses of cinacalcet (100 mg/day being the highest allowable dose, versus 180 mg for most other trials) to randomize Japanese patients to cinacalet versus placebo and found significant decreases in Ca, P and Ca × P, as well as a significantly greater number of subjects reaching 30% reductions in PTH and PTH ≤ 250 pg/mL.Citation15 Vitamin D analog use at baseline was reported to have no effect on attainment of goals, but a full description of activated vitamin D usage in the two groups was not reported.
Only two studies, to our knowledge, have attempted to minimize vitamin D analog dose in an attempt to determine whether cinacalcet might be used in lieu of, rather than in addition to, vitamin D analogs. Messa et al recently published the results of the OPTIMA study.Citation16 These investigators deliberately attempted to titrate down vitamin D analog dose, while preserving the provider’s ability to dose as they saw fit. A total of 552 subjects were enrolled in an open-label study. These authors found cinacalcet to be superior in achieving goals for PTH (≤300 pg/mL), Ca (<9.5 mg/dL), P (<5.5 pg/mL), Ca × P (≤55 mgCitation2/dLCitation2), and Ca × P plus PTH endpoints. While there was only a modest relative dose change in vitamin D between the groups (a 6% decrease in the cinacalcet group versus a 14% increase in the vitamin D-alone group), in the subgroup of subjects receiving vitamin D analogs at baseline (68% of the total study sample), there was a 22% decrease in relative vitamin D analog dose in the cinacalcet arm as opposed to a 3% increase in the control arm; whether this was statistically significant was not reported. Finally, the ACHIEVE study, currently under peer review, randomized approximately 178 HD patients to cinacacet and low-dose vitamin D analogs or vitamin D alone. This study showed that cinacalcet-treated subjects resulted in significantly higher numbers of subjects reaching targets for PTH, P, and Ca × P KDOQI targets, although there was more hypocalcemia in the cinacalcet arm.
The majority of evidence now demonstrates that cinacalcet is an extremely effective agent for the suppression of PTH, and that it has a clear role in therapy for SHPT. Such conclusions emerge readily upon collectively reading individual reports, while confirmation of this is provided in the findings of the recent metaanalysis. Even more significantly, the OPTIMA and ACHIEVE studies provide convincing evidence that cinacalcet can have a vitamin D analog-minimizing effect while achieving important clinical targets, suggesting that cinacalcet is a viable candidate for primary treatment for SHPT.
Other clinical studies of cinacalcet
As cinacalcet is increasingly used in clinical practice, other investigators are reporting on their long-term experiences with this agent, particularly in situations where cinacalcet-based regimens have supplanted vitamin D analog-based ones in clinical practices. Moe et al reported on 59 subjects who completed 100 weeks of an open-label trial of cinacalcet.Citation17 They demonstrated long-term control of PTH, in which the level of PTH at 52 weeks was little changed at 100 weeks, with PTH at both timepoints being significantly better than at baseline. Sterrett et al reported on 1 year of cinacalcet use in 210 HD patients, demonstrating a significantly greater decrease in percent change of PTH, percent change of Ca × P, attainment of PTH ≤ 250 pg/mL, and ≥30% reduction in PTH.Citation18
Two recent studies involved paradigms in which deliberate attempts were made to reduce vitamin D analog dose. Chertow et al examined 72 subjects with elevated Ca × P, all of whom were taking vitamin D analogs, in an open-label prospective study.Citation19 Subjects had their vitamin D analog dose reduced to “physiologic” levels of calcitriol (eg, paricalcitol 2 μg thrice weekly) while cinacalcet was initiated and subsequently titrated upwards as needed. Percent change in PTH, Ca, P, and Ca × P, and percent of subjects reaching Ca × P goal were all significantly greater in the cinacalcet arm (p < 0.0001 for all), while mean vitamin D dose was halved (and discontinued outright in 21% of subjects), demonstrating the ability of cinacalcet to “spare” vitamin D analogs. An open-label study by Block et al involved 375 subjects in which doses were reduced to low levels at study initiation.Citation20 Mean vitamin D dose was also halved in this study, and percent change in PTH, Ca, P, and Ca × P were significantly different between arms (p < 0.0001), again demonstrating that cinacalcet can be effective therapy for SHPT even in the face of lowering vitamin D dose.
Cinacalcet has also been tested in patients with extremely high levels of PTH. Arenas et al treated 28 HD patients with refractory SHPT (mean PTH 829 pg/mL), in whom treatment with vitamin D analogs was compromised by hypercalcemia or hyperphosphatemia, with cinacalcet.Citation21 They observed robust decreases in PTH, Ca, and Ca × P product, while P did not change. No decrement in vitamin D dose was necessarily sought, since the patients were by definition difficult to control with vitamin D analogs alone. After 9 months, all subjects achieved PTH ≤ 500 pg/mL, demonstrating cinacalcet’s usefulness is refractory SHPT.
Finally, in analyses of small single-center cohorts, other investigators have studied the effects of changes in treatment protocols. Lazar et alCitation22 and Speigel et alCitation23 initiated new cincacalcet-based paradigms, and compared attainment of KDOQI Clinical Practice Guideline targets before and after conversion of their treatment paradigms. While the studies were modest in size (35 and 61 subjects, respectively), and statistical power therefore limited, the former study demonstrated a significant increase in subjects attaining at least three mineral metabolic targets, and the latter a significant improvement in subjects attaining PTH goals. In the former study, the investigators reported that there was a 33% increase in subjects administered paricalcitol, presumably due to either hypocalcemia or because lowered cinacalcet-induced Ca × P permitted reintroduction of vitamin D analogs. In the latter study, vitamin D doses did not change in the cinacalcet-treated subjects.
Other evidence for benefits of cinacalcet
As reviewed definitively by Brown and MacLeod,Citation24 the CASR is found in a variety of tissues. Many of these are unlikely to play a major role in mineral metabolism, such as the brain, pancreas, bone marrow, pituitary, skin (keratinocytes) and breast (ductal cells). However, the CASR is found in other locations which may have direct impact on mineral metabolism, and, as such, morbidity in dialysis patients. Three such tissues are the parathyroid gland (PTG), the bone, and the vasculature.
In the PTG, the CASR regulates PTH secretion acutely and PTH gene transcription chronically.Citation24,Citation25 It also regulates PTG cell growth.Citation26 In both humans with neonatal severe hyperparathyroidismCitation27 and in the analogous homozygous casr animal knockouts,Citation28 there are increases in PTH, serum Ca, and hyperplasia of the PTG, while in uremia-induced SHPT, the CASR is downregulated in humans and animals.Citation29–Citation32 As reviewed by Drueke at al,Citation33 the effects of calcimimetcs have been extensively studied in rodents, where cinacalcet has consistently been shown to prevent to development of, or mitigate the effects of, PTG hyperplasia.Citation34,Citation35 Cinacalcet has even been shown to reverse hyperplasia.Citation35 Calcimimetics have also been shown to upregulate the CASR in rodent models of renal failure.Citation36
The effects of cinacalcet on calcium-mediated PTH release have been examined by de Francisco et al, who studied 10 subjects with extreme SHPT (mean PTH 1116 pg/mL).Citation37 Subjects were placed on alternating low-Ca and high-Ca dialysate baths to maximally stimulate and suppress the PTG, exposed to a mean of 13 weeks of cinacalcet, then tested again in the same manner to determine if there was any effect of cinacalcet on the PTH release setpoint (the Ca level associated with 50% maximal PTH stimulation). Cinacalcet reduced the setpoint and the maximal PTH release, an important finding because this setpoint may be a marker for the severity of SHPT and of PTG mass. More direct, but still rather preliminary, evidence of a potential association between cinacalcet and PTG histology comes from Lomonte et al.Citation38 These investigators examined PTG glands and found that nodular hyperplasia was more common in vitamin D analog-treated and in cinacalcet plus vitamin D-treated individuals than in individuals treated with binders alone. Because no subjects were exposed to cincacalcet monotherapy, they relied on linear regression to attempt to isolate the independent effects of cinacalcet, and found that the drug was associated with a significant increase in oxyphil to chief cell ratio. Oxyphil cells have been reported to proliferate at a lower rate than chief cells,Citation39 perhaps indicating that cinacalcet could slow the histologic progression of SPTH. However, the study was small, and the results should be considered hypothesis-generating.
Only one report, to our knowledge, has investigated the potential effects of calcimimetics in bone histology of humans.Citation40 Cinacalet use was associated with improved bone histomorphometry. While vitamin D analog use was not controlled for, the investigators attempted to minimize the dose. Cinacalcet-treated subjects had reduced markers of bone turnover and bone turnover rates by histology. However, they also experienced improvement in PTH, so improvements in bone histology may have been the result of better mineral metabolic control, rather than of any specific benefit attributable to cinacalcet per se.
Of great importance in ESRD patients are the potential effects of calcimimetics on the cardiovascular system. In vitro models demonstrate that functional CASR is present in rat cardiac myocytes.Citation41,Citation42 When such cells were exposed to calcium, intracellular inosotol phosphate concentrations increased, while exposure to cinacalcet shifted the curve to the left, implicating the CASR in this pathway.Citation42 Cinacalcet also reduced DNA synthesis in proliferating neonatal cardiac myocytes, indicating that the CASR may play a role in cardiac hypertrophy.Citation42 The CASR also appears to be present in blood vessels. While not all studies have demonstrated this,Citation43 several investigators have reported the CASR in adventitiaCitation44 and endothelium.Citation45,Citation46 In human aorta, the CASR agonist spermine increased intracellular Ca and NO production.Citation47 Taken together with data showing that dietary Ca causes vascular relaxation, it is plausible to suggest that the CASR plays a role in vascular relaxation.Citation48 In vivo models are also promising in this regard. In the uremic rodent model, calcimimetics were associated with decreased cardiac remodeling,Citation49 raising the possibility that cinacalcet could provide protection against cardiac remodeling and vascular calcification in humans.
Whether calcimimetics might demonstrate survival beneficial effects in ESRD patients though these actions on the PTG, bone, or the vasculature, requires much future investigation. However, some tantalizing evidence for this possibility has recently emerged. In study of 4 similarly-designed trials comprising 1184 subjects, Cunningham et al found significant associations between use of cinacalcet and decreased risk of parathyroidectomy and cardiovascular hospitalization, although not with mortality risk.Citation50 While the study was a retrospective and relied on pooled data, it provides intriguing preliminary evidence that cinacalcet might be a useful in reducing the need parathyroidectomy, and, more profoundly, might also have cardioprotective effects.
What evidence exists that vitamin D analogs improve mineral metabolic control in SHPT?
The clinical trial evidence for the ability of vitamin D analogs to improve mineral metabolic parameters is somewhat less than that for cinacalcet, which is probably a reflection that the former’s demonstrable ability to lower PTH allowed it to become standard-of-care without evidence from multiple RCTs. There have been a few RCTs which have tested the efficacy of vitamin D analogs versus placebo for treatment of SHPT. For example, Martin et alCitation51 and Delmez et alCitation52 tested paricalcitol and calcitriol, respectively, against placebo, and found significant improvements in PTH in response to exposure to the activated vitamin D compound. Several trials have been designed to test one (ie, a non-selective) agent against another (ie, a selective agent) over timeframes ranging from a few daysCitation53,Citation54 to 32 weeks,Citation55 although longer-term studies of cohorts exposed to single agents in non-placebo-controlled studies have been conducted over a year or more.Citation56–Citation58 Control of mineral metabolic parameters, and not mortality, was their principle endpoint.
However, there are been several large observational studies that have shown a consistent positive association between treatment of ESRD patients with vitamin D analogs and survival. Using the Fresenius Medical Care database, Teng et al examined over 67,000 HD patients to compare the effects of paricalcitol, a selective vitamin D analog, with calcitriol, a first-generation non-selective agent.Citation59 They observed an adjusted hazard ratio (HR) of 0.84 (95% confidence intervals [CI] 0.79–0.90) for paricalcitol relative to calcitriol. In a subsequent study, these investigators investigated whether use of any vitamin D analog provided survival benefits relative to no use. Over 50,000 Fresenius Medical Care dialysis patients were examined for 2-year survival.Citation60 They observed a 20% survival improvement for vitamin D analogs (HR 0.80, 95% CI 0.76–0.83). The investigators used sophisticated statistical techniques, including treatment of the exposure as time-dependent (to appropriately credit exposure risks and benefits to actual exposure), use of Cox regression models augmented by stratum-specific hazard ratios, sensitivity analyses to test for consistency of results under various assumptions, and, in a secondary analysis, the relatively novel technique of inverse probability of treatment weighting (IPTW), in which subjects are assigned a probability of receiving an exposure (vitamin D analogs) in a time-dependent fashion. Through these numerous analyses, the survival benefit attributable to vitamin D analogs was generally in the 20% to 27% range; that multiple statistical approachs yielded a similar magnitude of benefit strengthens their conclusions.
Kalentar-Zadeh et al examined over 58,000 incident and prevalent HD patients, and also found a significant survival advantage associated with paricalcitol use.Citation61 These investigators also used time-dependent Cox models, and used models adjusted for both case mix (a surrogate for baseline comorbidites, to which the authors did not have access) and a malnutrition-inflammation index created by the authors. This study, however, identified dose-dependent effects of vitamin D analogs, such that individuals receiving high (≥15 μg of paricalcitol per week) had worse survival, consistent with possible toxic effects of high dose vitamin D analogs. An alternative possibility, which cannot be discounted, is that patients with refractory SHPT, in whom large doses of vitamin D analogs were prescribed as a result, had a much higher mortality than those with less florid SHPT. The other large study examining this issue was that of Tentori et al who studied over 7700 prevalent HD patients from the Dialysis Clinics Incorporated database.Citation62 While median followup was relatively short (37 weeks), these investigators also found a 20% improvement in mortality associated with use of any vitamin D analog.
Despite this evidence, caution should be used in extrapolating these results too broadly. No technique of statistical adjustment is likely to remove all residual confounding, and the results could also be explained by confounding by intent (non-random treatment allocation bias). In many cases, decisions to treat or not to treat with vitamin D analogs in ESRD involves a physician’s bedside anecdotal knowledge of the patient’s medical history and comorbidities, and is unlikely to be fully captured by knowledge of either comorbidites (as recorded in databases) or through case-mix adjustment. Given the disappointing history of the discordance between observational studies and RCTs in the dialysis population, a RCT is needed to demonstrate the survival advantage of vitamin D analogs.
Controversies in therapy for SHPT: Is there a biologically plausible rationale to select one class of agents over another?
In the absence of prospective trials of dialysis patients demonstrating whether either calcimimetics or vitamin D analogs improve mortality, a variety of other considerations should inform prescribing practices. Here we examine several controversies in SHPT treatment, including whether reduced 1,25(OH)2 D in advanced kidney disease is pathological or an adaptive condition requiring treatment, whether either agent could be expected to have direct effects on PTG function through their respective receptors, and whether “non-classical” actions of vitamin D have relevance for patients with advanced kidney disease.
Is calcitriol deficiency in kidney disease a pathological state or an adaptive response?
The focus of the traditional understanding of SHPT has centered on diminished renal synthesis of 1,25(OH)2 D. According to this view, chronic kidney disease (CKD) represents a functional vitamin D-deficient state,Citation63,Citation64 resulting from the inability of the decreased renal mass to convert 25(OH) D (calcidiol) to 1,25(OH)2 D (calcitriol). This traditional framework has been shaped by an understanding of “true” vitamin D-deficient states, and holds that impaired gastrointestinal Ca absorption results from a reduction in 1,25(OH)2 D levels, and also results in stimulated PTH secretion. PTH has several targets: (1) bone, to increase Ca efflux; and (2) kidney, to stimulate 1,25(OH)2 D production, increase tubular Ca absorption, and inhibit P reabsorption. Elevated PTH levels maintain circulating Ca and P levels until compensatory mechanisms fail, during which time PTG hypertrophy and hyperplasia ensue. The role of diminished calcidiol availability is controversial: it may or may not be due to nutritional factors,Citation65 dysregulated liver metabolism,Citation66 or decreased substrate delivery to the kidney resulting from low glomerular filtration rate.Citation63 Diminished calcidiol likely contributes to decreased 1,25(OH)2 D levels in CKD. This view, which centers around the cardinal importance of vitamin D, would suggest that primary treatment of SHPT should consist of active vitamin D analogs to suppress PTH, possibly in conjunction with supplementation by nutritional vitamin D calcidiol.
The traditional view that CKD is a state of true vitamin D deficiency may be challenged by several observations. First, phosphate retention in CKD appears to be the initial abnormality inciting the development of SHPT. This is evidenced by the decreased capacity of the kidney to excrete phosphate, as well as the observation that reduction in dietary phosphate alone, in proportion to declining GFR, can prevent the development of SHPT in models of CKD.Citation67 Second, nutritional vitamin D deficiency is more commonly associated with hypophosphatemia,Citation68 rather than hyperphosphatemia resulting from impaired renal phosphate clearance. Third, the principal function of the vitamin D/PTH axis is not to prevent hyperphosphatemia, but rather to protect the organism from hypocalcemia by stimulating bone Ca efflux, 1,25(OH)2 D production, and renal Ca reabsorption. The role of PTH on P excretion is a secondary, mainly designed to excrete the P accompanying gastrointestinal Ca absorption and Ca efflux from the bone. Together, these strongly suggest that elevated PTH secretion in both vitamin D deficiency and advanced kidney disease have more relevance to the maintenance of circulating Ca levels than with P homeostasis.
Fourth, there is some evidence of refractoriness to treatment of calcidiol deficiency in CKD. While this issue is an active area of investigation in which more study evidence is likely to come to light, provision of nutritional vitamin D supplementation in the form of ergocalciferol to many CKD individuals with calcidiol deficiency leads to disappointing results. Some CKD patients appear refractory to nutritional vitamin D supplementation, as evidenced by the difficulty achieving “normal” levels of calcidiol using ergocalciferol in these patients. For example, Zisman et al administered ergocalciferol to deficient stage 3 and 4 CKD subjects according to the KDOQI suggested protocol.Citation69 After a long mean treatment period of over 7 months, subjects increased their calcidiol levels only to the 30 to 35 ng/mL range, with about one-third of subjects failing to reach levels >30 ng/mL. In another study, insufficient and deficient stage 3 and 4 CKD subjects were treated with an intense ergocalciferol regimen (50,000 IU weekly for 12 weeks, then 50,000 IU monthly for 3 months).Citation70 Calcidiol levels increased from an average of 16.6 ng/mL to only 27.2 ng/mL, and 45% demonstrated trivial increases of calcidiol (<5 ng/mL). It is certainly possible that ergocalciferol is not the optimal form of nutritional vitamin D supplementation. However, while one study demonstrated greater success by using cholecalciferol, resulting in a greater improvement in calcidiol levels, this was not accompanied by a statistically-significant suppression of PTH.Citation71
The discovery of FGF23, and its function as a 1,25(OH)2 D counter-regulatory hormone,Citation72,Citation73 provides a new conceptual framework for understanding the pathogenesis of SHPT. Made in bone, FGF23 is involved in adaptive responses which have evolved to protect the organism from hyperphosphatemia and vitamin D intoxication. Both PTH and FGF23 have phosphaturic actions, but they have opposite effects on 1α-hydroxylase/24-hydroxylase enzyme systems: PTH stimulates 1,25(OH)2 D production and inhibits its degradation, whereas FGF23 inhibits productionCitation74–Citation76 and increases degradation. FGF23 acts as a “counter-regulatory” hormone, to decrease 1α-hydroxylase and increase 24-hydroxylase (diminishing calcitriol levels). In this way, the FGF23-bone-kidney axis may be an effector of a phosphate “trade-off” that compensates for limited renal P excretion. Reduced renal P excretion may be the initial stimulus for a cascade of events, in which FGF23-dependent suppression of renal 1,25(OH)2 D production is an adaptive response, limiting gastrointestinal P absorption, rather than a functionally-deficient state requiring treatment. Thus, small increases in FGF23 are a very early event in CKD to maintain neutral P balance.Citation77–Citation79 FGF23 also has direct effects on the PTG to suppress PTH secretion,Citation74 which leads to the removal of PTH-mediated stimulatory effects on 1α-hydroxylase, and to further endogenous suppression of 1,25(OH)2 D production. As such, PTH elevation is almost certainly a later event following FGF23-mediated reductions in 1,25(OH)2 D.
Therapeutic approaches to SHPT would therefore differ depending on which conceptualization of SHPT pathogenesis is correct. If kidney disease represents functional calcitriol deficiency, then administration of vitamin D analogs to suppress PTH is rational. However, if P retention leads to increases in FGF23, suppression of 1α-hydroxylase, and stimulation of 24-hydroxylase leading to a fall of calcitrol levels, then the primary treatment of SHPT would be P restriction rather than vitamin D analog administration, since the latter would serve to increase Ca and P absorption, hyperphosphatemia, and further stimulation of FGF23. If this is true, it is fair to ask why treatment with vitamin D analogs in stage 4 and 5 CKD suppresses PTH without raising serum P.Citation80 The answer may be that vitamin D itself stimulates production of FGF23 levels, in turn increasing phosphaturia in the setting of decreasing renal function. In ESRD patients, in contrast, treatment with active vitamin D analogs worsens hyperphosphatemia, probably reflecting the unopposed effect of increase gastrointestinal phosphate absorption,Citation62 while cinacalcet results in a slight decrease in serum phosphate levels.Citation12,Citation16 The mechanism of this latter effect is uncertain, but may be due to a decrement in PTH-mediated P efflux from bone.
What is the relative importance of CASR and VDR in regulating PTG function?
The CASR regulates PTH gene transcription and secretionCitation24,Citation25 and cell proliferation of the PTG.Citation26 In humans with neonatal severe hyperparathyroidismCitation27 and, analogously, in homozygous casr knockout mice,Citation28 there are increases in PTH and serum Ca, as well as hyperplasia of the PTG, in spite elevations in circulating 1,25(OH)2 D.Citation33,Citation81–Citation83 In contrast, while ablation of the VDR also results in severe hyperparathyroidism, normalization of serum Ca (leading to activation of the CASR) is sufficient to normalize PTH secretion and suppress PTG hyperplasia.Citation84 That calcitriol is ineffective in suppressing PTH in the absence of the CASR, while Ca is sufficient to normalize PTG function in the absence of VDR, is evidence that the CASR is the dominant regulator of PTG function. The principal direct function of the VDR in the PTG, then, is to suppress PTH gene transcription; the VDR has an indirect function on the PTG via its actions in the gastrointestinal tract to increase Ca absorption and elevate serum Ca levels, which serves to affect PTG function via the CASR.
That the CASR appears to have a more dominant role over VDR in PTG hyperplasia does not trivialize the role of the VDR is unimportant. Complex interrelationships between the CASR and VDR exist. Both the CASRCitation29–Citation32 and the VDRCitation85–Citation89 can be downregulated in humans and animals with severe SHPT, making the gland resistant to treatment with vitamin D analogs. In rodent models of renal failure, calcimimetics upregulate the CASR.Citation36 There is also data that the VDR upregulates the CASR.Citation90–Citation92 While in the setting of adequate Ca the VDR is not necessary for the short-term survival of the organism, in the setting of more complex environments and over the long-term, a physiologically replete vitamin D state may be important.
Putative actions of vitamin D beyond mineral metabolism: relevance to patients with kidney disease
There is a growing body of literature on potential non-classical actions of vitamin D, ie, actions beyond those directly involved in mineral metabolism. Much of this data are derived from observational studies of individuals with normal renal function who are nutritionally vitamin D-deficiency (and who therefore have presumed impairments of VDR-dependent signaling), and from studies that investigate the extra-renal production of 1,25(OH)2 D and its role in innate immunity. Of particular relevance to ESRD patients are the putative effects of extrarenal production of 1,25(OH)2 D on innate immunity.Citation93 As reviewed by Peterlik et al,Citation94 activated vitamin D (calcidiol) is involved in many immune functions, such as induction of monocyte chemotaxis,Citation95 macrophage differentiation,Citation96,Citation97 upregulation of Fc receptors,Citation98 and production of the respiratory burstCitation99 and of nitric oxide.Citation100 Perhaps the most compelling evidence for the role of calcidiol emerges from studies of tuberculosis, where both in vitro and in vivo studies, as well as in epidemiologic investigations, calcidiol status is important in defense against this organism.Citation101 While tuberculosis is uncommon in ESRD patients in developed countries, other types of infections are extremely common; whether a state of physiologic 1,25(OH)2 D repletion would be a defense against infection is uncertain.
However, in addition to substrate, extrarenal production of 1,25(OH)2 D requires activation of Toll-like receptors necessary for upregulation of 1α-hydroxylase and downregulation of 24-hydroxylase.Citation93 Given the low levels of peripheral conversion of 25(OH) D to 1,25(OH)2 D,Citation102 it is not clear if the extrarenal production of 1,25(OH)2 D has the same significance in the setting of renal failure, where the vitamin D axis is suppressed by FGF23, as it does in nutritionally vitamin D-insufficient patients with normal kidney function.
Also of great potential importance is the possible role of vitamin D and its analogs in cardioprotection. Levels of calcidiol have been shown to be correlated with insulin sensitivity in epidemiologic studies,Citation103–Citation105 and may be associated with the metabolic syndrome. Additionally, there may be direct cardioprotective effects of calcitriol on the cardiovascular system, with evidence that calcitriol antagonizes cardiac myocyte hypertrophy in ratsCitation106 and down-regulates the renin-angiotensin system.Citation107 Again, whether these findings have clinical relevance in humans requires further study.
Given the collective body of evidence, what therapeutic approaches might be most suitable for the treatment of SHPT?
In , we illustrate two potential therapeutic approaches to SHPT (one calcimimetic-based, the other vitamin D analog-based), and list relevant advantages and disadvantages of each. The Figure represents less a specific protocol than an overall conceptualization of possible treatment strategies. Patients with low calcium levels (ie, <8.4 mg/dL) might best avoid the potential for cinacalcet-induced hypocalcemia, at least until low doses of activated vitamin D compounds increase serum calcium to the normal range, while those with high serum calcium ought to avoid large doses of activated vitamin D compounds. For patients whose calcium levels fall within the “acceptable” NKF K/DOQI range, namely 8.4 to 9.5 mg/dL, the approaches have many similarities. In both, dietary phosphorous restriction is important, and oral phosphate binders critical. Non-calcium-based binders are generally preferred in either approach, given the mounting evidence that non-calcium based binders are associated with less vascular calcificationCitation4–Citation7,Citation108 and perhaps improved survival,Citation109 although the latter finding requires replication and extension in true blinded RCTs.
Figure 1 Competing therapeutic strategies for treatment of secondary hyperparathyroidism, with advantages and disadvantages.
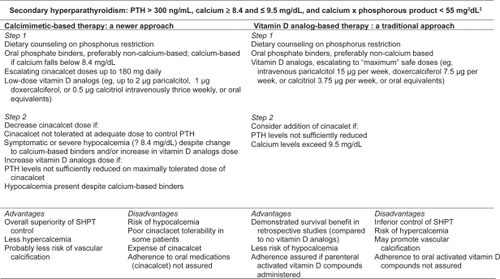
However, the cinacalcet-based approach relies on calcimimetics as the principal agent for PTH reduction, while still maintaining a role for low doses of vitamin D analogs to defend against hypocalcemia while contributing potential non-mineral-metabolism-related survival benefits that may be responsible for the findings of other studies.Citation60,Citation61 In a cinacalcet-based approach, doses of activated vitamin D analogs can be increased if cinacalcet is ineffective or not tolerated at adequate doses. Because of poor tolerability in some patients as well as the expense for individuals without drug insurance coverage, cinacalcet treatment poses adherence challenges. Nevertheless, the potential benefits of a cinacalcet-based strategy, specifically better overall control of SHPT, as demonstrated in multiple RCTs, and the likelihood of less hypercalcemia, make this the preferred therapeutic approach at the current time. A vitamin D analog-based strategy, in which cinacalet is added only if large doses of activated vitamin D is ineffective at controlling PTH or if hypercalcemia results, is less likely to lead to optimal SHPT control and may lead to a higher risk of vascular calcification and, ultimately, mortality. As such, the latter is a less attractive therapeutic approach for most patients.
Conclusions: time for a new treatment paradigm of SHPT in ESRD?
Treatment paradigms for SHPT must consider the pathogenesis of the disease as well as the results of clinical studies. In general, we believe that a cinacalcet-based approach is superior for most patients with SHPT because (1) clinical trials have demonstrated the superior suppression of PTH and control of Ca × P product in ESRD patients with calcimimetics, relative to vitamin D analogs, both when used as adjunctive therapy superimposed on vitamin D analogs and also as “primary” therapy with low doses of vitamin D analogs; (2) decreased levels of calcitriol appear to be an adaptive response in advanced renal disease to limit the toxic effects of hyperphosphatemia; (3) molecular human and mouse genetic studies demonstrate the greater importance of the CASR, relative to the VDR, in the regulation of PTG function; and (4) the use of cinacalcet may result in less calcium loading than therapy with vitamin D analogs. Calcium loading with calcium-based binders, in the setting of concurrent activated vitamin D compound therapy, has been associated with increased vascular calcifications.Citation4
Although survival benefits of vitamin D analogs in ESRD have not been demonstrated in an RCT, such benefits are biologically plausible, and physiological “replacement” doses of vitamin D analogs (eg, paricalcitol 2 μg or doxercalciferol 1 μg thrice weekly) appear warranted in the majority of patients without obvious contraindications. In contrast, long-term use of high-dose vitamin D analogs probably results in a more positive Ca balance in ESRD than similar treatment with cinacalcet, although studies that assess the risk reduction of cinacalcet currently exist solely in animal models.Citation110 Low doses of vitamin D analogs, as adjunctive therapy to cinacalet, are likely to emerge as the standard of care. These tonic doses of activated vitamin D therapy could be increased when (1) calcimimetics have been tried at maximal doses and failed to control SHPT, (2) calcimimetics have intolerable side effects at effective doses, or (3) calcimimetic use results in hypocalcemia, an effect which can frequently be remedied by vitamin D analogs. Whether nutritional vitamin D supplementation, in addition to treatment with activated vitamin D compounds, is of benefit is uncertain at the current time.
The nephrology community has dire need for data from well-designed RCTs to determine whether calcimimetics provide mortality benefits relative to vitamin D analogs. While a study in which cinacalcet is tested directly against a vitamin D analog would provides for a more rigorous experimental design, widespread use of vitamin D analogs, as well as their benefit in protection against cinacalcet-induced hypocalcemia, probably makes employment of such a design impractical. However, a trial in which low-dose vitamin D analogs are administered in the cinacalet arm would reflect clinical practice realities and increase the likelihood that subjects randomized to cinacalcet would be consistently exposed to the drug over a lengthy treatment period, a likely requirement if the trial is to show unambiguous differences in cumulative event rates. In the absence of clinical trial data, calcimimetics, along with dietary phosphate control, should be considered primary therapy in most ESRD patients with SHPT.
Disclosures
JBW receives honoraria and research funding from Amgen, Inc. LDQ receives honoraria from, and serves on the advisory board of, Amgen, Inc.
References
- BlockGAHulbert-ShearonTELevinNWPortFKAssociation of serum phosphorus and calcium × phosphate product with mortality risk in chronic hemodialysis patients: a national studyAm J Kidney Dis19983146076179531176
- GaneshSKStackAGLevinNWHulbert-ShearonTPortFKAssociation of elevated serum PO(4), Ca × PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patientsJ Am Soc Nephrol200112102131213811562412
- GoodmanWGGoldinJKuizonBDYoonCGalesBSiderDCoronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysisN Engl J Med2000342201478148310816185
- ChertowGMBurkeSKRaggiPSevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patientsKidney Int200262124525212081584
- ChertowGMRaggiPMcCarthyJTSchulmanGSilberzweigJKuhlikAThe effects of sevelamer and calcium acetate on proxies of atherosclerotic and arteriosclerotic vascular disease in hemodialysis patientsAm J Nephrol200323530731412915774
- FerramoscaEBurkeSChasan-TaberSRattiCChertowGMRaggiPPotential antiatherogenic and anti-inflammatory properties of sevelamer in maintenance hemodialysis patientsAm Heart J2005149582082515894962
- BlockGASpiegelDMEhrlichJMehtaRLindberghJDreisbachAEffects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysisKidney Int20056841815182416164659
- GoodmanWGHladikGATurnerSABlaisdellPWGoodkinDALiuWThe Calcimimetic agent AMG 073 lowers plasma parathyroid hormone levels in hemodialysis patients with secondary hyperparathyroidismJ Am Soc Nephrol20021341017102411912261
- QuarlesLDSherrardDJAdlerSRosanskySJMcCaryLCLiuWThe calcimimetic AMG 073 as a potential treatment for secondary hyperparathyroidism of end-stage renal diseaseJ Am Soc Nephrol200314357558312595492
- LindbergJSMoeSMGoodmanWGCoburnJWSpragueSMLiuWThe calcimimetic AMG 073 reduces parathyroid hormone and calcium × phosphorus in secondary hyperparathyroidismKidney Int200363124825412472790
- LindbergJSCulletonBWongGBorahMFClarkRVShapiroWBCinacalcet HCl, an oral calcimimetic agent for the treatment of secondary hyperparathyroidism in hemodialysis and peritoneal dialysis: a randomized, double-blind, multicenter studyJ Am Soc Nephrol200516380080715689407
- BlockGAMartinKJde FranciscoALTurnerSAAvramMMSuranyiMGCinacalcet for secondary hyperparathyroidism in patients receiving hemodialysisN Engl J Med2004350151516152515071126
- StrippoliGFPalmerSTongAElderGMessaPCraigJCMeta-analysis of biochemical and patient-level effects of calcimimetic therapyAm J Kidney Dis200647571572616632010
- MartinKJJuppnerHSherrardDJGoodmanWGKaplanMRNassarGFirst- and second-generation immunometric PTH assays during treatment of hyperparathyroidism with cinacalcet HClKidney Int20056831236124316105056
- FukagawaMYumitaSAkizawaTUchidaETsukamotoYIwasakiMCinacalcet (KRN1493) effectively decreases the serum intact PTH level with favorable control of the serum phosphorus and calcium levels in Japanese dialysis patientsNephrol Dial Transplant200823132833517717030
- MessaPMacarioFYaqoobMBoumanKBraunJvon AlbertiniBThe OPTIMA study: assessing a new cinacalcet (Sensipar/Mimpara) treatment algorithm for secondary hyperparathyroidismClin J Am Soc Nephrol200831364518178780
- MoeSMCunninghamJBommerJAdlerSRosanskySJUrena-TorresPLong-term treatment of secondary hyperparathyroidism with the calcimimetic cinacalcet HClNephrol Dial Transplant200520102186219316030053
- SterrettJRStromJStummvollHKBahnerUDisneyASorokaSDCinacalcet HCI (Sensipar/Mimpara) is an effective chronic therapy for hemodialysis patients with secondary hyperparathyroidismClin Nephrol2007681101717703830
- ChertowGMBlumenthalSTurnerSRoppoloMSternLChiEMCinacalcet hydrochloride (Sensipar) in hemodialysis patients on active vitamin D derivatives with controlled PTH and elevated calcium × phosphateClin J Am Soc Nephrol20061230531217699221
- BlockGAZeigSSugiharaJChertowGMChiEMTurnerSACombined therapy with cinacalcet and low doses of vitamin D sterols in patients with moderate to severe secondary hyperparathyroidismNephrol Dial Transplant2008In press
- ArenasMDAlvarez-UdeFGilMTMoledousAMalekTNunezCImplementation of ‘K/DOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease’ after the introduction of cinacalcet in a population of patients on chronic haemodialysisNephrol Dial Transplant20072261639164417277339
- LazarEHebertKPomaTStankusNLong-term outcomes of cinacalcet and paricalcitol titration protocol for treatment of secondary hyperparathyroidismAm J Nephrol200727327427817429197
- SpiegelDMCaseyLBellSParkerMChoncholMAchieving targets for bone and mineral metabolism: the impact of cinacalcet HCl in clinical practiceHemodial Int200610Suppl 2S242717022747
- BrownEMMacLeodRJExtracellular calcium sensing and extracellular calcium signalingPhysiol Rev2001811239297
- BrownEMClinical lessons from the calcium-sensing receptorNat Clin Pract Endocrinol Metab20073212213317237839
- SilverJLeviRRegulation of PTH synthesis and secretion relevant to the management of secondary hyperparathyroidism in chronic kidney diseaseKidney Int Suppl200595S81215882315
- PollakMRBrownEMChouYHHebertSCMarxSJSteinmannBMutations in the human Ca(2+)−sensing receptor gene cause familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidismCell1993757129713037916660
- HoCConnerDAPollakMRLaddDJKiforOWarrenHBA mouse model of human familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidismNat Genet19951143893947493018
- GogusevJDuchambonPHoryBGiovanniniMGoureauYSarfatiEDepressed expression of calcium receptor in parathyroid gland tissue of patients with hyperparathyroidismKidney Int19975113283368995751
- KiforOMooreFDJrWangPGoldsteinMVassilevPKiforIReduced immunostaining for the extracellular Ca2+-sensing receptor in primary and uremic secondary hyperparathyroidismJ Clin Endocrinol Metab1996814159816068636374
- BrownAJRitterCSFinchJLSlatopolskyEADecreased calcium-sensing receptor expression in hyperplastic parathyroid glands of uremic rats: role of dietary phosphateKidney Int19995541284129210200992
- RitterCSFinchJLSlatopolskyEABrownAJParathyroid hyperplasia in uremic rats precedes down-regulation of the calcium receptorKidney Int20016051737174411703591
- DruekeTMartinDRodriguezMCan calcimimetics inhibit parathyroid hyperplasia? Evidence from preclinical studiesNephrol Dial Transplant20072271828183917449493
- CollotonMShatzenEMillerGStehman-BreenCWadaMLaceyDCinacalcet HCl attenuates parathyroid hyperplasia in a rat model of secondary hyperparathyroidismKidney Int200567246747615673294
- ChinJMillerSCWadaMNaganoNNemethEFFoxJActivation of the calcium receptor by a calcimimetic compound halts the progression of secondary hyperparathyroidism in uremic ratsJ Am Soc Nephrol200011590391110770968
- MizobuchiMHatamuraIOgataHSajiFUdaSShiizakiKCalcimimetic compound upregulates decreased calcium-sensing receptor expression level in parathyroid glands of rats with chronic renal insufficiencyJ Am Soc Nephrol200415102579258715466262
- de FranciscoALIzquierdoMCunninghamJPineraCPalomarRFresnedoGFCalcium-mediated parathyroid hormone release changes in patients treated with the calcimimetic agent cinacalcetNephrol Dial Transplant2008423
- LomonteCVernaglioneLChimientiDBrunoACocolaSTeutonicoADoes vitamin D receptor and calcium receptor activation therapy play a role in the histopathologic alterations of parathyroid glands in refractory uremic hyperparathyroidism?Clin J Am Soc Nephrol20083379479918322048
- YamaguchiSYachikuSMorikawaMAnalysis of proliferative activity of the parathyroid glands using proliferating cell nuclear antigen in patients with hyperparathyroidismJ Clin Endocrinol Metab199782826812689253354
- MallucheHHMonier-FaugereMCWangGFrazaOJCharytanCCoburnJWAn assessment of cinacalcet HCl effects on bone histology in dialysis patients with secondary hyperparathyroidismClin Nephrol200869426927818397701
- WangRXuCZhaoWZhangJCaoKYangBCalcium and polyamine regulated calcium-sensing receptors in cardiac tissuesEur J Biochem200327012268026812787035
- Tfelt-HansenJHansenJLSmajilovicSTerwilligerEFHaunsoSSheikhSPCalcium receptor is functionally expressed in rat neonatal ventricular cardiomyocytesAm J Physiol Heart Circ Physiol20062903H1165117116243911
- Farzaneh-FarAProudfootDWeissbergPLShanahanCMMatrix gla protein is regulated by a mechanism functionally related to the calcium-sensing receptorBiochem Biophys Res Commun2000277373674011062022
- WangYBukoskiRDDistribution of the perivascular nerve Ca2+ receptor in rat arteriesBr J Pharmacol19981257139714049884066
- WonnebergerKScofieldMAWangemannPEvidence for a calcium-sensing receptor in the vascular smooth muscle cells of the spiral modiolar arteryJ Membr Biol2000175320321210833530
- SmajilovicSHansenJLChristoffersenTELewinESheikhSPTerwilligerEFExtracellular calcium sensing in rat aortic vascular smooth muscle cellsBiochem Biophys Res Commun200634841215122316919596
- ZiegelsteinRCXiongYHeCHuQExpression of a functional extracellular calcium-sensing receptor in human aortic endothelial cellsBiochem Biophys Res Commun2006342115316316472767
- SmajilovicSTfelt-HansenJCalcium acts as a first messenger through the calcium-sensing receptor in the cardiovascular systemCardiovasc Res200775345746717466291
- OgataHRitzEOdoniGAmannKOrthSRBeneficial effects of calcimimetics on progression of renal failure and cardiovascular risk factorsJ Am Soc Nephrol200314495996712660330
- CunninghamJDaneseMOlsonKKlassenPChertowGMEffects of the calcimimetic cinacalcet HCl on cardiovascular disease, fracture, and health-related quality of life in secondary hyperparathyroidismKidney Int20056841793180016164656
- MartinKJGonzalezEAGellensMHammLLAbboudHLindbergJ19-Nor-1-alpha-25-dihydroxyvitamin D2 (Paricalcitol) safely and effectively reduces the levels of intact parathyroid hormone in patients on hemodialysisJ Am Soc Nephrol199898142714329697664
- DelmezJAKelberJNorwoodKYGilesKSSlatopolskyEA controlled trial of the early treatment of secondary hyperparathyroidism with calcitriol in hemodialysis patientsClin Nephrol200054430130811076106
- CoyneDWGrieffMAhyaSNGilesKNorwoodKSlatopolskyEDifferential effects of acute administration of 19-Nor-1,25-dihydroxy-vitamin D2 and 1,25-dihydroxy-vitamin D3 on serum calcium and phosphorus in hemodialysis patientsAm J Kidney Dis20024061283128812460048
- JoistHEAhyaSNGilesKNorwoodKSlatopolskyECoyneDWDifferential effects of very high doses of doxercalciferol and paricalcitol on serum phosphorus in hemodialysis patientsClin Nephrol200665533534116724654
- SpragueSMLlachFAmdahlMTaccettaCBatlleDParicalcitol versus calcitriol in the treatment of secondary hyperparathyroidismKidney Int20036341483149012631365
- BrandiLDaugaardHTvedegaardENielsenPKEgsmoseCStormTLong-term suppression of secondary hyperparathyroidism by intravenous 1 alpha-hydroxyvitamin D3 in patients on chronic hemodialysisAm J Nephrol19921253113181488999
- LindbergJMartinKJGonzalezEAAcchiardoSRValdinJRSoltanekCA long-term, multicenter study of the efficacy and safety of paricalcitol in end-stage renal diseaseClin Nephrol200156431532311680662
- AkizawaTSuzukiMAkibaTNishizawaYOhashiYOgataELong-term effect of 1,25-dihydroxy-22-oxavitamin D(3) on secondary hyperparathyroidism in haemodialysis patients. One-year administration studyNephrol Dial Transplant200217 Suppl 10283612386266
- TengMWolfMLowrieEOfsthunNLazarusJMThadhaniRSurvival of patients undergoing hemodialysis with paricalcitol or calcitriol therapyN Engl J Med2003349544645612890843
- TengMWolfMOfsthunMNLazarusJMHernanMACamargoCAJrActivated injectable vitamin D and hemodialysis survival: a historical cohort studyJ Am Soc Nephrol20051641115112515728786
- Kalantar-ZadehKKuwaeNRegidorDLKovesdyCPKilpatrickRDShinabergerCSSurvival predictability of time-varying indicators of bone disease in maintenance hemodialysis patientsKidney Int200670477178016820797
- TentoriFHuntWCStidleyCARohrscheibMRBedrickEJMeyerKBMortality risk among hemodialysis patients receiving different vitamin D analogsKidney Int200670101858186517021609
- Al-BadrWMartinKJVitamin D and Kidney DiseaseClin J Am Soc Nephrol200851
- BrownAJSlatopolskyEDrug insight: vitamin D analogs in the treatment of secondary hyperparathyroidism in patients with chronic kidney diseaseNat Clin Pract Endocrinol Metab200723213414417237840
- MehrotraRKermahDBudoffMSaluskyIBMaoSSGaoYLHypovitaminosis D in Chronic Kidney DiseaseClin J Am Soc Nephrol2008416
- ZehnderDLandrayMJWheelerDCFraserWBlackwellLNuttallSCross-sectional analysis of abnormalities of mineral homeostasis, vitamin D and parathyroid hormone in a cohort of pre-dialysis patients. The chronic renal impairment in Birmingham (CRIB) studyNephron Clin Pract20071073c1091617890873
- SlatopolskyEBrownADussoARole of phosphorus in the pathogenesis of secondary hyperparathyroidismAm J Kidney Dis20011371 Suppl 2S54711158862
- RowePSThe wrickkened pathways of FGF23, MEPE and PHEXCrit Rev Oral Biol Med200415526428115470265
- ZismanALHristovaMHoLTSpragueSMImpact of ergocalciferol treatment of vitamin D deficiency on serum parathyroid hormone concentrations in chronic kidney diseaseAm J Nephrol2007271364317215573
- Al-AlyZQaziRAGonzalezEAZeringueAMartinKJChanges in serum 25-hydroxyvitamin D and plasma intact PTH levels following treatment with ergocalciferol in patients with CKDAm J Kidney Dis2007501596817591525
- ChandraPBinongoJNZieglerTRSchlangerLEWangWSomerenJTCholecalciferol (vitamin D3) therapy and vitamin D insufficiency in patients with chronic kidney disease: a randomized controlled pilot studyEndocr Pract2008141101718238736
- SaitoHKusanoKKinosakiMItoHHirataMSegawaHHuman fibroblast growth factor-23 mutants suppress Na+−dependent phosphate co-transport activity and 1alpha, 25-dihydroxyvitamin D3 productionJ Biol Chem200327842206221112419819
- LiuSTangWZhouJStubbsJRLuoQPiMFibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin DJ Am Soc Nephrol200651751305131516597685
- KrajisnikTBjorklundPMarsellRLjunggrenOAkerstromGJonssonKBFibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cellsJ Endocrinol2007195112513117911404
- ShimadaTHasegawaHYamazakiYMutoTHinoRTakeuchiYFGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasisJ Bone Miner Res200419342943515040831
- PerwadFZhangMYTenenhouseHSPortaleAAFibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1alpha-hydroxylase expression in vitroAm J Physiol Renal Physiol20072935F1577158317699549
- LarssonTNisbethULjunggrenOJuppnerHJonssonKBCirculating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteersKidney Int20036462272227914633152
- ShigematsuTKazamaJJYamashitaTFukumotoSHosoyaTGejyoFPossible involvement of circulating fibroblast growth factor 23 in the development of secondary hyperparathyroidism associated with renal insufficiencyAm J Kidney Dis200444225025615264182
- GutierrezOIsakovaTRheeEShahAHolmesJColleroneGFibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney diseaseJ Am Soc Nephrol20051672205221515917335
- CoburnJWMaungHMElangovanLGermainMJLindbergJSSpragueSMDoxercalciferol safely suppresses PTH levels in patients with secondary hyperparathyroidism associated with chronic kidney disease stages 3 and 4Am J Kidney Dis200443587789015112179
- GoodmanWGCalcimimetics: a remedy for all problems of excess parathyroid hormone activity in chronic kidney disease?Curr Opin Nephrol Hypertens200514435536015931004
- KosCHKaraplisACPengJBHedigerMAGoltzmanDMohammadKSThe calcium-sensing receptor is required for normal calcium homeostasis independent of parathyroid hormoneJ Clin Invest200311171021102812671051
- TuQPiMKarsentyGSimpsonLLiuSQuarlesLDRescue of the skeletal phenotype in CasR-deficient mice by transfer onto the Gcm2 null backgroundJ Clin Invest2003111710293712671052
- LiYCAmlingMPirroAEPriemelMMeuseJBaronRNormalization of mineral ion homeostasis by dietary means prevents hyperparathyroidism, rickets, and osteomalacia, but not alopecia in vitamin D receptor-ablated miceEndocrinology199813910439143969751523
- MartinLNKayathMJVieiraJGNose-AlbertiVParathyroid glands in uraemic patients with refractory hyperparathyroidism: histopathology and p53 protein expression analysisHistopathology199833146519726048
- FukudaNTanakaHTominagaYFukagawaMKurokawaKSeinoYDecreased 1,25-dihydroxyvitamin D3 receptor density is associated with a more severe form of parathyroid hyperplasia in chronic uremic patientsJ Clin Invest1993923143614438397225
- WangXSunBZhouFHuJYuXPengTVitamin D receptor and PCNA expression in severe parathyroid hyperplasia of uremic patientsChin Med J (Engl)2001114441041411780466
- TokumotoMTsuruyaKFukudaKKanaiHKurokiSHirakataHReduced p21, p27 and vitamin D receptor in the nodular hyperplasia in patients with advanced secondary hyperparathyroidismKidney Int20026241196120712234290
- YanoSSugimotoTTsukamotoTChiharaKKobayashiAKitazawaSDecrease in vitamin D receptor and calcium-sensing receptor in highly proliferative parathyroid adenomasEur J Endocrinol2003148440341112656660
- BrownAJZhongMFinchJRitterCMcCrackenRMorrisseyJRat calcium-sensing receptor is regulated by vitamin D but not by calciumAm J Physiol19962703 Pt 2F4544608780248
- CanaffLHendyGNHuman calcium-sensing receptor gene. Vitamin D response elements in promoters P1 and P2 confer transcriptional responsiveness to 1,25-dihydroxyvitamin DJ Biol Chem200227733303373035012036954
- TokumotoMTaniguchiMMatsuoDTsuruyaKHirakataHIidaMParathyroid cell growth in patients with advanced secondary hyper-parathyroidism: vitamin D receptor, calcium sensing receptor, and cell cycle regulating factorsTher Apher Dial20059Suppl 1S273416109139
- LiuPTStengerSLiHWenzelLTanBHKrutzikSRToll-like receptor triggering of a vitamin D-mediated human antimicrobial responseScience200631157681770177316497887
- PeterlikMCrossHSVitamin D and calcium deficits predispose for multiple chronic diseasesEur J Clin Invest200535529030415860041
- GirasoleGWangJMPedrazzoniMPioliGBalottaCPasseriMAugmentation of monocyte chemotaxis by 1 alpha, 25-dihydroxyvitamin D3. Stimulation of defective migration of AIDS patientsJ Immunol19901458245924642212648
- ProvvediniDMDeftosLJManolagasSC1,25-Dihydroxyvitamin D3 promotes in vitro morphologic and enzymatic changes in normal human monocytes consistent with their differentiation into macrophagesBone19867123283083846
- OrikasaMKawaseTSuzukiAInduction of macrophagic and granulocytic differentiation of murine bone marrow progenitor cells by 1,25-dihydroxyvitamin D3Calcif Tissue Int19935331932008242472
- Boltz-NitulescuGWillheimMSpittlerALeutmezerFTempferCWinklerSModulation of IgA, IgE, and IgG Fc receptor expression on human mononuclear phagocytes by 1 alpha,25-dihydroxyvitamin D3 and cytokinesJ Leukoc Biol19955822562627643018
- CohenMSMeslerDESnipesRGGrayTK1,25-Dihydroxyvitamin D3 activates secretion of hydrogen peroxide by human monocytesJ Immunol19861363104910533079794
- SlyLMLopezMNauseefWMReinerNE1alpha, 25-Dihydroxyvitamin D3-induced monocyte antimycobacterial activity is regulated by phosphatidylinositol 3-kinase and mediated by the NADPH-dependent phagocyte oxidaseJ Biol Chem200127638354823549311461902
- NnoahamKEClarkeALow serum vitamin D levels and tuberculosis: a systematic review and meta-analysisInt J Epidemiol200837111311918245055
- GallieniMKamimuraSAhmedABravoEDelmezJSlatopolskyEKinetics of monocyte 1 alpha-hydroxylase in renal failureAm J Physiol19952684 Pt 2F7467537733332
- PereiraMAJacobsDRJrVan HornLSlatteryMLKartashovAILudwigDSDairy consumption, obesity, and the insulin resistance syndrome in young adults: the CARDIA StudyJAMA2002287162081208911966382
- FordESAjaniUAMcGuireLCLiuSConcentrations of serum vitamin D and the metabolic syndrome among US adultsDiabetes Care20052851228123015855599
- LiuSSongYFordESMansonJEBuringJERidkerPMDietary calcium, vitamin D, and the prevalence of metabolic syndrome in middle-aged and older US womenDiabetes Care200528122926293216306556
- WuJGaramiMChengTGardnerDG1,25(OH)2 vitamin D3, and retinoic acid antagonize endothelin-stimulated hypertrophy of neonatal rat cardiac myocytesJ Clin Invest1996977157715888601621
- LiYCKongJWeiMChenZFLiuSQCaoLP1,25-Dihydroxyvi-tamin D(3) is a negative endocrine regulator of the renin-angiotensin systemJ Clin Invest2002110222923812122115
- SpiegelDMRaggiPSmitsGBlockGAFactors associated with mortality in patients new to haemodialysisNephrol Dial Transplant200722123568357217617651
- BlockGARaggiPBellasiAKooiengaLSpiegelDMMortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patientsKidney Int200771543844117200680
- LopezIMendozaFJAguilera-TejeroEPerezJGuerreroFMartinDThe effect of calcitriol, paricalcitol, and a calcimimetic on extraosseous calcifications in uremic ratsKidney Int200873330030718004298