Abstract
Rationale
The role of the immune response to caterpillar exposure is not well described. This case study is the first to report a patient who presented with an allergic reaction after exposure to the larvae of the sycamore tussock moth, Halysidota harrisii Walsh, 1864.
Methods
Blood was collected from an allergic asthmatic adult (m/42 y/o) at 2 hrs – 2 wks after contact urticaria with associated dyspnea after exposure to the larvae of the sycamore tussock moth, Halysidota harrisii Walsh, 1864. Distributions of blood lymphocytes (CD4+, CD8+, CD8+CD60+, CD19+, CD23+, CD16/56+, CD25, CD45RA+, CD45RO+), monocytes (CD1d+), levels of serum immunoglobulins (IgM, IgG, IgA, IgE), and cytokines (IFN-γ, IL-4, TNF-α) were studied (flow cytometry, nephelometry, UniCAP Total IgE Fluoroenzymeimmunoassay, cytokine ELISA, clinical toxicology).
Results
Numbers of CD4+ T cells, CD25+ cells, CD19+ B cells, and CD1d+ monocytes decreased (22, 27, 33, 20%, respectively) one week post reaction, CD45RA+ naïve T cells decreased at 36 hours (21%),while CD8+CD60+ T cells and CD23+ cells decreased 48 hrs (33, 74%, respectively) post reaction. In contrast, numbers of CD16/56+ NK precursor cells increased (60%) 12 hrs, then decreased (65%) 48 hrs post reaction; other lymphocyte subsets were unaffected. Serum IgM, IgG and IgA were within normal range; however, serum IgE demonstrated a bimodal elevation at 2 hrs (15%) and one week post reaction. Levels of IFN-γ, IL-4, and TNF-α were not detected in serum pre-exposure (<1.0–4.0 pg/mL). However, high levels of IFN-γ (187–319 pg/mL) and TNF-α (549–749 pg/mL) were detected in serum 24–36 hrs and 3.5–24 hrs post reaction, respectively. In contrast, levels of IL-4 were undetected (<1.0 pg/mL) in serum at all time points.
Conclusions
Exposure to the larvae of the sycamore tussock moth, Halysidota harrisii Walsh, 1864 may result in increased cytokine levels and blood CD16/56+ NK precursor cells.
Introduction
It has been reported that caterpillar exposure results in symptoms including: local effects (dermatitis),Citation1,Citation2–Citation5 local pain,Citation1,Citation6 and systemic effects, including anaphylaxis, depending on the species.Citation1,Citation6–Citation9 Caterpillar dermatitis or lepidopterism is a toxic irritant whose reaction is triggered by the release of histamine thaumetopoein and other kinins from the hairs of caterpillars and butterflies.Citation10 In central Europe, the two main causes of caterpillar dermatitis are the oak and pine processionary caterpillar.Citation10 It has been observed that patients may develop cutaneous reactions, conjunctivitis, bronchitis and in some instances, anaphylactic reactions.Citation10 With the exception of bee and wasp venom allergies, immediate-type allergic reactions to mosquitoes, flies, ticks, bed bugs, moths, caterpillars and spiders are rare.Citation11
Other studies have reported gypsy moth caterpillar dermatitis after direct contact with the first instar larva of the gypsy moth (Lymantria dispar).Citation12 The pathogenesis of this dermatitis most likely involves histamine release by the caterpillar and a delayed hypersensitivity reaction in its host.Citation12
Exposure to hairs from ‘itchy’ caterpillars are usually nonvenomous, but a contact reaction may produce mechanical irritationCitation1,Citation13 and dermatitis.Citation1,Citation3,Citation5,Citation14 Studies of Vega et al have reported allergic reactions to allergens from the pine processionary caterpillar Thaumetopoea pityocampa, which induces dermatitis by a toxic-irritative mechanism;Citation15 these reactions were confirmed by positive skin prick tests and specific IgE determination (immunoblot) using crude larval extracts.Citation15 However, a specific low molecular weight 15-kDa IgE-binding protein, Tha p 1, was later identified as a major caterpillar allergen.Citation16 Patients with suspected contact urticaria from pine processionary caterpillars displayed allergic reactions/symptoms that included angiodema, conjunctivitis, and anaphylaxis. Citation15 The wheals were localized mainly on the neck and forearms.Citation15 Cutaneous reactions to pine processionary caterpillar have also been reported in children.Citation17 However, these cases are frequent in pinery zones, and only in some cases, due to an IgE-mediated allergic mechanism caused by pine processionary caterpillar proteins.Citation17,Citation18 Systemic allergic reactions to the larvae of the sycamore tussock moth, Halysidota harrisii Walsh, 1864 in humans have not been reported.
In the current study, we are the first to describe the clinical and immunological responses following contact with the larvae of the sycamore tussock moth, Halysidota harrisii Walsh, 1864, in Brooklyn, NY. Even though reactions to Lepidoptera (moths, butterflies, and caterpillars) are usually mild and self-limited, reactions in sensitive individuals and to specific species can be severe.Citation19
Because the reported responses to caterpillar exposure are variable and the habitat of Halysidota harrisii Walsh, 1864 is prevalent in eastern parts of the United States as well as southeast regions of Canada,Citation20 the potential importance of cells and cytokines post exposure in atopic individuals may be underrepresented. As such this case demonstrates the complexity of immune reactions to caterpillar exposure. Awareness and further understanding of caterpillar exposure may be important for atopic individuals with caterpillar sensitivity.
Materials and methods
Patient history
A 42-year-old man presented to his private doctor’s office (Brooklyn, NY) with a severe allergic reaction from contact with a caterpillar which fell on his arm, when he was entering his car which was parked under a tree in front of his house (Brooklyn, NY). A more detailed description of the patient’s symptoms is listed in the Results section.
In order to further characterize the systemic immune response to the caterpillar, informed consent was obtained, and blood and serum samples were drawn at various time points after diagnosis (eg, 2, 3.5, 12, 24, 36, 48 hrs; 1, 2 wks).
Caterpillar identification
The caterpillar observed at the time of the exposure was obtained from the patient for identification. Identification was performed at the Dept of Entomology, Cornell University (Geneva, NY) according to standard clinical taxonomic methodology.
Immunoglobulin determination
Blood was collected and immunoglobulin (Ig) levels (IgG, IgM, IgA) were detected in serum (Quest Diagnostics, Teterboro, NJ). All serum IgE determinations were carried out in the Clinical Diagnostic Laboratory at SUNY Downstate Medical Center (UniCAP Total IgE fluoroenzymeimmunoassay, Pharmacia and Upjohn Diagnostics, Kalamazoo, MD) which was performed according to manufacturer’s recommendation.
Flow cytometry
For flow cytometry studies, blood was collected into ethylenediaminetetraacetic Monoject tubes (Sherwood Medical Company, St. Louis MO) (EDTA) and retained for up to 2 hr at room temperature.
Antibodies
Mouse antihuman monoclonal antibodies directly conjugated to fluorescein isothiocyanate (FITC) (IgG1 anti-CD45RA, CD25, CD23; IgM anti-CD60); phycoerythrin (PE) (IgG1 anti-CD4, CD8, CD1d; IgG2a anti-CD45RO), Simultest (FITC/PE-conjugated) reagents (CD3/CD4, CD3/CD8, CD3/CD19, CD3/CD16+CD56), and appropriately matched isotype control monoclonal antibodies (FITC-conjugated IgG1, PE-conjugated IgG1 and IgG2a, Simultest control γ1/γ2a, FITC-conjugated IgM). All antibodies were purchased from BD Biosciences (San Jose, CA), except IgM anti-CD60 which was purchased from Ancell, (Bayport, MN); and IgG1 anti- CD1d, which was purchased from BD Pharmingen (San Diego, CA). All were used according to manufacturers’ recommendation.
Assay
For labeling studies, conjugated antibodies (10 μL) (or 80 μL of titrated anti-CD60) directed against 1–3 markers, were added to blood (100 μL) in 12 × 75 mm (5 mL) tubes (Fisher Scientific, Springfield, NJ) and incubated for 10 mins at room temperature, after which erythrocytes were lysed with whole blood lysing reagent (Immunoprep; Beckman Coulter, Hialeah, FL), and the cells counted. Lymphocyte distributions were determined with a Coulter Epics XL/MCL Flow Cytometer with System II software (Coulter), and CytoComp (Coulter) QC Windows (Flow Cytometry Systems, San Juan, Puerto Rico) used to ensure consistent instrument settings. Absolute lymphocyte numbers are calculated from the total lymphocytes. Data are expressed as total lymphocytes per cubic millimeter (mm3) or mean percentage (%) of positive cells.
Cytokine determination
Serum cytokines (interferon gamma [IFN-γ], interleukin-4 [IL-4], Interleukin-12 [IL-12], Tumor necrosis factor-alpha [TNF-α]) were determined by sandwich ELISA (Biosource; Camarillo, CA) according to the manufacturer’s protocol.
Results
Patient symptoms
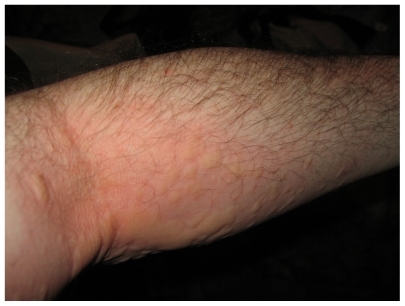
Patient symptoms began immediately after exposure to caterpillar larvae; the local effects included redness, swelling, pain, and severe itchiness with medium size urticaria (3–5 mm with flare) on his left forearm (), which spread to his upper chest and neck area. He complained about breathing difficulty with shortness of breath. The patient had a history of moderate asthmatic episodes (mild-intermittent) and atopy (environmental and food), with high serum IgE levels (208 IU/mL). He had no known drug allergies; his social history was unremarkable. Treatment was initiated immediately (doctor’s office) (within 10–15 min) with the antihistamine diphenhydramine HCl (75 mg po), and albuterol (mdi, 2 puffs, po). Patient’s symptoms resolved uneventfully, contact urticaria and itchiness resolved 48 hrs later while breathing difficulty resolved within 1–2 hrs. No persistent symptoms were observed over the next couple of days.
Taxonomic evaluation
The caterpillar was identified as the larvae of the sycamore tussock moth, Halysidota harrisii Walsh, 1864 ().
Serum immunoglobulins Total IgM, IgG, IgA, and IgE
Serum IgM, IgG, IgA were within normal range (see materials and methods), while serum IgE levels were high. Even though no differences were observed between any of the Igs, serum IgE levels demonstrated a slight bimodal elevation at 2 hrs and one week post reaction ().
Table 1 Immunoglobulin levels in serum of a patient with an allergic response to caterpillar exposure
Distributions of blood lymphocyte subpopulations
Distributions of blood lymphocytes (CD4+, CD8+, CD8+CD60+, CD19+, CD23+, CD16/56+, CD25, CD45RA+, CD45RO+) and monocytes (CD1d+) were studied in peripheral blood. Absolute numbers of CD4+ T cells, CD25+ cells, CD19+ B cells, and CD1d+ monocytes decreased (22, 27, 33, 20%, respectively) one week post reaction, while CD8+CD60+ T cells and CD23+ cells decreased 48 hrs (33, 74%, respectively) post reaction and CD45RA+ naïve T cells decreased (21%) 36 hours post reaction (, ). In contrast absolute numbers of CD16/56+ NK precursor cells increased (60%) 12 hrs, then decreased (65%) 48 hrs post reaction; other lymphocyte subsets were unaffected.
Table 2 Distributions of lymphocyte subpopulations in peripheral blood of a patient with an allergic response to caterpillar hair
Table 3 Distributions of lymphocyte subpopulations in peripheral blood of a patient with an allergic response to caterpillar exposure
Cytokines in serum
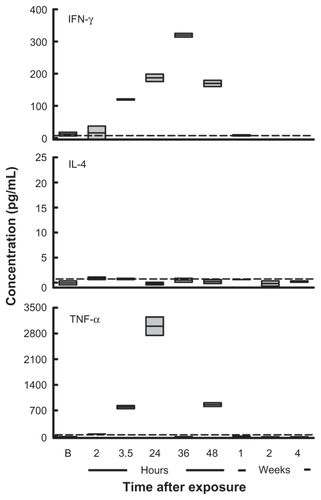
Levels of IFN-γ, IL-4, and TNF-α were not detected in serum pre-exposure (<1.0–4.0 pg/mL). However, high levels of IFN-γ (187–319 pg/mL) and TNF-α (549–749 pg/mL) were detected in serum 24–36 hrs and 3.5–24 hrs post reaction, respectively (). In contrast, levels of IL-4 were undetected (<1.0 pg/mL) in serum at all time points. All cytokines returned to baseline levels one year post reaction ().
Discussion
This is the first study of an allergic reaction resulting from caterpillar exposure that demonstrates that contact/exposure to the larvae of the Halysidota harrisii Walsh, 1864. The sycamore tussock moth (Halysidota harrisii) is a species of moth of the Arctiidae family, which is found in southeastern Canada and the eastern parts of the United States.Citation20 Depending on its location, the moth flies either from May to June or from July to August,Citation20 the latter which was reported in our study. Larvae are typically very hairy,Citation20 as observed (). Systemic allergic responses to contact reactions with the larvae of Halysidota harrissii have not been previously described.
Studies of Maier et al have reported dermatitis in children who came in contact with the urticating haris (setae) of oak processionary caterpillars, the larvae of Thaumetopoea processionea L., in a suburb of Vienna.Citation21 An increase in the number of cases of reported lepidopterism, an airborne disease caused by the setae of the oak processionary caterpillar, has caused public health concerns.Citation22 Studies of Jans and FranssenCitation23 described direct animal exposure to the urticating hairs of the oak processionary caterpillar due to ingestion and inhalation; symptoms included labial angioedema, ptyalism, sloughing, tongue swelling, stomatitis, conjunctivitis and respiratory distress.Citation23
Other caterpillars, including the pine processionary caterpillar, which is responsible for dermatitis, contact urticaria, and, rarely, respiratory and anaphylactic reactions through IgE-mediated or non-IgE-mediated mechanisms,Citation24 can also cause significant local reactions and airway swelling if ingested.Citation24 Accidents with the caterpillar Lonomia obliqua have been described, which are associated with a coagulation disorder and hemorrhagic syndrome in humans, caused by envenomation with this caterpillar.Citation25 Antivenin is available in the case of potentially fatal Lonomia envenomation.Citation19
In general, lepidopterism outbreaks are rare.Citation26 However, Redd et alCitation26 investigated a lepidopterism outbreak associated with caterpillars of the Douglas-fir tussock moth (Orgyia pseudotsugata) at a Boy Scout summer camp in New Mexico. Contact exposure included symptoms of itch, rash, or hives.Citation26 The authors concluded that avoiding areas of heavy infestation can reduce illness risk, as well as modifying behaviors associated with lepidopterism.Citation26 Allergic reactions to Lepidoptera can be treated with removal of offending hairs, followed by topical steroids, and oral antihistamines.Citation19
In the present study, our patient suffered a severe allergic reaction when he came in contact with the larva from the Halysidota harrisii Walsh. While we did not obtain serum tryptase levels in order to confirm anaphylaxis, the rapid development of contact urticaria and acute asthma are consistent with a systemic type 1 allergic response.
Our subject, an allergic asthmatic who had elevated serum IgE (>100 IU/mL), had normal immunoglobulin levels (IgG, IgM, IgA). However, his serum IgE levels slightly increased (15%), 2 hrs and then again one week post exposure. Numbers of blood CD16/56+ NK precursor cells increased (60%) 12 hrs post exposure, and then decreased (65%) 48 hrs post reaction. Further, the observed decreases in absolute numbers of T and B cell subsets at one week with yet earlier decreases (48 hours post reaction) occurring for CD8+CD60+ T cells and CD23+ cells may relate to a cellular interplay pertaining to IgE modulation. Earlier work in our laboratory has demonstrated the presence of CD8+CD60+ T cells in the placenta of twins with a family history of asthma and atopy.Citation27 Others have shown that CD23+ cells, which express the low affinity receptor for IgE (FcɛRII), can be activated by IgE sensitization and potentiate TNFα and MIP-1α cytokine expression.Citation28 The stoichiometric relationship between changes in immune cell subsets, IgE/antigen specific IgE and clinical severity after caterpillar exposure remains to be determined.
Serum IFN-γ levels were high, but IL-4 was not detected, postulating a non-IL-4-mediated allergic response. It is well known that IgE can survive without allergen contact for long periods bound to Fc receptors of mast cells or basophilic granulocytes.Citation29 It could be that our patient was presensitized to caterpillar antigen, and that exposure induced mast cell degranulation and induction of massive IFN-γ levels but total serum IL-4 and IgE levels remained basically low or unchanged, due to cross linking, not induction. The levels of TNF-α observed may result from mast cell degranulation, maybe in the early phase and late phase reactions but may also be in response to direct sensitization of IgE.Citation28 It could be that our patient demonstrated an allergic reaction to the larva, either by direct contact with hair or other proteinaceous components, which was induced by a non IgE-dependent mechanism that had a mast-cell dependent mechanism. However, the molecular mechanisms underlying the inflammatory effect of caterpillar antigen(s) remain to be elucidated.
As a case study of a single subject, this study has other limitations including the lack of confirmation of anaphylaxis as tryptase levels were not obtained, possible affects of antihistamine therapy on the immune response, and the lack of confirmation of IgE anti-larvae by classical methods (ELISA, Western blot). Finally, the lack of standardized caterpillar extract for skin testing and for the determination of specific IgE.Citation11 Nonetheless, since severe responses to caterpillar exposure are infrequent and vary based on species, continued awareness is critical to further the understanding of such responses.
In summary, our descriptive data provides detailed information on the immune response to the larvae of the sycamore tussock moth, Halysidota harrisii Walsh, 1864. Even though these exposures may be uncommon, it is important to recognize the potential clinical sequelae, including anaphylaxis, of contact with these larvae. Further, since the habitat of Halysidota harrisii Walsh, 1864 comprises eastern regions of the United States and southeastern Canada, the paucity of reports could be due to under-representation. Although our findings are based on one patient and extrapolation of the immunological findings to other patients or to other species of caterpillar would be premature, increased awareness of caterpillar exposure in atopic individuals may prove useful in identifying caterpillar sensitivities and clinical sequelae related to such exposure.
Acknowledgment
We express our appreciation to Dr Paul Robins, PhD, Dept of Entomology, Cornell University (Geneva, NY) for his contribution to the study.
Disclosure
The authors declare no competing financial interest.
References
- BalitCRGearyMJRussellRCIsbisterGKProspective study of definite caterpillar exposureToxicon200342665766214602121
- BurdmannEAAntunesISaldanhaLBAbdulkaderRCMRSevere acute renal failure induced by the venom of Lonomia caterpillarsClin Nephrol19964653373398953124
- ClelandJBPapulo-urticarial rashes caused by the hairlets of caterpillars of the moth (Euproctis edwardsi newm)Med J Aust19201169170
- ScholzARussellRGearyMInvestigation of caterpillar dermatitis in school childrenNSW Public Health Bull1993466566
- BalitCRGearyMJRussellRCIsbisterGKClinical effects of exposure to the white-stemmed gum moth (Chelepteryx collesi)Emerg Med Australas2004161748115239759
- EversonGWChapinJBNormannSACaterpillar envenomations: a prospective study of 112 casesVet Human Toxicol1990322114119
- Arocha-PinangoCLde BoschNBTorresASix new cases of a caterpillar-induced bleeding syndromeThromb Haemost19926744024071378651
- BalitCRPtolemyHCGearyMJRussellRCIsbisterGKOutbreak of caterpillar dermatitis caused by airborne hairs of the mistletoe browntail moth (Euproctis edwardsi)Med J Aust200117564164311837874
- IsbisterGKGrayMRA prospective study of 750 definite spider bites, with expert spider identificationQJM2002951172373112391384
- UtikalJBookenNPeitschWKKemmlerNGoebelerMGoerdtSCaterpillar dermatitis: an increasing dermatologic problem in warmer regions of GermanyHautarzt2009601485018654752
- BircherAJSystemic immediate allergic reactions to arthropod stings and bitesDermatology2005210211912715724094
- AllenVTMillerOF3rdTylerWBGypsy moth caterpillar dermatitis revisitedJ Am Acad Dermatol.1991246 Pt 19799811869687
- IsbiterGKWhelanPIEnvenomation by the billygoat plum stinging caterpillar (Thosea penthima)Med J Aust.200017311/1265465511379521
- PitettiRDKuspisDKrenzelokEPCaterpillar: an unusual source of ingestionPediatr Emerg Care1999151333610069310
- VegaJMMoneoIArmentiaAVegaJDe la FuenteRFernandezAPine processionaray caterpillar as a new cause of immunologic contact urticariaContact Dermatitis200043312913210985627
- MoneoIVegaJMCaballeroMLVegaJAldayEIsolation and characterization of Tha p 1, a major allergen from the pine processionary caterpillar Thaumetopoea pityocampaAllergy2003581343712580804
- VegaMLVegaJVegaJMMoneoISanchezEMirandaACutaneous reactions to pine processionary caterpillar (Thaumetopoea pityocampa) in pediatric populationPediatr Allergy Immunol200314648248614675477
- Fuentes AparicioVZapatero RemonLMartinez MoleroMIAlonso LebrerosEBeitia MazuecosJMBartolome ZavalaBAllergy to pine processionary caterpillar (Thaumetopoea pityocampa) in childrenAllergol Immunopathol (Madr)2006342596316606547
- HosslerEWCaterpillars and mothsDermatol Ther200922435336619580579
- WagnerDLCaterpillars of Eastern North America: A guide to identification and natural history (Princeton Field Guides)Princeton, NJPrinceton University Press2005443450
- MaierHSpeigelWKinaciyanTHonigsmannHCaterpillar dermatitis in two siblings due to the larvae of Thaumetopoea processoinea L., the oak processionary caterpillarDermatology20042081707314730242
- GottschlingSMeyerSAn epidemic airborne disease caused by the oak processionary caterpillarPediatr Dermatol2006231646616445416
- JansHWFranssenAEThe urticating hairs of the oak processionary caterpillar (Thaumetopoea processionea L.), a possible problem for animals?Tijdschr Diergeneeskd20081331042442918561703
- InalAAltintasDUGuvenmezHKYilmazMKendirliSGLife- threatening facial edema due to pine caterpillar mimicking an allergic eventAllergol Immunopathol (Madr)200634417117316854350
- VeigaABRibeiroJMGuimaraesJAFrancischettiIMA catalog for the transcripts from the venomous structures of the caterpillar Lonomia oliqua: identification of the proteins potentially involved in the coagulation disorder and hemorrhagic syndromeGene2005355112716023793
- ReddJTVoorheesRETorokTJOutbreak of lepidopterism at a Boy Scout campJ Am Acad Dermatol200756695295517368636
- Smith-NorowitzTANorowitzKSilverbergJCD8+ CD60+ T cells, cells expressing epsilon specific mRNA, and Th1/Th2 cytokines in cord blood and at 7 months of ageScand J Immunol20086852653318822110
- EzeamuzieCIAl-AttiyahRShihabPKAl-RadwanRLow-affinity IgE receptor (FcepsilonRII)-mediated activation of human monocytes by both monomeric IgE and IgE/anti-IgE immune complexInt Immunopharmacol200991110111419505590
- AsaiKKitauraJKawakamiYRegulation of mast cell survival by IgEImmunity20011479180011420048