 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Bisphosphonates are the mainstay of treatment for postmenopausal women with osteoporosis. Despite numerous clinical trials documenting efficacy, tolerability, and safety of bisphosphonate therapy, long-term persistence and adherence to these agents remains low. This has serious consequences for patients with osteoporosis in that medication non-compliance is associated with significantly higher fracture risk. This review explores the unique physicochemical properties of bisphosphonates that allow more convenient intermittent dosing and whether less frequent dosing regimens improve compliance. Bisphosphonates are now available as oral drugs (taken daily, weekly, or monthly) or as intravenous preparations (given every 3 months or annually). The safety and efficacy of these various preparations are reviewed and compared, with particular emphasis on the newest agent to be approved, once-monthly risedronate. In contrast to monthly oral ibandronate, risedronate is the first and only monthly oral bisphosphonate to offer both vertebral and non-vertebral fracture reduction, based upon non-inferiority trials. Whether the greater convenience of this monthly oral bisphosphonate will translate into improved compliance and lower fracture risk is yet to be determined.
Introduction
Osteoporosis is characterized by a reduction in bone density and strength, and an increase in risk of fractures with minimal trauma. It is estimated that in 2010, 12 million individuals in the United States will have osteoporosis and 40 million more will have low bone density.Citation1 Worldwide, 200 million people have osteoporosis, and as the population ages, these numbers will continue to rise.Citation2
Fractures associated with osteoporosis have a major impact on quality of life, mortality, and health care costs. Over 2 million osteoporosis-related fractures occurred in 2005 in the United States.Citation3 Spine and hip fractures are particularly debilitating and costly. Within the first year after a hip fracture, mortality is increased 10% to 20% and long-term disability is common. Half of those who sustain a hip fracture are no longer able to walk independently, and up to one-third remain in a long-term care facility.Citation4 Vertebral fractures also result in excess mortality with the effect persisting beyond 1 year after the event.Citation4 Kyphosis and height loss caused by vertebral fractures contribute to reduced activity levels and cardiopulmonary morbidity. The risk of subsequent fractures at any site after a vertebral fracture is dramatically increased, with hip fractures 2 to 3 times more frequent.Citation5 The costs associated with acute treatment, rehabilitation, and nursing home facilities following osteoporotic fractures are approximately US$20 billion per year in the US.Citation3,Citation4 Hip fractures account for 72% of the economic burden though only comprising 14% of osteoporotic fractures.Citation3
A variety of pharmacologic agents are available for the prevention and treatment of osteoporosis, including bisphosphonates (BPs), selective estrogen receptor modulators, calcitonin, and teriparatide. Estrogen/progestin therapy is also effective against osteoporosis but its use is limited to women with menopausal symptoms. Because of significant efficacy against fractures and good tolerability, bisphosphonates have become the cornerstone of therapy for osteoporosis.
Unique pharmacokinetics of bisphosphonates
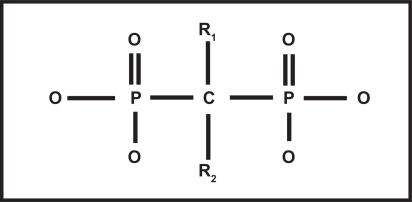
Chemically, all bisphosphonates contain a phosphate-carbon-phosphate (P-C-P) bond that is resistant to biological degradation.Citation6 Various substitutions at positions R1 and R2 on the carbon atom define the specific pharmacologic properties and mechanisms of action of the different bisphosphonates (see ). The unique chemical structure of the BPs acts as a ‘bone hook’, allowing rapid and widespread distribution of BPs onto bone mineral surfaces.Citation7 The specific structure of the R2 side chain determines the biological activity and antiresorptive potency of the BP molecule. Bisphosphonates containing nitrogen moieties at the R2 site, including risedronate, ibandronate, alendronate and zoledronate, are much more potent as antiresorptive agents compared with non-nitrogen-containing BPs, such as etidronate.Citation8
Figure 1 Structure of bisphosphonates: phosphate-carbon-phosphate (P-C-P) backbone, hydroxyl (-OH) groups, and substitutions at R1 and R2. Chemical group at R1 along with 2 phosphonate groups form the “bone hook” that is essential for binding to bone mineral; the three dimensional structure of the chemical group at R2 is critical for the biologic activity and antiresorptive potency of the molecule.
As a class, BPs are very poorly absorbed from the intestine with 50% of the absorbed dose taken up by the skeleton and the rest excreted in the urine.Citation6 Though cleared from the plasma within 6 to 10 hours after administration,Citation9 the portion bound to bone is slowly released back into the circulation over months or years.Citation10 Bisphosphonates are distributed into two bone compartments, one at the bone surface, where they exert their action, and the second, deeper within the bone matrix, where they remain biologically inert until released by later bone resorption.Citation6 The uptake and retention of bisphosphonates within the skeleton depend upon many factors including renal function, rate of bone turnover, number of available binding sites, and specific binding properties and potencies of the different bisphosphonates.Citation6
The activity of bisphosphonates is dependent on the continuous bone-remodeling cycle that “renews” the skeleton throughout life. Quiescent cells on the surface of bone are “activated” to begin bone remodeling at a specific area of the skeleton. Over a period of 2 weeks, activated osteoclasts remove old or damaged bone, leaving microscropic pits known as “lacunae” on the bone surface. As osteoclasts regress, bone-forming osteoblasts are recruited to secrete new osteoid (unmineralized bone) into the lacunae. This new bone is laid down and mineralized over the next 3 to 4 months, completing the bone remodeling cycle.Citation11
Potent nitrogen-containing bisphosphonates directly inhibit osteoclastic bone resorption resulting in a net gain in bone mass as the resorption pits are filled in with new bone. They also cause a decrease in bone turnover and a reduction in the rate of new fractures.Citation12 The process begins as bisphosphonates selectively bind to bone mineral at active sites of bone remodeling. During osteoclastic bone resorption, osteoclasts “ingest” the BP which induces a cascade of intracellular changes causing apoptosis (cellular death) of the osteoclasts.Citation6 As osteoclastic bone resorption slows down, the osteoblasts have more time to fill in empty lacunae and to restore bone structure and bone strength. Ultimately, though, osteoblastic bone formation also slows down as the two processes (resorption and formation) are “coupled.” As measured by biochemical markers of bone formation and bone resorption, the decrease in bone formation is slower and lags behind the decrease in bone resorption by several months. With continued BP administration, both bone resorption and bone formation reach new, lower steady states.Citation13
Since BPs have very high affinity for bone tissue and long half-lives, they can remain active at the surface of bone for extended periods of time between doses.Citation12,Citation14 For this reason, weekly administration of BPs may be categorized as continuous rather than truly intermittent therapy.Citation9 The efficacy of longer dosing intervals such as monthly or yearly administration of BPs, suggests that the inert drug buried beneath the bone surface must be recycled and reactivated by osteoclastic bone resorption.Citation15
Skeletal retention varies between BPs due to differences in binding properties so that not all drugs in this class may be able to be dosed at long, intermittent intervals.Citation6 The difference in binding affinities is mainly due to differences in the R2 side chain with a rank order of (from highest binding affinity to lowest) as follows:Citation16
The binding affinities of bisphosphonates for bone affect many important biological properties including uptake and retention in the skeleton, diffusion of the drug within bone, release of adsorbed drug from bone, potential recycling of the drug back onto bone surfaces and effects on mineral dynamics and cellular function within bone.Citation7 The ability of the BP to attach to bone mineral also contributes to its potency and duration of action. Higher affinity BPs, such as zoledronic acid, ibandronate and alendronate have more rapid uptakes, lower detachment rates, higher re-attachment rates and lower diffusion rates in the bone compared to risedronate.Citation16
Compliance and importance of less frequent drug dosing
Despite proven efficacy, good tolerability, and safety, patient compliance with BPs for osteoporosis remains poor. Reasons for suboptimal compliance with all osteoporosis therapies include the cost of medications, concerns about drug-related side effects, lack of understanding or motivation on the part of the patient, difficulty in treating an asymptomatic disease, and inconvenience.Citation17–Citation19 A number of side effects associated with oral bisphosphonates may also limit optimal compliance. These include gastritis, esophagitis, reflux, ulcers, abdominal discomfort, and musculoskeletal pains. In addition to side effects, convenience or lack thereof may play a particularly important role in adherence to oral bisphosphonate therapy.Citation18 The special dosing requirements for oral bisphosphonates include the need to take the drugs after an overnight fast, on a completely empty stomach, with 6 to 8 ounces (200 to 250 mL) of water, in an upright position, with no other food, pills, or beverages for at least 30 minutes. The drugs are contraindicated in patients with swallowing problems or difficulties remaining upright for at least 30 to 60 minutes. These strict requirements, necessary for the optimal absorption, effectiveness, and tolerability of these drugs, may be inconvenient and in some cases, impossible to meet.
A recent review of 14 different international pharmacy databases found that a high percentage of women with osteoporosis are not optimally compliant or persistent with their medication regimen.Citation20 In a study of 8822 new users of bisphosphonates, only 58% were compliant after 1 year.Citation21 Those on weekly oral bisphosphonates had consistently higher rates of compliance and persistence than those on daily regimens but still fell well below acceptable levels.Citation20 An analysis of a broad US retail pharmacy database with over 211,000 patients on oral bisphosphonates, found that adherence to therapy over a 12-month period was significantly higher among patients on weekly versus daily therapy.Citation22 However, only one-third of those receiving daily bisphosphonates and 45% of those on weekly bisphosphonates achieved adequate adherence levels.Citation22 In general, patients on weekly oral bisphosphonate regimens are 1.5 times more likely to persist with therapy compared to those on daily regimens.Citation20,Citation21,Citation23
If weekly therapy leads to better medication compliance than daily, would monthly oral therapy achieve even better results? In a prospective cross-over trial, 342 postmenopausal women with osteoporosis were randomized to either once-monthly ibandronate 150 mg followed by once-weekly alendronate 70 mg, or the opposite sequence, for a total of 6 months. Overall, 66% of women preferred the once-monthly regimen over the once-weekly regimen, mainly due to convenience and “ease of following a treatment regimen for a long time.”Citation24 When 248 women on weekly bisphosphonate therapy were switched to monthly ibandronate for 6 months, 95% stated a preference for the convenience of the monthly drug.Citation25 However, in an Internet-based survey of nearly 1000 postmenopausal women diagnosed with or at risk for osteoporosis, drug effectiveness against fractures was ranked as the most important attribute influencing drug preference while dosing frequency and dosing procedure were ranked much lower in importance.Citation26 Another online survey of 617 current bisphosphonate users found that patients preferred weekly therapies (risedronate or alendronate) over monthly therapy (ibandronate) by a very wide margin (82% vs 18%, respectively) when informed that the weekly therapies had broader anti-fracture efficacy.Citation27 Almost identical results were found in a European study using face-to-face or telephone interviews with similar prompting.Citation28 The studies by Gold and Keen were both sponsored by Procter and Gamble Pharmaceuticals and Sanofi-Aventis, the manufacturers of risedronate.
Gold et al found that among 240,000 patients in a pharmacy database receiving either weekly risedronate or monthly ibandronate, rates of persistence, adherence, and compliance were significantly higher with the weekly therapy.Citation27 The authors speculate that very infrequent dosing may result in skipping doses. They also suggest that earlier studies showing a preference for monthly dosing were biased by the presence of patient support programs for users of the monthly drug.Citation27 In a separate analysis of 166,000 women aged 50 years or older who were newly prescribed weekly alendronate or monthly ibandronate, ibandronate users were 10% more likely to discontinue therapy within the first year compared with those prescribed alendronate.Citation29 Ibandronate requires a higher co-pay which is offset by discount vouchers for the first pill. After taking the first month’s pill, 54% of patients on ibandronate failed to renew the prescription compared with 46% of alendronate users.Citation29 Once this initial effect was eliminated, however, there were very few differences in persistence between the groups by the end of one year.Citation29
There appear to be many determinants of patient persistence with medication. Weiss et al argue that dosing frequency is clearly not the only driver and may not even be an important driver of persistence with oral bisphosphonates.Citation29 Other important factors such as experience with side effects, practical difficulties with the dosing regimen, out of pocket costs, skepticism about drug efficacy or treatment benefit, lack of motivation, and concern about both short-term and long-term drug safety may be of far greater importance.Citation29
Consequences of poor compliance and adherence
Randomized clinical trials are critical for determining the safety and efficacy of drugs for osteoporosis. Unfortunately, they are not the best measures of “real world” conditions, particularly with respect to patient adherence to therapy. Even the most potent therapies for osteoporosis may “fail” if patients do not take medications properly or consistently over prolonged periods of time. Lack of adherence to oral bisphosphonate therapy has been associated with inadequate suppression of bone turnover markers as well as less than optimal gains in bone mineral density.Citation30–Citation32 Most importantly, poor adherence has been linked to higher fracture rates.
Bisphosphonate users with inconsistent adherence had 33% higher 10-year fracture rates than consistent users.Citation32 In a retrospective analysis of a pharmacy and medical claims database comprising over 35,000 women on oral bisphosphonates, compliant women had 21% fewer fractures relative to non-compliant women (p < 0.001).Citation33 Women who persisted with bisphosphonate therapy over 24 months had reductions of 40%, 29%, and 45% in vertebral, non-vertebral, and hip fractures, respectively, compared to non-persistent women (p < 0.001).Citation33 Overall, less than half of the women in this population were compliant with bisphosphonate therapy and only 1 in 6 persisted with therapy for 24 months.Citation33
A variety of international studies have found similar results. Among 44,000 British men and women on oral bisphosphonates, hip/femur fractures were reduced 22% in those who persisted with therapy for 1 year or more.Citation34 In a population from the Netherlands, patients who persisted with therapy for 2 years had 32% fewer fractures than those who stopped therapy before 2 years.Citation35 Among 8822 new female users of daily or weekly oral bisphosphonates in the Netherlands, compliance less than 80% was associated with a 40% increased risk of osteoporotic fracture compared with those with higher compliance rates.Citation36 Fracture risk increased steadily as the compliance rate decreased. Those with the very lowest compliance had an 80% higher risk of fracture than those with the highest compliance.Citation36 Finally, in a nested case control study from a large database in Quebec, women who sustained non-vertebral fractures while taking oral bisphosphonates for osteoporosis (cases) were matched with up to 20 age-matched women (controls) from the same database who were on bisphosphonates but did not fracture. Compared to controls, cases were frailer, had more risk factors for fracture, and were less compliant with bisphosphonate therapy. Even after controlling for confounding variables, lower compliance among the cases was associated with a 27% increased risk of fracture compared with controls.Citation37
Clinical trials with oral bisphosphonates
A number of randomized placebo-controlled prospective fracture trials supported the efficacy of once-daily oral BPs including alendronate, risedronate, and ibandronate in the treatment of postmenopausal osteoporosis.Citation38–Citation44 All of these drugs, when given once per day, significantly reduced the relative risk (RR) of vertebral fractures by 41% to 62%. In addition, daily oral alendronate reduced the RR of non-vertebral fractures by 47% (p = 0.021) in a group of women with low bone mineral density (BMD) (lumbar spine T-score of −2.0 or lower).Citation45 In a posthoc analysis of 4 clinical trials involving 620 postmenopausal women with osteopenia (T-score at the femoral neck between −1 and −2.5), 5 mg daily oral risedronate reduced the RR of all fragility fractures by 73% compared with placebo (p = 0.023).Citation45 These results suggest that both daily alendronate and risedronate are effective in reducing fractures in postmenopausal women with osteopenia as well as osteoporosis. With respect to hip fractures, daily oral risedronate reduced the RR of hip fracture by 30% in 5445 elderly women with osteoporosis at the femoral neck.Citation46 However, in this same study, oral daily risedronate did not reduce hip fractures in elderly women who had clinical risk factors for hip fracture but were not necessarily osteoporotic.Citation46
Ibandronate given daily (2.5 mg) or intermittently (20 mg every other day for 12 doses every three months) reduced vertebral fractures significantly when compared to placebo.Citation42 Neither treatment group showed a significant decrease in non-vertebral fractures, except for a subset of patients with very low BMD at the femoral neck (T-score < −3).Citation42 The ibandronate trials were the first to show that truly intermittent oral BP regimens could reduce fractures as well as daily regimens.
Decreasing the frequency or number of doses of a drug is a common strategy for enhancing adherence for all medications.Citation47 Among the oral drugs, weekly dosing of alendronate and risedronate, monthly ibandronate, and, most recently, risedronate given on two consecutive days a month or once per month, have all been approved for use based on non-inferiority trials. Non-inferiority trials with new dosing regimens have used BMD, not incidence of fracture, as the primary endpoint. Because BMD is inversely correlated with fracture risk, the FDA considers it to be an acceptable surrogate when approving new dosing regimens.
Until recently, ibandronate had been the only oral bisphosphonate offered in a monthly form. A randomized non-inferiority trial (MOBILE trial) found that after one year, treatment with monthly ibandronate, when compared to daily dosing, improved BMD similarly at both lumbar spine and hip in postmenopausal women with osteoporosis. Those who received 150 mg monthly had superior gains in lumbar spine BMD compared with 2.5 mg daily (4.9% for 150 mg monthly vs 3.9% in 2.5 mg daily).Citation48
Intermittent oral risedronate therapy
Risedronate has also been studied as an intermittent therapy. Weekly dosing of risedronate was studied in a 2-year randomized clinical trial of postmenopausal osteoporotic women.Citation49 Brown et al found that there was no significant difference in lumbar spine (LS) BMD improvement between risedronate 5 mg daily, 35 mg weekly, or 50 mg weekly. The three doses had similar effects on secondary endpoints including total hip, femoral neck, and trochanter BMD, bone turnover marker (BTM) levels, and incidence of vertebral fractures. Adverse side effect profiles were also similar among the three treatment groups.Citation49 These results led to approval of 35 mg weekly risedronate in 2002.
A 6-month pilot study compared 5 mg daily risedronate with 50 mg on 3 consecutive days a month.Citation50 One-hundred-fifty women aged 65 to 80 with low BMD (T-scores ≤ −2) were randomized to received 5 mg daily, 50 mg for 3 consecutive days a month, or 15 mg daily loading dose for a month followed by 50 mg on 3 consecutive days a month.Citation50 Serum markers of bone turnover including N-telopeptide (NTX), C-telopeptide (CTX), and serum bone specific alkaline phosphatase (BAP) served as evidence for efficacy over the 6-month period, with NTX serving as a primary endpoint. BMD was measured at baseline and after 6 months.
Levels of NTX, CTX, and BAP were reduced significantly from baseline in all three groups and there was no significant difference between the groups at 6 months. Mean percent changes from baseline at LS BMD at month 6 were similar among treatment groups as well as were adverse events. While this study showed promise in the efficacy and safety of risedronate monthly, the small sample size and brief length of follow-up were not powered to confirm non-inferiority.Citation50
Recently, two well-powered international, multicenter, randomized, double blind trials have investigated the efficacy and safety of intermittent risedronate. In one, postmenopausal women with osteoporosis were randomized to risedronate 5 mg daily (n = 613) or 75 mg on 2 consecutive days a month (2CDM) (n = 616) for 2 years.Citation51 The primary endpoint was percent change from baseline in LS BMD at 12 months of treatment. Mean percent change in LS BMD was 3.4% ± 0.16% in the 75 mg 2CDM group, and 3.6% ± 0.15% in the 5 mg daily group. These both represented significant increases in BMD, without significant difference between the two regimens, thereby confirming non-inferiority of the 75 mg 2CDM regimen.
Secondary efficacy was measured with mean percent change from baseline in LS, total hip, trochanter, and femoral neck BMD, and BTMs. Both groups had a significant effect on these measures, without a significant difference between the two groups. Incidence of new vertebral fractures was also assessed, by comparing radiographs at baseline and at 12 months. There was no difference between groups in new vertebral fractures (6 fractures in the 75 mg 2CDM and 7 fractures in the 5 mg daily).Citation51
There were no clinically relevant differences in safety and tolerability between the two groups and a similar number of subjects in each group experienced treatment-emergent adverse events (TEAEs). The most common TEAEs in both groups (ie, >10% of subjects) were arthralgias and back pain. Withdrawal occurred in 9% of subjects in both groups, and was most commonly related to gastrointestinal symptoms. Acute phase reactions (fever or flu-like illness within the first 5 days of treatment) were experienced in a small, but higher, number of patients in the 75 mg on 2CDM group (4 subjects) than in the 5 mg daily group (0 subjects). The authors of the study concluded that 75 mg of risedronate on 2CDM was at least as effective and safe as 5 mg daily.Citation51
In a 2nd randomized trial, Delmas et al found that 150 mg of risedronate monthly had similar efficacy and tolerability as 5 mg daily after the first year of a 2-year study.Citation52 Postmenopausal women with osteoporosis were randomized to receive 150 mg of risedronate as one dose a month (with placebo pills for the rest of the month) (n = 650), or 5 mg daily (n = 642). The primary endpoint was the mean percent change from baseline in LS BMD after one year. The mean percent change in LS BMD was 3.4% (95% CI 3.30%–3.82%) in the 5 mg daily group and 3.5% (95% CI 3.15%–3.93%) in the 150 mg monthly group; a difference of −0.1% (95% CI −0.5%–0.27%) between groups. These numbers represented a significant improvement in LS BMD in both groups, without difference in efficacy between groups, and satisfied pre-determined criteria for non-inferiority.Citation52
Change from baseline BMD at the total proximal femur, femoral neck, and trochanter were also measured and found to be significantly increased in both groups, with no significant difference between groups at any point in time. Bone turnover markers (NTX, CTX, BAP) were decreased significantly and to similar degrees in both groups at endpoint. The incidence of vertebral fractures was measured by comparing radiographs at baseline to radiographs after the first 12 months of treatment and was found to be equal: 8 subjects in each group had a new vertebral fracture.Citation52
Safety and tolerability were considered to be comparable between the two groups as well. Adverse events considered to be of special interest for bisphosphonates (including clinical vertebral and non-vertebral fractures, upper gastrointestinal AEs, and musculoskeletal events) were reported at similar frequency in both groups. Diarrhea and influenza were the only AEs reported more frequently in the monthly group, with 5 patients withdrawing due to diarrhea. Most cases of influenza occurred more than 90 days after treatment and all were considered mild or moderate in severity. No subjects withdrew as a result. The incidence of potential acute phase reactions (influenza or flu-like illness and/or pyrexia within 3 days of start of treatment) was slightly higher in the monthly group (1.4% in the monthly group and 0.2% in the daily group), but only one event, in the monthly group, was considered severe. Furthermore, only one subject withdrew as a result of a reaction.Citation52
Intermittent intravenous therapy for osteoporosis
Despite efforts to make oral BPs more convenient by moving to intermittent, less frequent dosing, a number of patients are still unable to tolerate these drugs. As noted earlier, the most common side effects are gastrointestinal including gastritis, esophagitis, reflux, abdominal pain and ulcers. The oral BPs may be poorly absorbed and ineffective in patients with intestinal malabsorption syndromes. The drugs are contraindicated in patients with swallowing problems or difficulties remaining upright for at least 30 to 60 minutes. Less frequent dosing does not eliminate the need to take the oral BPs properly, as outlined above, and may result in poor compliance and persistence, particularly among elderly patients with complicated medication regimens or cognitive difficulties.
The intravenous BPs eliminate a number of the drawbacks of oral BPs. Currently, two intravenous preparations are FDA-approved for osteoporosis in the United States. These include ibandronate 3 mg given as an intravenous (iv) injection over 15 to 30 seconds once every 3 monthsCitation53–Citation55 and zoledronic acid 5 mg given as a once-yearly iv infusion over 15 to 20 minutes.Citation56,Citation57 The ibandronate trials did not include fracture endpoints but simply looked at changes in BMD and bone resorption markers. In contrast, both zoledronic acid trials had vertebral and nonvertebral fracture endpoints. In the 3-year randomized, prospective placebo-controlled study of postmenopausal women with osteoporosis by Black et al an annual infusion of 5 mg zoledronic acid significantly reduced morphometric vertebral, clinical vertebral, hip and non-vertebral fractures by 70%, 77%, 41%, and 25%, respectively.Citation56 The HORIZON study conducted by Lyles et al was the first clinical trial to study secondary fracture prevention and other outcomes in patients with recent hip fractures.Citation57 Only 41% of this patient population had osteoporosis according to BMD measurements at the femoral neck while the rest had normal or osteopenic BMD at the hip. In this high-risk population, the authors reported a significant 35% reduction in all clinical fractures and a 28% risk reduction in all-cause mortality in the patients who received zoledronic acid versus placebo.Citation57
Comparisons between different bisphosphonates
There are no head-to-head antifracture studies comparing the various oral and intravenous bisphosphonates on the market. Without such studies, current evidence does not support a clear distinction in fracture reduction among the different agents. Surrogate markers, such as BMD changes and suppression of biochemical markers of bone turnover, have been compared between alendronate and risedronate users in a randomized double blind clinical trial (the FACT study).Citation58 In this 12-month study of 833 postmenopausal women with osteoporosis, 70 mg of weekly alendronate resulted in significantly greater BMD increases at all sites and significantly lower markers of bone turnover compared to 35 mg of weekly risedronate.Citation58 A 1-year extension of this study continued to show that alendronate “outperformed” risedronate on every measure including BMD increases, suppression of bone turnover markers, and number of responders.Citation59 Adverse events did not differ between the two groups.
The differences in antiresorptive efficacy between alendronate and risedronate may be related to the greater affinity of alendronate for hydroxyapatite and its longer retention in bone.Citation16 These properties, though beneficial in some respects, have raised concerns about potential over-suppression of normal bone activity in long-term alendronate users.Citation60 Several case reports have linked chronic use of alendronate, for 5 or more years, with sudden, low-energy, subtrochanteric fractures.Citation61–Citation63 However, a recent report using a cross-sectional and matched cohort national database found no increase in these atypical femur fractures among alendronate users.Citation64 Another concern is the relationship between bisphosphonates and osteonecrosis of the jaw (ONJ). Reports of ONJ occurring in cancer patients treated with iv bisphosphonates led to questions about the safety of oral bisphosphonates in treatment of osteoporosis. A literature review found that ONJ occurred rarely (23 cases) in patients taking oral bisphosphonates for osteoporosis, especially considering that millions of patients have been prescribed bisphosphonates for this purpose.Citation65 Furthermore, many cases had a history of invasive dental treatment with dental trauma at the site of ONJ. In the two largest trials of intravenous zoledronic acid for treatment of osteoporosis,Citation56,Citation57 there was no increased risk of ONJ in those receiving the bisphosphonate for up to 3 years. An extensive review of ONJ is beyond the scope of this paper.
Two short-term studies have compared annual intravenous zoledronic acid with weekly oral alendronate.Citation66,Citation67 In a 24-week trial comparing a single-infusion of zoledronic acid with weekly oral alendronate in postmenopausal women with osteoporosis, zoledronic acid caused a greater and more rapid reduction in bone turnover markers compared with weekly alendronate.Citation66 However, acute phase reactions consisting of flu-like symptoms and fever within 3 days of the infusion were seen much more frequently with zoledronic acid compared with alendronate (18.8% vs 5.1%). Despite this, most patients expressed a preference for annual iv therapy (66.4%) compared with weekly oral therapy (19.7%).Citation66
A second head-to-head comparison trial between annual iv versus weekly oral BP therapy was conducted by McClung et alCitation67 It was a 12-month trial involving postmenopausal women with osteoporosis who had received at least 1 year of alendronate, prior to randomization. Participants were randomized to receive either one 5-mg zoledronic acid infusion or 70 mg of weekly alendronate. The primary outcome was the percent change from baseline in lumbar spine BMD at the end of one year. Zoledronic acid maintained the therapeutic effect of prior alendronate. Moreover, the annual iv infusion was preferred by 79% of the patients at the end of the study. Adverse events were mild and similar between the groups. Interestingly, none of the patients who switched from alendronate to zoledronic acid experienced acute phase reactions suggesting that longer exposure to BPs causes a waning of this side effect.
Conclusion: monthly risedronate for osteoporosis
Bisphosphonates have become the mainstay of treatment in most patients with postmenopausal osteoporosis. By reducing excessive osteoclast activity, BPs are able to restore the rate of bone turnover to premenopausal levels, thereby preventing further deterioration of bone quality in patients with accelerated bone loss.Citation7 Oral alendronate, risedronate and ibandronate have all been shown to reduce the risk of vertebral fractures, but only alendronate and risedonate have been documented to reduce non-vertebral and hip fractures.
There are many causes for nonresponse to anti-osteoporosis therapy including co-morbid conditions, malabsorption, calcium and vitamin D deficiency, and poor compliance with therapy.Citation68 Suboptimal compliance with daily and even weekly oral BPs has been associated with higher fracture rates. In an effort to improve compliance and persistence with therapy, less frequent oral and iv dosing regimens have been developed. Monthly oral risedronate offers patients the convenience and ease of monthly self-administration. In contrast to monthly oral ibandronate, risedronate is the first and only monthly oral BP to offer both vertebral and non-vertebral fracture reduction (though this is based on non-inferiority studies, not actual fracture trials with the monthly preparation). There are no head-to-head studies comparing efficacy, tolerability, or patient preferences between oral monthly risedronate and once-yearly iv zoledronic acid, but studies comparing weekly alendronate and annual iv zoledronic acid suggest that patients may prefer the once per year iv infusion over a weekly oral therapy. Whether this same preference applies to monthly oral therapy remains unclear. In the end, other factors such as cost, insurance coverage, physician and patient preferences, familiarity with the iv drug administration,Citation7 and concerns about safety of the iv infusion, may be the most important issues determining the place of monthly risedronate in the armamentarium of osteoporosis drug therapies.
Disclosures
The authors have no conflicts of interest to disclose.
References
- National Osteoporosis Foundation102008 Available from: http://www.nof.org/advocacy/prevalence
- ReginsterJYBurletNOsteoporosis: A still increasing prevalenceBone2006382 Suppl 1S4S916455317
- BurgeRDawson-HughesBSolomonDHWongJ BKingATostesonAIncidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025J Bone Miner Res200722346547517144789
- CummingsSRMeltonLJEpidemiology and outcomes of osteoporotic fracturesLancet200235993191761176712049882
- van StaaTPLeufkensHGCooperCDoes a fracture at one site predict later fractures at other sites? A British cohort studyOsteoporos Int200213862462912181620
- CremersSCPillaiGPapapoulosSEPharmacokinetics/pharmacodynamics of bisphosphonates: use for optimization of intermittent therapy for osteoporosis. ReviewClin Pharmacokinet200544655157015932344
- BoonenSVanderschuerenDVenkenKMilisenKDelforgeMHaentjensPRecent developments in the management of postmenopausal osteoporosis with bisphosphonates: enhanced efficacy by enhanced complianceJ Intern Med200826431533218823505
- RussellRGCroucherPIRogersMJBisphosphonates: pharmacology, mechanisms of action and clinical usesOsteoporos Int19999Suppl 2S66S8010525729
- MillerPDOptimizing the management of postmenopausal osteoporosis with bisphosphonates: the emerging role of intermittent therapyClin Ther200527436137615922811
- LinJHBisphosphonates: a review of their pharmacokinetic propertiesBone199618275858833200
- FrostHMSome ABCs of skeletal pathophysiology. III: Bone balance and the delta B.BMUCalcif Tissue Int1989451311332505901
- DelmasPDTreatment of postmenopausal osteoporosisLancet200235993222018202612076571
- PapapoulosSbisphosphonates in the management of osteoporosisMarcusRFeldmanDKelseyJOsteoporosis22nd EdSan FranciscoAcademic Press2001631650
- Moro-AlvarezMJDiaz-CurielMRisedronate once monthly: a potential new regimen for the treatment of postmenopausal osteoporosisClin Intervent Aging2008132227232
- BaussFRussellRGIbandronate in osteoporosis: preclinical data and rationale for intermittent dosingOsteoporos Int200415642343315205712
- NancollasGHTangRPhippsRJNovel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatiteBone20063861762716046206
- RossiniMBianchiGDi MunnoODeterminants of adherence to osteoporosis treatment in clinical practiceOsteoporos Int20061791492116538553
- SegalETamirAIsh-ShalomSCompliance of osteoporotic patients with different treatment regimensIsr Med Assoc J2003585986214689753
- TostesonANGroveMRHammondCSEarly discontinuation of treatment for osteoporosisAm J Med200311520921612947959
- CramerJAGoldDTSilvermanSLLewieckiEMA systematic review of persistence and compliance with bisphosphonates for osteoporosisOsteoporos Int2007181023103117308956
- Penning-van BeestFJAGoettschWGErkensJAHeringsRMCDeterminants of persistence with bisphosphonates: a study in women with postmenopausal osteoporosisClin Ther20062823624216678644
- ReckerRRGallagherRMacCosbePEEffect of dosing frequency on bisphosphonate medication adherence in a large longitudinal cohort of womenMayo Clin Proc200580785686116007889
- Penning-van BeestFJAErkensJAOlsonMHeringsRMCDeterminants of noncompliance with bisphosphonates in women with postmenopausal osteoporosisCurr Med Res Opin20082451337134418380910
- EmkeyRKoltunWBeusterienKPatient preference for once-monthly ibandronate versus once-weekly alendronate in a randomized, open-label, cross-over trial: The Boniva Alendronate Trial in Osteoporosis (BALTO)Curr Med Res Opin200521121895190316368038
- KastelanDLozaPStamenkovicDPreference for weekly and monthly bisphosphonates among patients with postmenopausal osteoporosis: results from the Croatian PROMO studyClin Rheumatol.20081125 [Epub ahead of print]
- WeissTWGoldDTSilvermanSLMcHorneyCAAn evaluation of patient preferences for osteoporosis medication attributes: results from the PREFER-US studyCurr Med Res Opin200622594996016709316
- GoldDTSafiWTrinhHPatient preference and adherence: comparative US studies between two bisphosphonates, weekly risedronate and monthly ibandronateCurr Med Res Opin200622122383239117257452
- KeenRJodarEIolasconGEuropean women’s preference for osteoporosis treatment: influence of clinical effectiveness and dosing frequencyCurr Med Res Opin200622122375238117257451
- WeissTWHendersonSCMcHorneyCACramerJAPersistence across weekly and monthly bisphosphonates: analysis of US retail pharmacy prescription refillsCurr Med Res Opin20072392193220317686228
- EastellRGarneroPVrijensBInfluence of patient compliance with risedronate therapy on bone turnover marker and bone mineral density response: the IMPACT studyCalcif Tissue Int200372297
- YoodRAEmaniSReedJICompliance with pharmacologic therapy for osteoporosisOsteoporos Int20031496596814504697
- SebaldtRJShaneLGPhamBLonger term effectiveness outcomes of noncompliance and nonpersistence with daily reimen bisphosphonate therapy in patients with osteoporsois treated in tertiary specialist careOsteoporos Int200415Suppl 1S107 [abstract P391SA]
- SirisESHarrisSTRosenCJAdherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databasesMayo Clin Proc20068181013102216901023
- GallagherAMRietbrockSOlsonMvan StaaTPFracture outcomes related to persistence and compliance with oral bisphosphonatesJ Bone Miner Res200823101569157518505366
- Van den BoogaardCHABreekveldt-PostmaNSBorggreveSEPersistent bisphosphoante use and the risk of osteoporotic fractures in clinical practice: a database analysis studyCurr Med Res Opin2006221757176416968579
- Penning-van BeestFJAErkensJAOlsonMHeringsRMCLoss of treatment benefit due to low compliance with bisphosphonate therapyOsteoporos Int20081951151717874028
- BlouinJDragomirAMorideYLouis-GeorgeS-MFernandesJCPerreaultSImpact of noncompliance with alendraonte and risedronate on the incidence of nonvertebral osteoporotic fractures in elderly womenBr J Clin Pharmacol66111712718460036
- CummingsSRBlackDMThompsonDEEffect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results form the Fracture Intervention TrialJAMA1998280207720829875874
- HarrisSTWattsNBGenantHKEffects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy(VERT) Study GroupJAMA19992821344135210527181
- ReginsterJMinneHWSorensenOHRandomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study GroupOsteoporos Int200011839110663363
- SorensenOHCrawfordGMMulderHLong-term efficacy of risedronate: a 5-year placebo-controlled clinical experienceBone20033212012612633783
- ChesnutCHSkagAChristiansenCEffects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosisJ Bone Miner Res2004191241124915231010
- DelmasPDReckerRRChesnutCHDaily and intermittent oral ibandronate normalize bone turnover and provide significant reduction in vertebral fracture risk: results from the BONE studyOsteoporos Int20041579279815071723
- BoneHGHoskingDDevogelaerJPTen years’ experience with alendraonte for osteoporosis in postmenopausal womenN Engl J Med20043501189119915028823
- PolsHAFelsenbergDHanleyDAMultinational, placebo-controlled, randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: results of the FOSIT study. Fosamax International Trial Study GroupOsteoporos Int1999946146810550467
- SirisESSimonJABartonIPEffects of risedronate on fracture risk in postmenopausal women with osteopeniaOsteoporos Int200819568168617968610
- McClungMRGeusensPMillerPDEffect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study GroupN Engl J Med200134433334011172164
- ClaxtonAJCramerJPierceCA systematic review of the associations between dose regimens and medication complianceClin Ther2001231296131011558866
- MillerPDMcClungMRMacoveiLMonthly oral and ibandronate therapy in postmenopausal osteoporosis: 1-year results from the MOBILE studyJ Bone Miner Res2005201315132216007327
- BrownJPKendlerDLMcClungMRThe efficacy and tolerability of risedronate once a week for the treatment of postmenopausal osteoporosisCalc Tissue Int2002712103111
- RacewiczAJSchofieldPJCahallDLClineGABurgioDEMonthly dosing with risedronate 50 mg on three consecutive days a month compared with daily dosing with risedronate 5 mg: a 6-month pilot studyCurr Med Res Opin200723123079308917971285
- DelmasPDBenhamouCLManZMonthly dosing of 75 mg risedronate on 2 consecutive days a month: Efficacy and safety resultsOsteoporos Int20081971039104518087660
- DelmasPDMcClungMRZanchettaJREfficacy and safety of risedronate 150 mg once a month in the treatment of postmenopausal osteoporosisBone2008421364217920005
- ThiebaudDBurckhardtPKriegbaumHThree monthly intravenous injection of ibandronate in the treatment of postmenopausal osteoporosisAm J Med19971032983079382122
- DelmasPDAdamiSStrugalaCIntravenous ibandronate injections in postmenopausal women with osteoporosis: one-year results from the dosing intravenous administration studyArthritis Rheum2006541838184616729277
- EismanJACivitelliRAdamiSEfficacy and tolerability of intravenous ibandronate injections in postmenopausal osteoporosis: 2-year results from the DIVA studyJ Rheum20083548849718260172
- BlackDMDelmasPDEastellROnce-yearly zoledronic acid for treatment of postmenopausal osteoporosisN Engl J Med20073561809182217476007
- LylesKWColon-EmericCSMagazinerJSZoledronic acid and clinical fractures and mortality after hip fractureN Engl J Med20073571799180917878149
- RosenCJHochbergMCBonnickSLFosamax Actonel Comparison Trial Investigators. Treatment with once-weekly alendronate 70 mg compared with once-weekly risedronate 35 mg in women with postmenopausal osteoporosis: a randomized double-blind studyJ Bone Miner Res20052014115115619680
- BonnickSLSaagKGKielDPComparison of weekly treatment of postmenopausal osteoporosis with alendronate versus risedronate over two yearsJ Clin Endocr Metab20069172631263716636120
- OdvinaCVZerwekhJERaoDSSeverely suppressed bone turnover: a potential complication of alendronate therapyJ Clin Endocrinol Metab20059031294130115598694
- GohSKYangKYKohJSSubtrochanteric insufficiency fractures in patients on alendronate therapy: a cautionJ Bone Joint Surg Br200789334935317356148
- KwekEBGohSKKohJSPngMAHoweTSAn emerging pattern of subtrochanteric stress fractures: a long-term complication of alendronate therapy?Injury200839222423118222447
- LenartBALorichDGLaneJMAtypical fractures of the femoral diaphysis in postmenopausal women taking alendronateN Engl J Med2008358121304130618354114
- AbrahamsenBEikenPEastellRSubtrochanteric and diaphyseal femur fractures in patients treated with alendronate: a register-based national cohort studyJ Bone Miner Res20081229 [Epub ahead of print]
- PazianasMMillerPBlumentalsWABernalMKothawalaPA review of the literature on osteonecrosis of the jaw in patients with osteoporosis treated with oral bisphosphonates: prevalence, risk factors and clinical characteristicsClin Ther20072981548155817919538
- SaagKLindsayRKriegmanABeamerEZhouWA single zoledronic acid infusion reduces bone resorption markers more rapidly than weekly oral alendronate in postmenopausal women with low bone mineral densityBone2007401238124317347063
- McClungMReckerRMillerPIntravenous zoledronic acid 5 mg in the treatment of postmenopausal women with low bone density previously treated with alendronateBone20074112212817468062
- LewieckiEMNonresponders to osteoporosis therapyJ Clin Densitom20036430731414716042