Abstract
Until recently, autoimmune diseases had been categorized as either Th1- or Th2-mediated diseases. However, the discovery of a novel subset of helper T cells producing interleukin (IL)-17, ie, Th17 cells, changed this paradigm. Currently, IL-17 and Th17 cells are implicated in many autoimmune diseases, such as rheumatoid arthritis, psoriasis, multiple sclerosis, and inflammatory bowel diseases. Such conclusions were initially drawn from observations in animal models of autoimmune diseases, and accumulating data from clinical research also support the involvement of IL-17 in human diseases as well. Reagents targeting Th17-related molecules have been under clinical investigation for some diseases but have not always been effective in controlling disease activity. Consistent with this, it has become evident that there are substantial differences in the development of Th17 cells and in the way they function in autoimmune diseases between humans and experimental animals. Thus, further investigation is needed before we can draw any conclusions about the importance of IL-17 and Th17 cells in human autoimmune diseases.
Introduction
The introduction of biologicals for treatment of autoimmune disorders has dramatically changed the prognosis of these diseases. However, there are patients who are refractory to this treatment, and the frequency of patients who achieve drug-free remission is very low. This might indicate limitation of the current therapy targeting nonspecific inflammatory cytokines, such as tumor necrosis factor-alpha (TNFα). Therefore, development of a novel treatment strategy targeting molecules or cells that are closer to the etiology of autoimmune diseases is desired. Until recently, it was widely accepted that autoimmune diseases are categorized as Th1- or Th2-mediated diseases. The former includes Crohn’s disease (CD), psoriasis, rheumatoid arthritis (RA), and multiple sclerosis (MS), while the latter includes asthma, systemic lupus erythematosus (SLE), and ulcerative colitis (UC). However, the Th1/Th2 paradigm of autoimmune diseases included substantial discrepancies and was questioned by the discovery of a novel helper T cell subset, ie, Th17 cells, producing interleukin (IL)-17, firstly in mice, and a couple of years later in humans. Currently, many autoimmune diseases are believed to be Th17-mediated diseases, because the biologic functions of IL-17 are consistent with the chronic and destructive nature of inflammation. This review introduces accumulating evidence on the roles of IL-17 and Th17 cells in human autoimmune diseases.
Biology of IL-17
IL-17 (IL-17A) was discovered in 1993 originally as a rodent T cell cDNA transcript, cytotoxic T lymphocyte-associated antigen 8 (CTLA8).Citation1 Human IL-17 was subsequently identified. Citation2 To date, five additional members of the IL-17 family have been identified and termed IL-17B, C, D, E, and F. IL-17F is most closely related to IL-17, and can form a heterodimer with IL-17, while IL-17E, also named IL-25, is instead classified as a Th2 cytokine.Citation3 There are five receptors for the IL-17 family of cytokines, ie, IL-17RA, RB, RC, RD, and RE, of which IL-17RA and RC mediate the biologic activity of IL-17. While IL-17 is produced mainly by T cells, its receptor is expressed ubiquitously on various cell types, including myeloid cells, epithelial cells, and fibroblasts. Therefore, IL-17 exerts various biologic functions in vivo, which might be involved in the pathogenesis of a wide range of inflammatory disorders, as well as infectious conditions ().
Figure 1 Effects of IL-17 signaling on host defense, inflammation, and tissue destruction.
Abbreviations: BBB, blood-brain barrier; G-CSF, granulocyte colony stimulating factor; GM-CSF granulocyte macrophage colony stimulating factor; VE GF, vascular endothelial growth factor; MMPs, metalloproteinases; PGE2, prostaglandin E2; TNF-α, tumor necrosis factor-alpha.
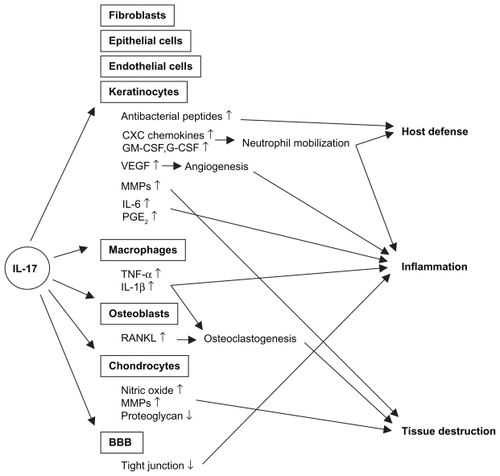
One of the well-defined functions of IL-17 is mobilization of neutrophils, which is mediated by the production of CXC chemokines, including IL-8 (CXCL8) and growth-regulated oncogene-alpha (GROα, CXCL1), and growth factors, including granulocyte colony-stimulating factor (G-CSF) and granulocyte macrophage colony-stimulating factor (GM-CSF), from epithelial cells and smooth muscle cells, as well as fibroblasts.Citation3,Citation4 In fact, murine models of infection showed the involvement of IL-17 in neutrophil-mediated host defense against extracellular bacteria and fungi, such as Klebsiella pneumoniae, Escherichia coli, and Candida albicans.Citation5 A role for IL-17 in mycobacterial infection, in which macrophage activation is important for host defense, was also reported. Furthermore, IL-17 participates in the elimination of pathogens by inducing antimicrobial peptides, such as β-defensins, especially in cooperation with IL-22.Citation6 Some studies suggest the role of IL-17 in human host defense mechanisms as well. Acosta-Rodriguez showed that human memory T cells specific for C. albicans produced IL-17.Citation7 Patients with hyper-IgE syndrome, who are defective in IL-17 production, are susceptible to bacterial and fungal infections.Citation8
IL-17 exerts various biologic activities, which potentially cause tissue destruction and degeneration during chronic inflammation. IL-17 stimulates macrophages to produce various inflammatory cytokines, such as IL-1β and TNFα.Citation9 Furthermore, IL-17 acts synergistically with TNFα in IL-6 and GM-CSF production from fibroblasts.Citation4 Of particular importance in the pathogenesis of RA is that IL-17 induces cartilage destruction via induction of metalloproteinases and inhibition of proteoglycan synthesis. IL-17 induces expression of RANKL on osteoblasts that mediates osteoclastogenesis, leading to bone destruction in RA.Citation10,Citation11 Regarding intestinal inflammation, IL-17 stimulated metalloproteinase, IL-6, and IL-8 production from cultured colonic subepithelial myofibroblasts.Citation12 IL-17 also upregulates production of GM-CSF, IL-6, and GRO-α from keratinocytes.Citation13 In the central nervous system, IL-17 was shown to disrupt the blood-brain barrier tight junction, which facilitated local migration of CD4 T cells.Citation14 All these findings suggest an involvement of IL-17 in the pathogenesis of various autoimmune diseases affecting a number of tissues.
Th17 cells
As is the case for most T cell-derived effector cytokines, an initial study demonstrated IL-17 production by CD45RO+ memory T cells.Citation4 Subsequently IL-17 was shown to be produced by Th1, Th0, and even Th2 cell clones.Citation13,Citation15 However, later studies in mice demonstrated by flow cytometric analysis of intracellular cytokine staining that IL-17-producing cells are characterized as a subset of helper T cells, which do not produce interferon gamma (IFNγ) or IL-4, the development of which was negatively regulated by IFNγ and IL-4, which are prototypic cytokines of Th1 and Th2 cells, respectively.Citation3 Thus, IL-17-producing CD4 T cells, which have been identified as a novel helper CD4 T cell subset distinct from Th1 and Th2 cells, were subsequently named Th17 cells. Identification of human Th17 cells was also reported a couple of years later.Citation7,Citation16
Since the discovery of Th17 cells, the molecular mechanisms for their differentiation have been extensively studied. Initial studies showed that IL-23, a member of the IL-12 family of cytokines that consists of the common IL-12/23p40 subunit and the unique IL-23p19 subunit, induced differentiation of mouse Th17 cells. However, it was later suggested that IL-23 is involved in the maintenance, expansion, and functional maturation of Th17 cells. On the other hand, it is now widely accepted that naive mouse CD4 T cells primed with antigenic stimulation in the presence of transforming growth factor beta (TGFβ) and IL-6 differentiate into Th17 cells. IL-21, which is produced by Th17 cells, can substitute for IL-6 in the differentiation of Th17 cells in an autocrine manner. Citation3 Resulting downstream signaling events that include activation of STAT3, induce the expression of RORγt and RORα, the master regulators of Th17 differentiation.Citation17 Human Th17 cells also express the human ortholog of murine RORγt, RORC2.Citation18 T cells in patients with mutated STAT3 are unable to differentiate into Th17 cells.Citation8 However, there seem to be substantial differences in the signal requirement for the differentiation of mouse and human Th17 cells. In mice, IL-23 does not directly induce the differentiation of Th17 cells. However, IL-23, in combination with IL-1β, was repeatedly reported to play central roles in the differentiation of human Th17 cells, while the requirement for TGFβ in human Th17 differentiation is still controversial.Citation3 Different sensitivity to TGFβ in the serum used for in vitro culture and different cellular sources used for the experiments are possible explanations for this discrepancy. Alternatively, TGFβ indirectly promotes Th17 development by inhibiting Th1 responses.Citation19 Interestingly, human Th17 cells were demonstrated to originate from CD161+ cells.Citation20 CD161 is the human homolog of NK1.1 in mice. However, neither mouse Th17 cells nor IL-17-producing invariant NKT cells express NK1.1.Citation21 Thus, there are substantial differences between human and mouse Th17 cells.
Expression of CCR6 is a common feature of mouse and human Th17 cells,Citation7,Citation16,Citation22 although it should be kept in mind that not all CCR6-positive cells are Th17 cells. Th17 cells migrate toward CCL20, the ligand for CCR6, but also secrete CCL20. Therefore, there might be a positive feedback loop of chronic accumulation of Th17 cells once inflammation is provoked. Th17 cells produce several kinds of inflammatory cytokines in addition to IL-17 and IL-17F, which include TNFα and IL-6, IL-21, IL-22, and IL-26. It is worth noting that not only IL-6 and TNFα, but also other cytokines, can be produced by other subsets of CD4 T cells. For example, IL-21 is more likely a product of follicular helper T cells, which can be derived from Th1 and Th2 cells as well.Citation23 In addition, not all IL-17-producing cells are positive for IL-21. Similarly, a large part of the IL-22-producing cell population does not belong to Th17 cells and is regarded as another independent subset of helper T cells, denoted as Th22 cells.Citation24 Nevertheless, it is important to note that IL-22 and IL-17 cooperatively enhance expression of antimicrobial peptides.Citation6 IL-22 is known to induce hyperplasia of keratinocytes involved in the pathogenesis of psoriasis (see below). IL-23 is also involved in IL-22 production. Thus, there are functional similarities between IL-17 and IL-22-producing T cells. As mentioned previously, IFNγ suppresses the differentiation of Th17 cells in vitro. However, presence of CD4 T cells producing both IL-17 and IFNγ has also been noted, especially in humans. Their origin and functions are not clarified yet, but phenotypic plasticity of Th17 to Th1 cells by IL-12-signaling has been demonstrated.Citation16
In addition to Th17 cells, other subset of T cells, including CD8 T, NK T, and TCRγδ T cells, have been demonstrated to produce IL-17 in mice. Even non-T cells, such as neutrophils and lymphoid tissue inducer-like cells, can also be an innate source of IL-17.Citation25 Among them, γδ T cells are the most well known as an important source of in vivo IL-17 production in some circumstances, not limited to infection conditions,Citation5 but even in autoimmune diseases.Citation26,Citation27 IL-17 production by γδ T cells as well as other populations of non-Th17 cells has also been reported in human, but its importance has yet to be determined.
Implication of IL-17 and Th17 cells in autoimmune diseases
Inflammatory bowel diseases
CD and UC are the two major inflammatory bowel diseases (IBDs). Although CD and UC share some features, there are clear differences in the areas of involvement as well as histology, which are at least in part explained by their different cytokine profiles. Thus, it has been widely accepted that CD and UC are Th1 and Th2 diseases, respectively. Citation28 Accordingly, animal models of IBD have also been subdivided into Th1 and Th2 types as the models of CD and UC, respectively. However, the discovery of Th17 cells have provided novel insight into the pathogenesis of IBD. It was revealed that IL-23 is essential for the development of T cell-dependent IBD models, in which IBD is induced by transferring naive CD4 T cells into RAG-deficient mice or developed spontaneously in IL-10-deficient mice.Citation29,Citation30 The latter is of particular interest, because it was recently reported that the CD-associated mutation of the NOD2 gene suppresses IL-10 transcription.Citation31 Neutralization of IL-23 exerted a therapeutic effect on the development of experimental colitis.Citation32 These findings suggest the involvement of Th17 cells in the development of IBD mouse models. However, a protective role of IL-17 was also reported in the naive CD4 T cell-transfer model of colitis,Citation33 although IL-17F might have opposite functions.Citation34 Thus, the disease-promoting effect of IL-23 might not necessarily mediate IL-17 or Th17 cells. Actually, it was observed that the lack of IL-23 also reduced Th1 responses, and that blocking IFNγ abrogated the development of the T cell transfer model of colitis.Citation35 Furthermore, IL-23 promoted even T cell-independent models of colitis.Citation29
Despite the controversies about the importance of the IL-23/IL-17 axis in IBD animal models, there have been reports showing overexpression of Th17-related molecules in human IBD lesions (see ). IL-17 mRNA was expressed in inflamed colonic tissue from CD or UC.Citation36 Expression of IL-17 in the intestine of CD and UC was also demonstrated by immunohistochemistry, in which highest levels of expression were detected in the active CD lesion.Citation37 Expression of IL-23p19 was increased in inflamed mucosa.Citation38,Citation39 There are studies addressing other Th17-related cytokines. IL-22 was shown to be overexpressed in inflamed mucosa and serum of CD patients.Citation40,Citation41 IL-17F mRNA was also expressed at a higher level especially in UC.Citation42 An involvement of IL-23- signaling in the pathogenesis of IBD was also suggested by its genetic association with IL23R polymorphisms.Citation43 Genetic associations of CD with Tyk2 and STAT3, both of which are involved in IL-23-signaling, were also shown in a Japanese population.Citation44
Table 1 Expression pattern of IL-17 and IL-23 transcripts in lesional tissue samples
Human Th17 cells were first reported using samples from IBD patients, and there was an increase of IL-17+ cells in the gut of CD patients.Citation16 Notably, many IL-17-producing T cells in the gut also produced IFNγ (about 40%). CD161+ Th17 cells are enriched in the lamina propria, even in healthy subjects, with a further increase in CD lesions.Citation45 The percentage of IL-17-producing cells in CD161 + CD4 T cells in the peripheral blood also increased in CD patients. Interestingly, it was reported earlier that CD4 T cells in UC were enriched with IL-13-producing cells, which expressed CD161.Citation46 Unfortunately, IL-17 production by CD161 + CD4 T cells was not expected at that time and therefore was not examined. Kobayashi et al demonstrated an increased frequency of Th17 cells in the lamina propria compared with peripheral blood.Citation47 Lamina propria CD4 T cells in UC produced more IL-17 than those in CD, while IFNγ production was higher in CD than in UC. The addition of IL-23 augmented both IL-17 and IFNγ production of LP CD4 T cells in vitro. It was also reported that the percentage of IL-17- and IFNγ-producing cells was higher in T cells in IBD lesions compared with control samples, but there was no difference between CD and UC.Citation48 The number of macrophages secreting IL-23, TNFα, and IL-6 increased in CD, and they affected IFNγ, but not IL-17, production of lamina propria mononuclear cells, suggesting the presence of an IL-23/IFNγ axis in the pathogenesis of CD.Citation49 Therefore, although the relative importance of Th17 cells in human CD and UC is still unclear, IL-23 likely plays a critical role in the pathogenesis, which is now proved by the results of clinical trials described later in this paper.
Psoriasis
Psoriasis is a chronic inflammatory disease of the skin characterized by epidermal hyperplasia, dermal angiogenesis, and inflammatory cell infiltrates. Psoriasis had been generally regarded as a Th1 disease, but recent data suggest the involvement of Th17 responses. Expression of IL-17 mRNA was detected in biopsies from lesional psoriatic skin.Citation50–Citation53 Serum levels of IL-17 correlated with disease severity.Citation54 Th17 cells were detected in inflamed skin,Citation51,Citation53 although an increase of Th1 cells was also observed.Citation55 The accumulation of Th17 cells in psoriatic skin could be anticipated, because it was reported even before the discovery of Th17 that expression of CCL20 was upregulated in psoriatic skin, and skin-homing T cells express its receptor, CCR6.Citation56 Keratinocytes, dermal fibroblasts, microvascular endothelial cells, and dendritic cells produce CCL20. As mentioned above, CCL20 is also produced by Th17 cells, which might account for the chronicity of the disease.
Upregulation of IL-23 in psoriatic lesions was also repeatedly reported. Increased expression of IL-23p19 and IL-12/ IL-23p40 was noted in the lesional skin of psoriasis patients.Citation52,Citation57,Citation58 Similar to IBD, association of the disease with the IL23R gene polymorphism was demonstrated.Citation59,Citation60 The importance of IL-23 in the pathogenesis of psoriasis is also supported experimentally. Intradermal injection of IL-23 in mice induced psoriasis-like lesions with epidermal hyperplasia and inflammatory cellular infiltrate.Citation52 Notably, TNFα, but not IL-17, was required for the response. It was later revealed that IL-22 mediates the IL-23-induced dermal pathology.Citation61 Ma et al also demonstrated the requirement of IL-22 in another psoriasis model, which was induced by transferring naive CD4 T cells into scid/scid mice.Citation62 The IL-22 receptor was not expressed on immune cells but rather on epithelial keratinocytes, in which IL-22 induced expression of antimicrobial peptides.Citation63 IL-22 also regulates migration, proliferation, and differentiation of keratinocytes, suggesting roles of IL-22 in wound healing as well as skin inflammation.Citation64 Furthermore, injecting IL-22 directly into the skin induced expression of antimicrobial peptide, and inflammatory cytokines and keratinocyte hyperplasia.Citation62 In agreement with these findings, serum IL-22 levels increased in psoriasis patients and were correlated with disease severity.Citation65 Local IL-22 expression increased in psoriatic lesions and was associated with increased expression of metalloproteinases and antimicrobial peptides.Citation53,Citation63,Citation65 Although Th17 cells can produce IL-22,Citation6,Citation61 IL-22 production by another independent subset of helper T cells in psoriatic lesions was recently reported by several groups.Citation24,Citation66,Citation67 These IL-22-producing cells produce neither IL-17 nor IFNγ, but share similarities with Th17 cells in the expression pattern of chemokine receptors. However, their detailed characteristics and functions are yet to be analyzed. These findings suggest the importance of IL-23 and IL-22, possibly more than IL-17, in the pathogenesis of psoriasis. It is also suggested that IL-17 and IL-22 have a distinct function in the pathogenesis of psoriasis, ie, IL-22 regulates keratinocyte differentiation, while IL-17 is more related to inflammation.Citation68
Rheumatoid arthritis
RA is characterized by chronic inflammation of systemic synovial tissue, which leads to joint destruction. The well known disease association with major histocompatibility complex Class II genes and infiltration of CD4 T cells in synovial tissue implies that activation of autoreactive CD4 T cells drives chronic inflammation. The requirement of CD4 T cells for the development of arthritis in animal models also supports the idea. Massive infiltration of activated macrophages in RA synovium suggests the involvement of Th1 cells, which are well known to activate macrophages in the host defense against intracellular pathogens. Expression of Th1 cytokines in RA joints was also demonstrated.Citation69 However, there has also been some data conflicting with the Th1 hypothesis, ie, mice blocking IFNγ-signaling were more susceptible to arthritis.Citation70 Nevertheless, disease resistance of IL-12-deficient mice strongly argues in favor of the importance of Th1. However, it was later revealed that IL-23, which shares the p40 subunit with IL-12, but not IL-12, was required for the development of collagen-induced arthritis in an animal model of RA.Citation71 Preserved IFNγ and defective IL-17 production in IL-23p19-deficient mice suggested the importance of IL-17 in the development of the disease. This was supported by findings that mice lacking IL-17 and mice in which IL-17 was blocked were less susceptible to collagen-induced arthritis.Citation72–Citation74 Thereafter, the importance of IL-17 was demonstrated in many, if not all, animal models of arthritis, including spontaneous arthritis in IL-1Rα-deficient mice and the SKG strain of mice.Citation75,Citation76 These results suggest that Th17 cells are the pathogenic CD4 T cells driving chronic inflammation in human RA.
There have been numerous studies examining IL-17 production in human RA. The first report by Chabaud et al detected bioactive IL-17 in the culture supernatants of synovial cells.Citation77 Immunohistochemical analysis demonstrated IL-17-positive cells in RA synovium, which accounted for about 1% of T cells. Consistent with this, a recent study demonstrated that the majority of IL-17-producing cells in RA synovium were mast cells, while there were relatively few IL-17-producing T cells.Citation78 An earlier study, in which the concentration of IL-17 was measured by using polyclonal antibodies against IL-17, showed very high levels of IL-17 in RA synovial fluid (SF) and serum.Citation79 Although more recent studies also showed increased IL-17 production in RA SF compared with osteoarthritis SF,Citation11,Citation22,Citation80,Citation81 the levels were not as high as in the earlier report. Furthermore, a considerable portion of RA SF did not contain a detectable amount of IL-17. The concentration of IL-17 was even comparable between RA and osteoarthritis SF, but was increased in reactive arthritis and juvenile idiopathic arthritis.Citation82,Citation83 Raza et al reported that IL-17 production increased in the SF of early but not established RA.Citation84 There are also several reports examining the expression of IL-17 transcripts in RA synovial membrane. Kirkham et al reported that increased levels of IL-17 mRNA expression predicted progression of joint damage,Citation85 which fits well with the known biologic activity of IL-17. However, IL-17 mRNA was only detected in 15 of 54 RA patients, whereas IFNγ mRNA was detected in 48 of 56 patients. Others also detected IL-17 mRNA in only about half of RA samples.Citation86,Citation87 No difference was found in the expression level of IL-17 mRNA between RA and osteoarthritis by qualitative real-time polymerase chain reaction. Citation88 These findings are supported by studies examining the prevalence of Th17 cells by flow cytometry. The frequency of Th17 cells found in the peripheral blood of RA patients did not differ from controls,Citation89,Citation90 but was significantly increased in patients with psoriatic arthritis. Furthermore, Th1 cells predominated over Th17 cells in the joints,Citation26,Citation89 although the percentage of Th17 in CD4 T cells can be higher in joints than in blood, possibly due to the enrichment of CD45RO+ memory CD4 T cells. This is supported by earlier reports showing no increase of CCR6 expression in memory CD 4T cells in RA joints.Citation91 In contrast with these findings, a recent report showed an increased proportion of Th17 cells in the peripheral blood of disease-modifying antirheumatic-drug naive RA patients.Citation92 Together with the report by Raza et al this suggests the importance of Th17 at an early stage of the disease.Citation84 A significant decrease of serum IL-17 after the onset of RA was also recently reported.Citation93
Although IL-23 is even more important than IL-17 for the development of animal models of arthritis, expression of IL-23 is not easily detected in RA. It was reported that the level of IL-23 protein in RA and osteoarthritis SF was comparably low, mainly due to defective IL-12/23p40 expression.Citation94 Stamp et al detected a low level of expression of IL-23p19 in RA synovial membrane, which was higher in the samples with IL-17 expression (13 of 25).Citation86 Therefore, unlike the case of CD or psoriatic lesions, IL-23 and IL-17 might not always be upregulated in RA synovium. In addition, it was reported that CD and psoriasis-associated variants of the IL23R gene are not associated with RA.Citation95,Citation96 Of particular interest is the observation made by Hillyer et al that the addition of anti- IL-23 monoclonal antibody (mAb) to the synovial cell culture only partly reduced IL-1β, TNFα, and IL-6 production.Citation87 Furthermore, the effect of anti-IL-17 mAb was even weaker than anti-IL-23 mAb. These results were clearly different from what was shown two decades ago, ie, blocking TNFα in a similar culture system completely suppressed inflammatory cytokine production.Citation97 Therefore, the importance of IL-17 in the pathogenesis of human RA could differ from that in animal models and remains an open question.
Multiple sclerosis
MS is an inflammatory demyelinating disease of the central nervous system. Experimental autoimmune encephalomyelitis (EAE) is an animal model of multiple sclerosis (MS) and is a prototype of T cell-mediated autoimmune disease. Thus, EAE is induced by either active immunization with myelin antigen or by adoptive transfer of myelin antigen-primed T cells, which enables us to address T cell functions easily. In fact, the importance of IL-23 in autoimmune inflammation was first demonstrated in this model,Citation98 which led to the discovery of Th17 cells. However, the importance of the Th1 response in the pathogenesis of EAE was reevaluated more recently. While adoptive transfer of myelin-specific Th17 cells induced EAE characterized by neutrophil infiltration, Th1 cells transferred EAE with a macrophage-rich infiltrate.Citation99 Expression of T-bet, irrespective of Th1 or Th17, was important for encephalitogenicity.Citation100 Furthermore, it was shown that IL-17, IL-17F, and IL-22 were dispensable for the development of EAE,Citation101,Citation102 although there are also conflicting results.Citation34 Thus, both Th1 and Th17 cells can be pathogenic in EAE.
Relatively few data are available on the involvement of IL-17 in the pathogenesis of human MS compared with mouse EAE or other human diseases, likely due to the difficulty in obtaining lesion samples. An increased frequency of mononuclear cells expressing IL-17 mRNA in the blood and spinal fluid of MS patients was reported.Citation103 Transcriptional profiling of the genes expressed in MS lesions also demonstrated upregulation of IL-6 and IL-17.Citation104 Ishizu et al reported increased levels of IL-17 and IL-8 in the spinal fluid of patients with the opticospinal form of MS, in which neutrophil infiltration is more prominent than in conventional MS.Citation105 The frequency of Th17 cells but not Th1 cells in the peripheral blood was significantly increased in active MS patients.Citation106 In another report, the frequency of Th17 cells in the spinal fluid, but not in peripheral blood, was increased during relapse.Citation107 Histologic analysis also showed expression of IL-17 in active lesions.Citation108 As for IL-23, peripheral blood monocyte-derived dendritic cells from MS patients had increased expression of IL-23p19.Citation109 IL23p19 as well as IL-12/23p40 was also detected in active MS lesions.Citation110 Although these results suggest the involvement of Th17 cells in the pathogenesis of human MS, there have also been reports showing the predominance of Th1 cells in myelin- specific T cells.Citation111,Citation112 The genetic association of MS with IL23R polymorphism is controversial.Citation113–Citation116 In addition, it was observed that exacerbations of MS in patients treated with altered peptide ligand were associated with an increased Th1 response.Citation117 MS was also exacerbated in patients treated with IFNγ.Citation118 Thus, similar to the case of mouse EAE, Th17 cells may not be the only pathogenic CD4 T cells in human MS.
Current therapies for autoimmune diseases and effect on IL-17 or Th17 cells
The treatment strategy and prognosis of autoimmune disease have greatly changed since the introduction of biologic agents, firstly anti-TNFα mAb for RA. Thereafter, anti-IL-6 and anti-CD20 mAbs and soluble CTLA4-Ig have been demonstrated to be highly effective in RA and are now available worldwide.Citation119 TNFα blockers have also been used in the treatment of CD and psoriasis.Citation120,Citation121 However, biological-based therapy is sometimes unsuccessful, even when a beneficial effect is expected. For example, blocking TNFα exerted deleterious effects on MS,Citation122 although local TNFα production was shown to be increased in active MS,Citation123 and TNFα has various biologic activities that can explain the pathogenesis of MS.Citation124 In addition, blocking TNFα was effective in the animal model of MS and EAE,Citation125 and TNFα-deficient mice were less susceptible to EAE.Citation126 These findings remind us of an important lesson, ie, the pathogenesis of animal models and real human diseases are not necessarily identical.
There have been studies addressing the effects of current therapies on Th17 responses. Kageyama et al observed a significant decline in the level of IL-23 in the serum of RA patients after treatment with anti-TNFα mAb, which was significantly correlated with improved disease activity, although the level of IL-17 was unaffected.Citation127 Yue et al reported, however, that the percentage of Th17 cells in peripheral blood tended to decrease after treatment with another antibody against TNFα.Citation128 It was also shown in psoriasis patients that TNFα inhibition reduced local expression of Th17-related molecules, including IL-23, IL-22, IL-17, CCL20, and β-defensin 4.Citation129 These results suggest a downregulatory role of TNFα blockers on Th17 responses in vivo, either directly or indirectly as the result of reduced inflammation. A mAb against IL-6 receptor also effectively controls disease activity in RA.Citation130 Because IL-6 was shown to be involved in the development of Th17 cells, an animal study anticipated that Th17 cells are one of the targets of therapy.Citation131 On the other hand, in vitro analysis of human T cells showed that IL-6 plays a relatively minor role in the development of Th17 cells.Citation3 In this regard, it is of interest to see the effect of blocking IL-1β, because it plays a critical role in the differentiation of human Th17 cells. However, IL-1β blockade exerted only a modest effect in RA.Citation132 In any case, the effects of IL-1β- or IL-6-targeted therapy on Th17 cells have not been reported so far. As for MS, IFNβ is widely used for its treatment, and some recent studies suggest the involvement of downregulation of Th17 responses. It was reported that Type I IFN receptor-deficient mice develop severe EAE with enhanced IL-17 production,Citation133 although conflicting data were also demonstrated. Citation134 In vitro experiments showed that IFNβ inhibits human Th17 differentiation directly or indirectly via dendritic cells.Citation135 However, Drulovic et al reported that while IFNβ treatment reduced the level of IFNγ and T-bet, it did not affect the level of IL-17 and RORc expression,Citation136 again leaving a question about the importance of Th17 cells in MS.
Clinical trials of reagents targeting Th17-related molecules in autoimmune diseases
A clinical trial is the only way to prove the importance of a given target, because it sometimes has results which are unexpected from in vitro analysis or animal studies. It was revealed that administration of an anti-IL-12/23p40 mAb (ABT-874) showed a therapeutic effect in patients with CD, with no increase of adverse events.Citation137 Treatment with ABT- 874 decreased IFNγ, IL-12, IL-6, and TNFα secretion by mononuclear cells in the lamina propria. Impaired IL-17 production of lamina propria T cells was also reported later.Citation138 A randomized trial of ustekinumab (CNTO 1275), another antihuman IL-12/23p40 mAb, in 104 patients with moderate to severe CD also showed a significant effect, even in patients who had previously been given antiTNFα mAb.Citation139 In addition to these antibody-based drugs, a small oral dose of an IL-12/23 inhibitor, apilimod mesylate (STA 5326), was tested for CD.Citation140 The action of apilimod includes selective inhibition of c-Rel translocation, which results in the suppressed transcription of IL-12/23p40 and IL-12p35. The results of a clinical trial showed that apilimod was well tolerated and clearly effective in moderate to severe CD. Blocking IL-12/23p40 is also highly effective for the treatment of psoriasis.Citation141,Citation142 For instance, a randomized, double-blind study revealed that ustekinumab was so effective that 66% of patients receiving 45 mg of ustekinumab but only 3% of patients receiving placebo achieved a 75% improvement of the psoriasis area and severity index.Citation143 Furthermore, it was recently reported that ustekinumab was even more effective than the soluble TNFα receptor, etanercept, for the treatment of psoriasis.Citation144 These results indicate that IL-12/23p40 plays a critical role in the pathogenesis of CD and psoriasis. However, as these reagents do not only affect Th17 responses, possible involvement of IFNγ or Th1 responses in the pathogenesis is not excluded. It is also possible that IL-12 or IL-23 participate in the disease pathogenesis in a T cell-independent manner.
In contrast with the success in CD and psoriasis, a clinical trial of ustekinumab had disappointing results in MS. Up to 180 mg of ustekinumab was administered weekly until three weeks, and every four weeks thereafter. The number of new lesions was evaluated at week 23, but there was no reduction of lesions in the treatment group. Furthermore, there was no difference in the number of clinical or objective relapses.Citation145 One may argue that the treatment should be applied at an early stage of the disease. However, administration of anti- IL-12/23p40 mAb as well as anti-IL-23p19 mAb was effective in EAE even after onset.Citation146 Furthermore, the apparent clinical effect of blocking IL-12/23p40 in psoriasis and CD, irrespective of the duration of the disease, also argues against the idea. Therefore, it is more reasonable to assume that the pathology of MS, especially in the context of the cytokine network, is largely different from CD or psoriasis. This also reminds us of the different effect of TNFα blockade between MS and CD or psoriasis. As for RA, there has been no report of the therapeutic effect of targeting IL-12/23p40. However, the results of a Phase I trial of anti-IL-17 mAb (LY2439821) in the treatment of RA were recently published.Citation147 Although a certain degree of improvement of disease activity has been shown, the effect does not seem to be as strong as for TNFα blockers. However, it might be too early to draw conclusions, because of the small subject number and relatively high improvement rates in the placebo group. There is another anti-IL-17 mAb (AIN457) being tested for RA, but detailed results of the trial have not been published so far.Citation119 In view of the biologic functions of IL-17, blocking IL-17 may exert a clearer beneficial effect in suppression of joint destruction during long-term follow-up. In this regard, future combination therapy including a TNFα blocker is of particular interest. To date, there has been no report of the effects of blocking IL-17 or other Th17-related cytokines in other human autoimmune diseases.
Conclusions
The discovery of IL-17 has led to novel insights into the pathogenesis of several autoimmune diseases, and the importance of IL-17 has been demonstrated in various animal models. However, it has become clear that there is a large variation in the therapeutic effect of targeting IL-17-related molecules in human autoimmune diseases. Further clinical trials might clarify the whole picture. At the same time, these findings also provoke the need for a better understanding of the differences between human and mouse immune systems, and for the development of better disease models, in order to avoid unnecessary clinical trials with their inevitable risk of adverse events.
Disclosure
The author reports no conflict of interest in this work.
References
- RouvierELucianiMFMatteiMGDenizotFGolsteinPCTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri geneJ Immunol199315012544554568390535
- YaoZPainterSLFanslowWCHuman IL-17: A novel cytokine derived from T cellsJ Immunol199515512548354867499828
- de JongESuddasonTLordGMTranslational mini-review series on Th17 cells: Development of mouse and human T helper 17 cellsClin Exp Immunol2009159214815819912248
- FossiezFDjossouOChomaratPT cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokinesJ Exp Med19961836259326038676080
- MatsuzakiGUmemuraMInterleukin-17 as an effector molecule of innate and acquired immunity against infectionsMicrobiol Immunol200751121139114718094532
- LiangSCTanXYLuxenbergDPInterleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptidesJ Exp Med2006203102271227916982811
- Acosta-RodriguezEVRivinoLGeginatJSurface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cellsNat Immunol20078663964617486092
- PaulsonMLFreemanAFHollandSMHyper IgE syndrome: An update on clinical aspects and the role of signal transducer and activator of transcription 3Curr Opin Allergy Clin Immunol20088652753318978467
- JovanovicDVDi BattistaJAMartel-PelletierJIL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophagesJ Immunol19981607351335219531313
- JovanovicDVMartel-PelletierJDi BattistaJAStimulation of 92-kd gelatinase (matrix metalloproteinase 9) production by interleukin- 17 in human monocyte/macrophages: A possible role in rheumatoid arthritisArthritis Rheum20004351134114410817568
- KotakeSUdagawaNTakahashiNIL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesisJ Clin Invest199910391345135210225978
- YagiYAndohAInatomiOTsujikawaTFujiyamaYInflammatory responses induced by interleukin-17 family members in human colonic subepithelial myofibroblastsJ Gastroenterol200742974675317876544
- AlbanesiCScarponiCCavaniAInterleukin-17 is produced by both Th1 and Th2 lymphocytes, and modulates interferon-gamma-and interleukin-4-induced activation of human keratinocytesJ Invest Dermatol20001151818710886512
- KebirHKreymborgKIferganIHuman TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammationNat Med200713101173117517828272
- AarvakTChabaudMMiossecPNatvigJBIL-17 is produced by some proinflammatory Th1/Th0 cells but not by Th2 cellsJ Immunol19991623124612519973376
- AnnunziatoFCosmiLSantarlasciVPhenotypic and functional features of human Th17 cellsJ Exp Med200720481849186117635957
- YangXOPappuBPNurievaRT helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gammaImmunity2008281293918164222
- Acosta-RodriguezEVNapolitaniGLanzavecchiaASallustoFInterleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cellsNat Immunol20078994294917676045
- SantarlasciVMaggiLCaponeMTGF-beta indirectly favors the development of human Th17 cells by inhibiting Th1 cellsEur J Immunol200939120721519130583
- CosmiLDe PalmaRSantarlasciVHuman interleukin 17-producing cells originate from a CD161+CD4+ T cell precursorJ Exp Med200820581903191618663128
- MichelMLKellerACPagetCIdentification of an IL-17- producing NK1.1(neg) iNKT cell population involved in airway neutrophiliaJ Exp Med20072045995100117470641
- HirotaKYoshitomiHHashimotoMPreferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal modelJ Exp Med200720412803281218025126
- NurievaRIChungYHwangDGeneration of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineagesImmunity200829113814918599325
- EyerichSEyerichKPenninoDTh22 cells represent a distinct human T cell subset involved in epidermal immunity and remodelingJ Clin Invest2009119123573358519920355
- TakatoriHKannoYWatfordWTLymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22J Exp Med20092061354119114665
- ItoYUsuiTKobayashiSGamma/delta T cells are the predominant source of interleukin-17 in affected joints in collagen-induced arthritis, but not in rheumatoid arthritisArthritis Rheum20096082294230319644886
- LeesJRIwakuraYRussellJHHost T cells are the main producers of IL-17 within the central nervous system during initiation of experimental autoimmune encephalomyelitis induced by adoptive transfer of Th1 cell linesJ Immunol2008180128066807218523270
- BoumaGStroberWThe immunological and genetic basis of inflammatory bowel diseaseNat Rev Immunol20033752153312876555
- HueSAhernPBuonocoreSInterleukin-23 drives innate and T cell-mediated intestinal inflammationJ Exp Med2006203112473248317030949
- YenDCheungJScheerensHIL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6J Clin Invest200611651310131616670770
- NoguchiEHommaYKangXNeteaMGMaXACrohn’s disease-associated NOD2 mutation suppresses transcription of human IL10 by inhibiting activity of the nuclear ribonucleoprotein hnRNP-A1Nat Immunol200910547147919349988
- ElsonCOCongYWeaverCTMonoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in miceGastroenterology200713272359237017570211
- O’ConnorWJrKamanakaMBoothCJA protective function for interleukin 17A in T cell-mediated intestinal inflammationNat Immunol200910660360919448631
- YangXOChangSHParkHRegulation of inflammatory responses by IL-17FJ Exp Med200820551063107518411338
- PowrieFLeachMWMauzeSMenonSCaddleLBCoffmanRLInhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cellsImmunity1994175535627600284
- NielsenOHKirmanIRudigerNHendelJVainerBUpregulation of interleukin-12 and -17 in active inflammatory bowel diseaseScand J Gastroenterol200338218018512678335
- FujinoSAndohABambaSIncreased expression of interleukin 17 in inflammatory bowel diseaseGut2003521657012477762
- SchmidtCGieseTLudwigBExpression of interleukin-12- related cytokine transcripts in inflammatory bowel disease: Elevated interleukin-23p19 and interleukin-27p28 in Crohn’s disease but not in ulcerative colitisInflamm Bowel Dis2005111162315674109
- HolttaVKlemettiPSipponenTIL-23/IL-17 immunity as a hallmark of Crohn’s diseaseInflamm Bowel Dis20081491175118418512248
- AndohAZhangZInatomiOInterleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblastsGastroenterology2005129396998416143135
- WolkKWitteEHoffmannUIL-22 induces lipopolysaccharide-binding protein in hepatocytes: A potential systemic role of IL-22 in Crohn’s diseaseJ Immunol200717895973598117442982
- SeidererJElbenIDiegelmannJRole of the novel Th17 cytokine IL-17F in inflammatory bowel disease (IBD): Upregulated colonic IL-17F expression in active Crohn‘s disease and analysis of the IL17F p.His161Arg polymorphism in IBDInflamm Bowel Dis200814443744518088064
- DuerrRHTaylorKDBrantSRA genome-wide association study identifies IL23R as an inflammatory bowel disease geneScience200631458041461146317068223
- SatoKShiotaMFukudaSStrong evidence of a combination polymorphism of the tyrosine kinase 2 gene and the signal transducer and activator of transcription 3 gene as a DNA-based biomarker for susceptibility to Crohn’s disease in the Japanese populationJ Clin Immunol200929681582519653082
- KleinschekMABonifaceKSadekovaSCirculating and gut- resident human Th17 cells express CD161 and promote intestinal inflammationJ Exp Med2009206352553419273624
- FussIJHellerFBoirivantMNonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitisJ Clin Invest2004113101490149715146247
- KobayashiTOkamotoSHisamatsuTIL23 differentially regulates the Th1/Th17 balance in ulcerative colitis and Crohn’s diseaseGut200857121682168918653729
- RovedattiLKudoTBiancheriPDifferential regulation of interleukin 17 and interferon gamma production in inflammatory bowel diseaseGut200958121629163619740775
- KamadaNHisamatsuTOkamotoSUnique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/ IFN-gamma axisJ Clin Invest200811862269228018497880
- TeunissenMBKoomenCWde Waal MalefytRWierengaEABosJDInterleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytesJ Invest Dermatol199811146456499764847
- LowesMAKikuchiTFuentes-DuculanJPsoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cellsJ Invest Dermatol200812851207121118200064
- ChanJRBlumenscheinWMurphyEIL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesisJ Exp Med2006203122577258717074928
- WilsonNJBonifaceKChanJRDevelopment, cytokine profile and function of human interleukin 17-producing helper T cellsNat Immunol20078995095717676044
- AricanOAralMSasmazSCiragilPSerum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severityMediators Inflamm20052005527327916258194
- KryczekIBruceATGudjonssonJEInduction of IL-17+ T cell trafficking and development by IFN-gamma: Mechanism and pathological relevance in psoriasisJ Immunol200818174733474118802076
- HomeyBDieu-NosjeanMCWiesenbornAUp-regulation of macrophage inflammatory protein-3 alpha/CCL20 and CC chemokine receptor 6 in psoriasisJ Immunol2000164126621663210843722
- LeeETrepicchioWLOestreicherJLIncreased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgarisJ Exp Med2004199112513014707118
- PiskinGSylva-SteenlandRMBosJDTeunissenMBIn vitro and in situ expression of IL-23 by keratinocytes in healthy skin and psoriasis lesions: Enhanced expression in psoriatic skinJ Immunol200617631908191516424222
- CaponFDi MeglioPSzaubJSequence variants in the genes for the interleukin-23 receptor (IL23R) and its ligand (IL12B) confer protection against psoriasisHum Genet2007122220120617587057
- CargillMSchrodiSJChangMA large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genesAm J Hum Genet200780227329017236132
- ZhengYDanilenkoDMValdezPInterleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosisNature2007445712864865117187052
- MaHLLiangSLiJIL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammationJ Clin Invest2008118259760718202747
- WolkKKunzSWitteEFriedrichMAsadullahKSabatRIL-22 increases the innate immunity of tissuesImmunity200421224125415308104
- BonifaceKBernardFXGarciaMGurneyALLecronJCMorelFIL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytesJ Immunol200517463695370215749908
- WolkKWitteEWallaceEIL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: A potential role in psoriasisEur J Immunol20063651309132316619290
- DuhenTGeigerRJarrossayDLanzavecchiaASallustoFProduction of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cellsNat Immunol200910885786319578369
- TrifariSKaplanCDTranEHCrellinNKSpitsHIdentification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cellsNat Immunol200910886487119578368
- NogralesKEZabaLCGuttman-YasskyETh17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathwaysBr J Dermatol200815951092110218684158
- SteinerGTohidast-AkradMWitzmannGCytokine production by synovial T cells in rheumatoid arthritisRheumatology (Oxford)1999383202213
- TesmerLALundySKSarkarSFoxDATh17 cells in human diseaseImmunol Rev20082238711318613831
- MurphyCALangrishCLChenYDivergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammationJ Exp Med2003198121951195714662908
- NakaeSNambuASudoKIwakuraYSuppression of immune induction of collagen-induced arthritis in IL-17-deficient miceJ Immunol2003171116173617714634133
- LubbertsEKoendersMIOppers-WalgreenBTreatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosionArthritis Rheum200450265065914872510
- BushKAFarmerKMWalkerJSKirkhamBWReduction of joint inflammation and bone erosion in rat adjuvant arthritis by treatment with interleukin-17 receptor IgG1 Fc fusion proteinArthritis Rheum200246380280511920418
- NakaeSSaijoSHoraiRSudoKMoriSIwakuraYIL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonistProc Natl Acad Sci U S A2003100105986599012721360
- HirotaKHashimotoMYoshitomiHT cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritisJ Exp Med20072041414717227914
- ChabaudMDurandJMBuchsNHuman interleukin-17: A T cell-derived proinflammatory cytokine produced by the rheumatoid synoviumArthritis Rheum199942596397010323452
- HueberAJAsquithDLMillerAMCutting edge: Mast cells express IL-17A in rheumatoid arthritis synoviumJ Immunol201018473336334020200272
- ZiolkowskaMKocALuszczykiewiczGHigh levels of IL-17 in rheumatoid arthritis patients: IL-15 triggers in vitro IL-17 production via cyclosporin A-sensitive mechanismJ Immunol200016452832283810679127
- HusseinMRFathiNAEl-DinAMAlterations of the CD4(+), CD8(+) T cell subsets, interleukins-1beta, IL-10, IL-17, tumor necrosis factor-alpha and soluble intercellular adhesion molecule-1 in rheumatoid arthritis and osteoarthritis: Preliminary observationsPathol Oncol Res200814332132818392953
- MoranEMMullanRMcCormickJHuman rheumatoid arthritis tissue production of IL-17A drives matrix and cartilage degradation: Synergy with tumour necrosis factor-alpha, Oncostatin M and response to biologic therapiesArthritis Res Ther.2009114R11319627579
- SinghRAggarwalAMisraRTh1/Th17 cytokine profiles in patients with reactive arthritis/undifferentiated spondyloarthropathyJ Rheumatol200734112285229017937463
- AgarwalSMisraRAggarwalAInterleukin 17 levels are increased in juvenile idiopathic arthritis synovial fluid and induce synovial fibroblasts to produce proinflammatory cytokines and matrix metal-loproteinasesJ Rheumatol200835351551918203309
- RazaKFalcianiFCurnowSJEarly rheumatoid arthritis is characterized by a distinct and transient synovial fluid cytokine profile of T cell and stromal cell originArthritis Res Ther200574R78478515987480
- KirkhamBWLassereMNEdmondsJPSynovial membrane cytokine expression is predictive of joint damage progression in rheumatoid arthritis: A two-year prospective study (the DAMAGE study cohort)Arthritis Rheum20065441122113116572447
- StampLKEassonAPetterssonLHightonJHessianPAMonocyte derived interleukin (IL)-23 is an important determinant of synovial IL-17A expression in rheumatoid arthritisJ Rheumatol200936112403240819797506
- HillyerPLarcheMJBowmaEPInvestigating the role of the interleukin-23/-17A axis in rheumatoid arthritisRheumatology (Oxford)2009481215811589
- KohnoMTsutsumiAMatsuiHInterleukin-17 gene expression in patients with rheumatoid arthritisMod Rheumatol2008181152218092129
- YamadaHNakashimaYOkazakiKTh1 but not Th17 cells predominate in the joints of patients with rheumatoid arthritisAnn Rheum Dis20086791299130418063670
- JandusCBioleyGRivalsJPDudlerJSpeiserDRomeroPIncreased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritidesArthritis Rheum20085882307231718668556
- BurmanAHaworthOHardieDLA chemokine-dependent stromal induction mechanism for aberrant lymphocyte accumulation and compromised lymphatic return in rheumatoid arthritisJ Immunol200517431693170015661933
- ColinEMAsmawidjajaPSvan HamburgJP1,25-dihydroxyvitamin D(3) modulates Th17 polarization and interleukin-22 expression by memory T cells from patients with early rheumatoid arthritisArthritis Rheum201062113214220039421
- KokkonenHSoderstromIRocklovJHallmansGLejonKRantapaa DahlqvistSUp-regulation of cytokines and chemokines predates the onset of rheumatoid arthritisArthritis Rheum201062238339120112361
- BrentanoFOspeltCStanczykJGayREGaySKyburzDAbundant expression of the interleukin (IL)23 subunit p19, but low levels of bioactive IL23 in the rheumatoid synovium: Differential expression and Toll-like receptor-(TLR) dependent regulation of the IL23 subunits, p19 and p40, in rheumatoid arthritisAnn Rheum Dis200968114315018276743
- ChangMSaikiRKCantaneseJJThe inflammatory disease-associated variants in IL12B and IL23R are not associated with rheumatoid arthritisArthritis Rheum20085861877188118512797
- ParkJHKimYJParkBLBaeJSShinHDBaeSCLack of association between interleukin 23 receptor gene polymorphisms and rheumatoid arthritis susceptibilityRheumatol Int200929778178619034457
- BrennanFMChantryDJacksonAMainiRFeldmannMInhibitory effect of TNF alpha antibodies on synovial cell interleukin-1 production in rheumatoid arthritisLancet1989286572442472569055
- CuaDJSherlockJChenYInterleukin-23 rather than interleukin- 12 is the critical cytokine for autoimmune inflammation of the brainNature2003421692474474812610626
- KroenkeMACarlsonTJAndjelkovicAVSegalBMIL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibitionJ Exp Med200820571535154118573909
- YangYWeinerJLiuYT-bet is essential for encephalitogenicity of both Th1 and Th17 cellsJ Exp Med200920671549156419546248
- HaakSCroxfordALKreymborgKIL-17A and IL-17F do not contribute vitally to autoimmune neuro-inflammation in miceJ Clin Invest20091191616919075395
- KreymborgKEtzenspergerRDumoutierLIL-22 is expressed by Th17 cells in an IL-23-dependent fashion, but not required for the development of autoimmune encephalomyelitisJ Immunol2007179128098810418056351
- MatuseviciusDKivisakkPHeBInterleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosisMult Scler19995210110410335518
- LockCHermansGPedottiRGene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitisNat Med20028550050811984595
- IshizuTOsoegawaMMeiFJIntrathecal activation of the IL-17/IL-8 axis in opticospinal multiple sclerosisBrain20051285988100215743872
- DurelliLContiLClericoMT-helper 17 cells expand in multiple sclerosis and are inhibited by interferon-betaAnn Neurol200965549950919475668
- Brucklacher-WaldertVStuernerKKolsterMWolthausenJTolosaEPhenotypical and functional characterization of T helper 17 cells in multiple sclerosisBrain2009132123329334119933767
- TzartosJSFrieseMACranerMJInterleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosisAm J Pathol2008172114615518156204
- Vaknin-DembinskyABalashovKWeinerHLIL-23 is increased in dendritic cells in multiple sclerosis and down-regulation of IL-23 by antisense oligos increases dendritic cell IL-10 productionJ Immunol2006176127768777416751425
- LiYChuNHuAGranBRostamiAZhangGXIncreased IL-23p19 expression in multiple sclerosis lesions and its induction in microgliaBrain2007130249050117003070
- PelfreyCMRudickRACotleurACLeeJCTary-LehmannMLehmannPVQuantification of self-recognition in multiple sclerosis by single-cell analysis of cytokine productionJ Immunol200016531641165110903775
- VoskuhlRRMartinRBergmanCDalalMRuddleNHMcFarlandHFT helper 1 (Th1) functional phenotype of human myelin basic protein-specific T lymphocytesAutoimmunity19931521371437692995
- BegovichABChangMCaillierSJThe autoimmune disease-associated IL12B and IL23R polymorphisms in multiple sclerosisHum Immunol2007681193493718082575
- NunezCDemaBCenitMCIL23R: A susceptibility locus for celiac disease and multiple sclerosis?Genes Immun20089428929318368064
- RoosIMKockumIHillertJThe interleukin 23 receptor gene in multiple sclerosis: A case-control studyJ Neuroimmunol20081941–217318018164077
- IllesZSafranyEPeterfalviA3′UTR C2370A allele of the IL-23 receptor gene is associated with relapsing-remitting multiple sclerosisNeurosci Lett20084311363818180107
- BielekovaBGoodwinBRichertNEncephalitogenic potential of the myelin basic protein peptide (amino acids 83–99 in multiple sclerosis: Results of a phase II clinical trial with an altered peptide ligandNat Med20006101167117511017150
- PanitchHSHirschRLHaleyASJohnsonKPExacerbations of multiple sclerosis in patients treated with gamma interferonLancet1987185388938952882294
- SenoltLVencovskyJPavelkaKOspeltCGaySProspective new biological therapies for rheumatoid arthritisAutoimmun Rev20099210210719328245
- TarganSRHanauerSBvan DeventerSJA short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn‘s disease. Crohn’s Disease cA2 Study GroupN Engl J Med199733715102910359321530
- ReichKNestleFOPappKInfliximab induction and maintenance therapy for moderate-to-severe psoriasis: A phase III, multicentre, double-blind trialLancet200536694941367137416226614
- van OostenBWBarkhofFTruyenLIncreased MRI activity and immune activation in two multiple sclerosis patients treated with the monoclonal anti-tumor necrosis factor antibody cA2Neurology1996476153115348960740
- ShariefMKHentgesRAssociation between tumor necrosis factor- alpha and disease progression in patients with multiple sclerosisN Engl J Med199132574674721852181
- SelmajKWRaineCSTumor necrosis factor mediates myelin and oligodendrocyte damage in vitroAnn Neurol19882343393463132891
- RuddleNHBergmanCMMcGrathKMAn antibody to lymphotoxin and tumor necrosis factor prevents transfer of experimental allergic encephalomyelitisJ Exp Med19901724119312002212948
- KornerHRimintonDSStricklandDHLemckertFAPollardJDSedgwickJDCritical points of tumor necrosis factor action in central nervous system autoimmune inflammation defined by gene targetingJ Exp Med19971869158515909348316
- KageyamaYKobayashiHKatoNInfliximab treatment reduces the serum levels of interleukin-23 in patients with rheumatoid arthritisMod Rheumatol200919665766219685204
- YueCYouXZhaoLThe effects of adalimumab and methotrexate treatment on peripheral Th17 cells and IL-17/IL-6 secretion in rheumatoid arthritis patientsRheumatol Int20091022 [Epub ahead of print]
- ZabaLCCardinaleIGilleaudeauPAmelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responsesJ Exp Med2007204133183319418039949
- MainiRNTaylorPCSzechinskiJDouble-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexateArthritis Rheum20065492817282916947782
- FujimotoMSeradaSMiharaMInterleukin-6 blockade suppresses autoimmune arthritis in mice by the inhibition of inflammatory Th17 responsesArthritis Rheum200858123710371919035481
- MertensMSinghJAAnakinra for rheumatoid arthritis: A systematic reviewJ Rheumatol20093661118112519447938
- GuoBChangEYChengGThe type I IFN induction pathway constrains Th17-mediated autoimmune inflammation in miceJ Clin Invest200811851680169018382764
- PrinzMSchmidtHMildnerADistinct and nonredundant in vivo functions of IFNAR on myeloid cells limit autoimmunity in the central nervous systemImmunity200828567568618424188
- RamgolamVSShaYJinJZhangXMarkovic-PleseSIFN-beta inhibits human Th17 cell differentiationJ Immunol200918385418542719783688
- DrulovicJSavicEPekmezovicTExpression of Th1 and Th17 cytokines and transcription factors in multiple sclerosis patients: Does baseline T-bet mRNA predict the response to interferon-beta treatmentJ Neuroimmunol20092151–2909519695714
- MannonPJFussIJMayerLAnti-interleukin-12 antibody for active Crohn’s diseaseN Engl J Med2004351202069207915537905
- FussIJBeckerCYangZBoth IL-12p70 and IL-23 are synthesized during active Crohn’s disease and are down-regulated by treatment with anti-IL-12 p40 monoclonal antibodyInflamm Bowel Dis200612191516374252
- SandbornWJFeaganBGFedorakRNA randomized trial of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn‘s diseaseGastroenterology200813541130114118706417
- BurakoffRBarishCFRiffDA phase 1/2A trial of STA 5326, an oral interleukin-12/23 inhibitor, in patients with active moderate to severe Crohn’s diseaseInflamm Bowel Dis200612755856516804392
- KruegerGGLangleyRGLeonardiCA human interleukin- 12/23 monoclonal antibody for the treatment of psoriasisN Engl J Med2007356658059217287478
- KimballABGordonKBLangleyRGMenterAChartashEKValdesJSafety and efficacy of ABT-874, a fully human interleukin 12/23 monoclonal antibody, in the treatment of moderate to severe chronic plaque psoriasis: Results of a randomized, placebo-controlled, phase 2 trialArch Dermatol2008144220020718283176
- LeonardiCLKimballABPappKAEfficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1)Lancet200837196251665167418486739
- GriffithsCEStroberBEvan de KerkhofPComparison of ustekinumab and etanercept for moderate-to-severe psoriasisN Engl J Med2010362211812820071701
- SegalBMConstantinescuCSRaychaudhuriAKimLFidelus-GortRKasperLHRepeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: A phase II, double-blind, placebo-controlled, randomised, dose-ranging studyLancet Neurol20087979680418703004
- ChenYLangrishCLMcKenzieBAnti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitisJ Clin Invest200611651317132616670771
- GenoveseMVan den BoschFRobersonSLY2439821, a humanized anti-IL-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritisArthritis Rheum201062492993920131262