Abstract
Space flight (SF) and dust inhalation in habitats cause hypertension whereas in SF (alone) there is no consistent hypertension but reduced diurnal blood pressure (BP) variation instead. Current pharmaceutical subcutaneous delivery systems are inadequate and there is impairment in the absorption, metabolism, excretion, and deterioration of some pharmaceuticals. Data obtained from the National Aeronautics and Space Administration through the Freedom of Information Act shows that Irwin returned from his 12-day Apollo 15 mission in 1971 and was administered a bicycle stress test. With just three minutes of exercise, his BP was >275/125 mm Hg (heart rate of only 130 beats per minute). There was no acute renal insult. Irwin’s apparent spontaneous remission is suggested to be related to the increase of a protective vasodilator, and his atrial natriuretic peptide (ANP) reduced with SF because of reduced plasma volume. With invariable malabsorption and loss of bone/muscle storage sites, there are significant (P < 0.0001) reductions of magnesium (Mg) required for ANP synthesis and release. Reductions of Mg and ANP can trigger pronounced angiotensin (200%), endothelin, and catecholamine elevations (clearly shown in recent years) and vicious cycles between the latter and Mg deficits. There is proteinuria, elevated creatinine, and reduced renal concentrating ability with the potential for progressive inflammatory and oxidative stress-induced renal disease and hypertension with vicious cycles. After SF, animals show myocardial endothelial injuries and increased vascular resistance of extremities in humans. Even without dust, hypertension might eventually develop from renovascular hypertension during very long missions. Without sufficient endothelial protection from pharmaceuticals, a comprehensive gene research program should begin now.
“The farther backward you can look, the farther forward you are likely to see.”
Introduction
Even without radiation the endothelium is vulnerable to dysfunction/injuries with space flight (SF) because of oxidative stress involving both the cardiovascular system as well as the systemic circulation in the “Apollo 15 space syndrome.”Citation1 This syndrome, which I described, was experienced by both Irwin and Scott on the first of three excursions to the lunar surface, even prior to dust inhalation in that habitat. My syndrome was characterized by very painful, apparently swollen, fingertips possibly secondary to peripheral vasospasm and compression by fluid trapped distally. Therefore it can be a warning that coronary vasospasm with ischemia (possibly silent) might also exist, predisposing the person to a myocardial infarction.
Similarly vasoconstriction of systemic blood vessels could be the underlying mechanism of a decrease of calf blood flow of 40%. Measurements taken between 4–12 SF-days showed calf vascular resistance doubling. The blood pressure (BP) increased during SF.Citation2 With SF, vicious cycles can occur between magnesium (Mg) ion deficits and catecholamine elevations and also between catecholamine elevations and ischemia. Furthermore vicious cycles can also develop between reduced nitric oxide (NO), coronary vasospasm with turbulence, and endothelial injuries.Citation3
Through information obtained by the Freedom of Information Act (FOIA), I acquired bicycle stress test data regarding Irwin on Apollo 15 showing extraordinary hypertension. The stress test data indicated a BP > 275/125 mm Hg with a heart rate of only 132 per minute after just three minutes of exercise. This test was probably performed on the day after his return from the 12-day mission. The resting BP was not shown, but at one minute the BP was 250/125, with the same heart rate. There was no evidence of an acute renal insult. On the other hand it is noteworthy that Shepard’s bicycle stress test data, on the day after his return from the Apollo 14 mission, revealed a relatively normal response, with a BP of 200/75 at the same heart rate as Irwin’s, but not reaching this level (132 per minute) until 13 minutes of exercise.
One mechanism for this discrepancy could be related to the variation in the chemical composition of the dust inhaled at various lunar landing site habitats.Citation4 Conrad’s stress test data – the only other deceased moon walker (Apollo 12) – is not available, after a “careful search” by National Aeronautics and Space Administration (NASA) in response to my FOIA request in 2008.
On the stress test, on the day of his return, Irwin showed “cyanosis of the finger tips” from the 18th–20th minutes with a BP of 200/90, consistent with venous blood trapped distally, which supports my syndrome on the likelihood of space syndrome. I postulated that the severe hypertension was a complication of dust inhalation. The inhalation had occurred for a total of almost seven days; about 40 hours in the lunar habitat, two days orbiting the moon, making the survey, dust brought into the command module and the three-day journey home.Citation4 The vulnerability to endothelial injuries is far greater in males and in addition the endothelium does not heal adequately after the age of 30 years.Citation3 Furthermore, assuming that some day the problems of hypertension related to dust, brought from the habitats on the moon, will be resolved with removal of even ultrafine dust,Citation4 there are the problems stemming from depletion of the reservoirs for water ie, primarily in skeletal muscle and Mg reservoirs in muscle and bone (about 60% in the latter). This loss stems from hypokinesia (decreased movement) compounded by decreased thirst and is conducive to oxidative stress-induced renovascular hypertension.Citation5 To prevent this, gene therapy may be necessaryCitation6,Citation7 since there are multiple problems with pharmaceuticals; there is invariable SF-malabsorption,Citation8 deterioration of some medications no longer meeting United States Pharmacopeia (USP) standardsCitation9 and impairment in pharmaceutical metabolism and excretion because of potential SF injuries to the liverCitation10 and kidneys.Citation11
Whereas Fritsch-Yelle and colleaguesCitation12 found reduced BP on shuttle missions of 5–10 days; Shiraishi and colleaguesCitation13 found that the waking BP was not different from preflight levels. On the other hand Watenpaugh and colleaguesCitation2 found that SF BP increased in comparison to postflight supine levels and they suggested that premission differences in baseline conditions could account for these discrepancies. However the absence of a significant drop (<10%) in nocturnal BPCitation12,Citation13 indicates that those during SF are “nondippers,” which portends to renal disease as discussed below.
Potential renovascular hypertension
There is a predisposition for progressive oxidative stress-induced renal diseaseCitation5 intensified by atrial natriuretic peptide (ANP) deficienciesCitation6,Citation14–Citation16 with a >40% reduction on Spacelab 2 and excessive levels of catecholaminesCitation1,Citation3,Citation4,Citation17,Citation18 and the combination of endothelinCitation19 and significant angiotensin I elevations (P < 0.0001)Citation17 conducive to sustained renal vasoconstriction.Citation20,Citation21
There is a potential synergism regarding endothelin and angiotensin II blockade which has been shown to improve endothelial function. Blockade of both has also been shown to increase renal blood flow in healthy subjects,Citation21 but, as emphasized above, pharmaceuticals can not be utilized. Proteinuria, considered an expression of endothelial dysfunctionCitation22 and a strong predictor of renal disease progression,Citation23–Citation25 with stimulation of endothelin expression in renal cells,Citation19 was shown after space missions.Citation17,Citation18 Proteinuria was more pronounced after prolonged missions but disappeared during the recovery periodCitation17 possibly related to reductions back to premission catecholamine levels.Citation23 SF reductions of vascular endothelial growth factor (VEGF) from thrombocytopenia with platelet aggregation from endothelial dysfunctionCitation26 and impaired VEGF expression as a result of SF insulin resistanceCitation27 would impair capillary repair in damaged glomeruli and contribute to proteinuria.Citation28,Citation29 Furthermore the reduced diurnal variabilities in blood pressures (“nondipper”) as noted previously increases the risk of renal dysfunction. The mechanisms may be related to increased sympathetic nervous system activity and possibly insulin resistance, both of which have been shown with SF.Citation1,Citation3,Citation4,Citation17,Citation30 Many cosmonauts have shown higher levels of blood creatinine during and after SF in comparison to their preflight levels, and there is decreased concentrating ability after SF.Citation17 It was also found that urine osmolarity in response to fluid deprivation was consistently lower after flight than before, but only if the flight lasted more than 30 days.Citation17
Studies after short missions and hypokinesia of rat heart tissue showed narrowing of the small vessels, some occlusions, and endothelial projections conducive to increased permeability.Citation18,Citation31 Leach Huntoon and colleaguesCitation17 postulated that increased permeability of capillary membranes may be the most important mechanism for SF-induced plasma volume reduction of >10% conducive to endothelial injuries with vicious cycles and angiotensin elevations and reduced secretion of ANP.
Too much or too little exercise will deplete Mg ionsCitation3 that are antioxidants as well as calcium (Ca) blockers.Citation27 On spacecraft, it has been postulated that there may be a shift of Ca into the cells complicating high carbon dioxide levels.Citation4 This may contribute to Ca overload of the mitochondria as shown in rats after SF.Citation31 Contributing to SF oxidative stressCitation32 and in turn Ca overload is the necessity of 100% oxygen for 1–2 hours prior to a space walk to prevent decompression sickness.Citation4 Mg could reduce this oxidative stress,Citation27,Citation33 but there is no suitable subcutaneous delivery device for Mg, since at this time a reliable subcutaneous microchip device can’t be replenished.Citation27 Invariable SF malabsorption and reduced storage sites could be responsible for significant SF Mg deficits (P < 0.0001) after shuttle missions.Citation17 In addition to the 200% increase of plasma angiotensin after, for example, the Skylab flights of up to 84 days,Citation17 there could be the predisposition for an active intrarenal renin–angiotensin system. SF is conducive to increased angiotensin effects from both reduced plasma volumeCitation17 and ANP deficits with reduced NOCitation15 and also from Mg ion deficits. Magnesium also affects the synthesis and release of ANP.Citation16 An intrarenal renin–angiotensin system resulting from SF-reduced NO could contribute to chronic kidney disease stemming from inflammation both directly and through interaction with oxidative stress mechanisms.Citation17,Citation18,Citation32,Citation34,Citation35 Elevated sympathetic nerve activity has been shown unequivocally in patients with renovascular hypertension,Citation36 but until a few years ago the catecholamine levels were not thought to be elevated with SF. This concept has now been shown to have been incorrect. Christensen and colleagues,Citation37 for example, have shown that plasma norepinephrine (NE) and plasma renin activityCitation38 were increased even to levels above those of the seated ground-based position. Plasma NE was approximately twice the value of the supine position on the ground, predisposing those in SF to nondipping, as noted above.Citation30 Furthermore, it was stated that the reason for this was unclear. Certainly one mechanism may be the reductions of SF Mg ion levels with, in turn, elevations of catecholamines, along with elevations of angiotensin and aldosterone (P = 0.0008) with ongoing vicious cycles.Citation1,Citation3,Citation4,Citation17,Citation18,Citation27,Citation39 Magnesium may also provide protection against the renal damaging effects of aldosterone, predisposing the person to fibrosis.Citation39–Citation41
Gender selection
Pertaining to the predisposition for renovascular hypertension, young females have several clear advantages. The endothelium is not adequately repaired after the age of 30 years,Citation3 and the cardiovascular mortality rate is six times higher in males than females under the age of 35 years. This could be related not only to estrogen’s vascular advantagesCitation3 and probably to the fact that males have no physiological way of losing iron, predisposing them to oxidative stress, but also could be related to other clear advantages in females. The levels of ANP in females are approximately twice those of young men.Citation42 During the 438-day mission of Polyakov and after just five months in space, his cyclic guanosine monophosphate (cGMP) levels were undetectable, which supports my hypothesis.Citation3,Citation43 cGMP is a second messenger of both NO and ANP, and Polyakov’s cGMP did not return to premission levels until three months after this mission. The cGMP–signaling pathway has been postulated as an important regulator of renal physiology.Citation15 ANP receptors are expressed on the surface of renal endothelial cells,Citation15 and ANP inhibits renin release and can reduce renal damage from an ischemic insult.Citation38
Metabolic balance studies of young adults showed that with marginal intakes of Mg, males tended to be in negative Mg balance whereas females remained in equilibrium. Catecholamine levels in males, in turn, are therefore higher with vicious cycles between Mg ion deficits and catecholamine elevationsCitation39 (). After SF of only 18–22 days, rats showed an increased activity of the juxtaglomerular apparatusCitation18 and similarly, experimental Mg deficiency has been shown to cause hypertrophy of this structure, resulting in aldosterone secretion, and in turn, an increased Mg loss and another vicious cycle.Citation27,Citation39 VEGF levels are significantly higher during the formative stage (early teens) in females, although not in adulthood,Citation44 which would enhance repair of the endothelium as well as increase the development of collateral circulation.Citation28,Citation29
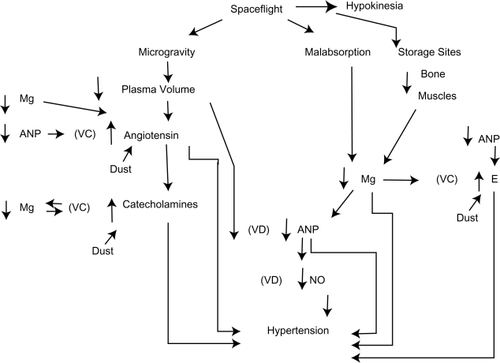
Figure 1 Proposed mechanisms showing how decreased atrial natriuretic peptide can cause severe hypertension secondary to dust inhalation, microgravity, and hypokinesia.
Gene therapy
How can we offset the SF reductions in cGMPCitation43 to maintain endothelial function by utilizing novel pharmacological compounds to prevent renal disease and in turn hypertension? How can we break self-perpetuating vicious cycles? Oxidative stress involving the kidneys and blood vessels triggers hypertension, whereas conversely hypertension has been shown to cause oxidative stress and inflammation in the kidney and cardiovascular tissue of experimental animals.Citation45 With very long missions, gene therapy may be the only option even without consideration of renal complications.Citation46 Gene therapy has been proposed, for example, to prevent invariable space anemia.Citation7 This, therefore, could be of vital importance in preventing ANP deficiencies.Citation6 There is a 10% red blood cell mass loss within one week of entering space.Citation17 Anemia may contribute to endothelial dysfunction by reducing oxygen delivery and intensifying oxidative stress since red blood cells have important antioxidant functions.Citation47 In addition there are SF reductions in platelets, the primary source of VEGF, which, in turn, play an important role in angiogenesis, function, and repair of the endothelium.Citation27 With SF there are reductions of water because of reduced thirst and loss of storage sites for water and Mg, and an impairment in red blood cell production. Therefore there is a reduction of three natural antioxidants conducive to endothelial dysfunction.Citation1,Citation3,Citation4,Citation48,Citation49 Furthermore, there is no subcutaneous device available to replenish Mg.Citation27
Without the availability of pharmaceuticals for use in SF, there is no alternative but the use of gene therapy. In addition to its use to correct invariable SF anemia,Citation7 gene therapy studies have been conducted to reduce BP.Citation6,Citation50,Citation51 NO is generated by NO synthase (NOS) enzymes and NOS gene transfer has been shown to reverse endothelial dysfunction in several diseases including hypertension and atherosclerosis. In addition NOS gene therapy has been shown to improve renal function and since NO is antifibrotic, this therapy may reduce the potential for renal fibrosis complicating aldosterone excess.Citation40,Citation52 A major stumbling block, however, has been finding a suitable vector to deliver the gene to the site of action and this may require several years (possibly decades) of SF research involving vector studies with progressively wider applications.Citation53
A number of viruses have been developed for gene transfer but the focus has been primarily on retroviruses, adenoviruses, adeno-associated viruses, and lentiviruses. The latter are currently of great interest; they are a type of retrovirus that can infect both dividing and nondividing cells because their virus “shelf ” can get through the intact membrane of the nucleus of the target cell. However because of persistent serious immune reactions with viruses, some researchers are directing their investigations to nonviral gene delivery. For example liposomal gene transfer has been investigated but there is a relatively low level of gene expression compared with that achieved from viral vectors.Citation52
It has been emphasizedCitation52 that gene therapy has not proven to be nearly as successful as had been predicted, which may be due to only transient transfection efficiencies. In addition if the expression of NOS gene therapy is not tightly regulated to the target site, there is the potential complication of pronounced hypotension with impending shock. McCarthy and colleaguesCitation52 conclude, by stating that although thousands of patients have been treated with gene transfer therapies, reproducibility of positive results has yet to be demonstrated conclusively.
I have stressed that one of the major concerns with SF is the vulnerability of the endothelium and, therefore, research must include the correction of ANP, NO, VEGF, and red blood cell deficiencies, the latter because of its antioxidant effect; this effect is shared by Mg administration,Citation33 which also has an anti-inflammatory and antifibrotic effect, and can prevent aldosterone-induced renal dysfunction (proteinuria).Citation40 Since transfection efficiency may be temporary,Citation52 gene therapy will have to be repeatedly reevaluated, depending upon the length of the mission. This will involve regularly scheduled diagnostic evaluation tests. How complex will these tests be and how much equipment and personnel will be required?
The gravity on the moon is 1/6 G whereas Mars is 0.38 G. Investigators have argued for decades as to how much <1 G is tolerable. Since we have Earth genes, I believe that any fraction <1 G may be ultimately intolerable. There is a solution, but we are only at the threshold of a process that may require many decades of gene therapy research before we are ready to embark on very long missions or colonize other planets. Is it “our destiny to colonize” before this research is complete? Some animals that spend all their lives in caves can’t adapt to the outside. Are we spared the restrictions of Nature? We share at least 80% of the same genes as rats.
Conclusions
Even though with relatively short missions (six months), the mean 24-hour BP is not elevated unless there is hypertension from inhalation of dust in the habitats without adequate protection devices, it seems reasonable to speculate that ultimately, astronauts will develop renovascular hypertension unless gene therapy is utilized. Until a suitable replenishable subcutaneous device is developed, Mg, necessary for the synthesis as well as the release of ANP, cannot be administered. Space missions of considerable length are more likely to succeed with a female crew. In addition to ongoing pharmaceutical research, a comprehensive gene research program should begin now. Specifically gene therapy may be necessary to suppress activity of an upregulated intrarenal renin–angiotensin system.
Disclosure
The author reports no conflicts of interest in this work. No grants or other support was received. The paper was presented in part at the European Magnesium Meeting. Paris–Cordeliers, May 15–17, 2008. The author greatly appreciates the assistance of retired NASA archivist, Lee Saegesser, who has provided a wealth of information for several years and for the conscientious work of all four librarians at the Medical University Of Ohio in Toledo who spent a portion of three days searching through NASA publications – in vain – as to whether Irwin’s extraordinary stress test data had ever been published.
References
- RoweWJThe Apollo 15 space syndromeCirculation1998971191209443446
- WatenpaughDEBuckeyJCLaneLDEffects of spaceflight on human calf hemodynamicsJ Appl Physiol2001901552155811247959
- RoweWJInterplanetary travel and permanent injury to normal heartActa Astronaut19974071971072211543600
- RoweWJMoon dust may simulate vascular hazards of urban pollutionJBIS200760133136
- HigashiYSasakiSNakagawaKMatsuuraHOshimaTChayamaKEndothelial function and oxidative stress in renovascular hypertensionN Engl J Med20023461954196212075056
- LinKFChaoJChaoLHuman atrial natriuretic peptide gene delivery reduces blood pressure in hypertensive ratsHypertension199526Part 18478537490138
- OhiSDeveloping protocols for recombinant adeno-associated virus-mediated gene therapy in spaceJ Gravit Physiol200076768
- AmidonGLDeBrincatGANajibNEffects of gravity on gastric emptying, intestinal transit, and drug absorptionJ Clin Pharmacol1991319689731761729
- DuJBayuseTMShahVPutchaLStability of pharmaceuticals during space flight [abstract]AAPS Pharm Sci2002T3153
- GraebeASchuckELLensingPPutchaLDerendorfHPhysiolgical, pharmacokinetic, and pharmacodynamic changes in spaceJ Clin Pharmacol20044483785315286087
- WadeCEMorey-HoltonEAlteration of renal function of rats following spaceflightAm J Physiol1998275R1058R10659756534
- Fritsch-YelleJMCharlesJBJonesMMWoodMLMicrogravity decreases heart rate and arterial pressure in humansJ Appl Physiol1996809109148964756
- ShiraishiMKamoTKamegaiMPeriodic structures and diurnal variation in blood pressure and heart rate in relation to microgravity on space station MIRBiomed Pharmacother200458S31S3415754836
- MatsukawaTManoTAtrial natriuretic hormone inhibits angiotensin II-stimulated sympathetic nerve activity in humansAm J Physiol19962712 Pt 2R464R4718770149
- CostaDMGonzalez BoscLVMajowiczMPVidalNABalaszczukAMArranzCTAtrial natriuretipeptide modifies arterial blood pressure through nitric oxide pathway in ratsHypertension2000351119112310818074
- WongNLMHuDCKWongEFCEffect of dietary magnesium on atrial natriuretic peptide releaseAm J Physiol19912615 Pt 2H1353H3571835306
- Leach HuntoonCSGrigorievAINatochinYVAmerican Astronaut SocietyFluid and Electrolyte Regulation in Spaceflight. (Science and Technology Series 94)San Diego, CAUnivelt1998
- AtkovOYBednenkoVSHypokinesia and Weightlessness: Clinical and physiologic aspectsMadison, WIInternational Universities Press1992167
- NeuhoferWPittrowDRole of endothelin and endothelin receptor antagonists in renal diseaseEur J Clin Invest200636788816919017
- LiaoTDYangXPLiuYHRole of inflammation in the development of renal damage and dysfunction in angiotensin II-induced hypertensionHypertension20085225626318541733
- DhaunNGoddardJWebbDJThe endothelin system and its antagonism in chronic kidney diseaseJ Am Soc Nephrol20061794395516540557
- PedrinelliRGiampietroOCarmassiFMicroalbuminuria and endothelial dysfunction in essential hypertensionLancet199434414187912295
- Mena-MartínFJMartín-EscuderoJCSimal-BlancoFCarretero-AresJLArzúa-MouronteDCastrodeza SanzJJHortega Study Investigators. Influence of sympathetic activity on blood pressure and vascular damage evaluated by means of urinary albumin excretionJ Clin Hypertens (Greenwich)2006861962416957423
- FutrakulNSridamaVFutrakulPMicroalbuminuria – A biomarker of renal microvascular diseaseRen Fail20093114014319212911
- WangTJEvansJCMeigsJBLow-grade albuminuria and the risks of hypertension and blood pressure progressionCirculation20051111370137615738353
- RadomskiMWPalmerRMJMoncadaSEndogenous nitric oxide inhibits human platelet adhesion to vascular endotheliumLancet198728567105710582889967
- RoweWJThe case for a subcutaneous magnesium product and delivery device for space missionsJ Am Coll Nutr200423525S528S15466957
- HaraAWadaTFuruichiKBlockade of VEGF accelerates proteinuria, via decrease in nephrin expression in rat crescentic glomerulonephritisKidney Int2006691986199516641924
- MasudaYShimizuAMoriTVascular endothelial growth factor enhances glomerular capillary repair and accelerates resolution of experimentally induced glomerulonephritisAm J Pathol200115959960811485918
- DavidsonMBHixJKVidtDGBrotmanDJAssociation of impaired diurnal blood pressure variation with a subsequent decline in glomerular filtration rateArch Intern Med200616684685216636209
- PhilpottDEPopovaIAKatoKStevensonJMiquelJSappWMorphological and biochemical examination of Cosmos 1887 rat heart tissue: Part I – UltrastructureFASEB J1990473782295379
- SteinTPSpace flight and oxidative stressNutrition20021886787112361781
- GarciaLADejongSCMartinSMSmithRSBuettnerGRKerberREMagnesium reduces free radicals in an in vivo coronary occlusion reperfusion modelJ Am Coll Cardiol1998325365399708488
- GracianoMLCavaglieri RdeCDellêHIntrarenal renin-angiotensin system is upregulated in experimental model of progressive renal disease induced by chronic inhibition of nitric oxide synthesisJ Am Soc Nephrol2004151805181515213268
- CampbellRCThe renin-angiotensin system: a 21st century perspectiveJ Am Soc Nephrol2004151963196415213288
- GrassiGAssessment of sympathetic cardiovascular drive in human hypertensionHypertension20095469070619720958
- ChristensenNJDrummerCNorskPRenal and sympathoadrenal responses in spaceAm J Kidney Dis20013867968311532706
- BrennerBMBallermanBJGunningMEZeidelMLDiverse biological actions of atrial natriuretic peptidePhysiol Rev1990706656992141944
- SeeligMCardiovascular consequences of magnesium deficiency and loss: pathogenesis prevalence and manifestations-magnesium and chloride loss in refractory potassium repletionAm J Cardiol1989634G21G
- SontiaBMontezanoACIParaviciniTTabetFTouyzRMDown-regulation of renal TRPM7 and increased inflammation and fibrosis in aldosterone-infused mice: effects of magnesiumHypertension20085191592118268139
- RemuzziGCattaneoDPericoNThe aggravating mechanisms of aldosterone on kidney fibrosisJ Am Soc Nephrol2008191459146218550649
- ClarkBAElahiDEpsteinFHThe influence of gender, age, and the menstrual cycle on plasma natriuretic peptideJ Clin Endocrin Metab199070349352
- RosslerANoskovVLaszloZPolyakowVVHinghoffer-SzalkayHGPermanent depression of plasma cGMP during long-term space flightPhysiol Res200150839011300230
- Malamitsi-PuchnerATziotisJTsonouAProtonotariouESarandakouACreatsasGChanges in serum levels of vascular endothelial growth factor in males and females throughout lifeJ Soc Gynecol Investig20007309312
- VaziriNDRodriguez-IturbeBMechanisms of disease: oxidative stress and inflammation in the pathogenesis of hypertensionNat Clin Pract Nephrol2006258259317003837
- RoweWJExtraordinary hypertension after a lunar missionAm J Med2009122e119854309
- DzieciuchowiczLChecinskiPKraussHHeparin reduces oxidative stress in the postoperative periodMed Sci Monit20028CR657CR66012218949
- MaierJAMMalpuech-BrugèreCZimowskaWRayssiguierYMazurALow magnesium promotes endothelial cell dysfunction: implications for atherosclerosis, inflammation and thrombosisBiochim Biophys Acta20041689132115158909
- WeglickiWBPhillipsTMFreedmanAMCassidyMMDickensBFMagnesium-deficiency elevates circulating levels of inflammatory cytokines and endothelinMol Cell Biochem19921101691731584207
- LinKFChaoLChaoJProlonged reduction of high blood pressure with human nitric oxide synthase gene deliveryHypertension199730Part 13073139314409
- SchillingerKJTsaiSYTaffetGERegulatable atrial natriuretic peptide gene therapy for hypertensionProc Natl Acad Sci U S A2005102137891379416162668
- McCarthyHOCoulterJARobsonTHirstDGGene therapy via inducible nitric oxide synthase: a tool for the treatment of a diverse range of pathological conditionsJ Pharm Pharmacol200860999101718644193
- KohnDBCandottiFGene therapy fulfilling its promiseN Engl J Med200936051852119179320