Abstract
Background
Age-related declines in testosterone and growth hormone (GH) are associated with increased adiposity and decreases in lean mass and bone mineral density (BMD). A long-term retrospective study examined the effects of testosterone and/or GH supplementation on body composition and quality of life (QoL).
Methods
A database survey assessed the records of 91 men and 97 women (ages 25–82) in treatment groups based on their hormonal status: dehydroepiandrosterone but no hormonal supplementation (control); testosterone only (Tes); GH only (GH); and testosterone plus GH (Tes+GH). Pre-and post-treatment assessments recorded changes in fat and lean mass, BMD, and QoL.
Results
After an average of 3 years of treatment, weight decreased in women in the control and Tes+GH groups but remained stable in men in all groups. Tes and Tes+GH produced statistically significant increases in lean mass, reductions in fat mass, and improvements in BMD in both sexes; GH produced similar changes in women. QoL and mood improved in all groups. Treatments were generally safe and well tolerated.
Conclusions
In this retrospective survey, treatment with testosterone and/or GH was associated with favorable effects in men and women across a wide age range.
Background
Since the second century, when Galen observed that castration led to declines in sexual function and general health and recommended eating animals’ testicles as a way to improve vitality, medicine has progressed to a point where isolated and purified testosterone can be used clinically. Today, however, the goal is not only to enhance libido and sexual function, especially in men, but also to optimize body composition – to offset age-related hormonal changes that may contribute to reduced bone mass (osteopenia), reduced muscle mass (sarcopenia), and increased adiposity. Increased visceral adiposity has been associated with increased risk of coronary artery disease, type 2 diabetes, and other types of morbidity (CitationNicklas et al 2004; CitationLebovitz and Banerji 2005; CitationJensen 2006).
Testosterone levels are positively correlated with measures of body composition such as bone mineral density (BMD) and lean muscle mass. Improved BMD is associated with a decreased risk for osteoporosis and fracture. The correlation between testosterone levels and lean muscle mass is seen across a wide range of age and health status (CitationBross et al 1998). Optimal lean mass is associated with increased strength and coordination and reduced injury from falls. Reduced body fat is associated with decreased actuarial health risk, especially for coronary artery disease and type 2 diabetes mellitus.
The most obvious beneficiaries of testosterone supplementation would be hypogonadal men. All patients in this reported study in the testosterone-only group had been diagnosed as hypogonadal when they began testosterone treatment. Because testosterone levels generally decline as men age, with free testosterone concentrations declining by about 50% between age 25 and 75, elderly men are at higher risk of becoming hypogonadal. The over-65 male population is expected to double (to over 31 million) by 2030, with the incidence of low testosterone levels increasing from 30% in the seventh decade of life to 70% in the eighth decade (CitationHijazi and Cunningham 2005). Accordingly, it is important to understand whether testosterone supplementation improves measures of health.
As in men, androgens in women affect body composition, mood, libido, and general well-being. Deficiency in young women may result from ovarian or adrenal dysfunction, hypothalamic amenorrhea, ovarian failure, oophorectomy, or wasting from acquired immunodeficiency syndrome. Deficiency in testosterone may occur secondary to use of estrogen, oral contraceptives, or corticosteroids. However, this condition is difficult to recognize (declining libido may be the only symptom), and the few data on testosterone therapy in premenopausal women reveal mainly the expected adverse effects of reversible hirsutism and acne; more data are needed to identify women who would be candidates for testosterone therapy and to establish therapeutically useful regimens in this population (CitationKalantaridou and Calis 2006).
Like patients who have low androgen levels, individuals with growth hormone (GH) deficiency have increased adiposity. All patients in this study who received GH had clinically documented GH deficiency. Results of studies designed to determine the effects of GH on body composition in deficient adults have been mixed. Various trials have shown improvement in BMD in men (CitationBravenboer et al 2005; CitationSnyder et al 2007), decreased fat mass and increased lean mass in both men and women, along with significant improvements in serum lipids but not in BMD (CitationHoffman et al 2004), and beneficial reductions in waist:hip ratio and serum low density lipoprotein cholesterol (CitationFranco et al 2006).
The objective of the retrospective survey of clinical data was to assess the effects of testosterone and GH supplementation on body composition and quality of life (QoL) in men and women who had been diagnosed as deficient in androgens and/or GH across a wide age range.
Methods
This study examined the records of patients treated at the Cenegenics® Medical Institute (Las Vegas, NV) during the period 1999 to 2006.
The records of 91 men were assessed in the following treatment groups: dehydroepiandrosterone (DHEA; an adrenal precursor to both estrogens and androgens) but no testosterone or GH (control; n = 31; age range 40–82); testosterone only (Tes; n = 17; age range 40–79); GH only (GH; n = 20; age range 42–70); and testosterone plus GH (Tes+GH; n = 23, age range 36–81).
The records of 97 women were assessed in the same defined treatment groups: control (n = 27; age range 25–60); Tes (n = 26; age range 38–69); GH (n = 12; age range 42–71); and Tes+GH (n = 32, age range 29–75).
The average length of treatment was 3 years.
outlines the hormonal regimen used in male and female patients with their consent after explanation of clinical and laboratory goals to be achieved.
Table 1 Hormonal regimens; in addition to the use of testosterone and GH, other hormonal treatments were offered as needed to achieve normalization of clinical and laboratory status
Measures of body composition (BMD and body mass index) and QoL were compared in patients who received hormonal treatment versus patients who received DHEA supplementation but no hormonal treatment. Other therapies, given adjunctively as needed to optimize clinical and laboratory parameters, included DHEA, thyroid hormone, melatonin (for antioxidant and sleep-stabilizing properties), human chorionic gonadotropin in men (to facilitate weight loss and stimulate endogenous testosterone production), and estradiol and progesterone in women.
In addition to the hormonal regimens, all patients were placed on a low-glycemic diet to improve the lipid profile and increase insulin sensitivity and an exercise program to increase lean muscle mass and decrease fat mass.
All patients had comprehensive baseline and annual physical examinations as well as laboratory assessments at 4-to 6-month intervals. Dual X-ray absorptiometry scans were obtained annually to assess BMD at the hip and lumbar spine; fat mass and lean muscle mass were also assessed annually. QoL outcomes were rated using standardized scales (the Beck Depression Inventory and the Holmes and Dickerson linear analog self-assessment scale) to assess mood and functionality.
Data were expressed as the mean ± standard error. Between-group differences were assessed using the Mann-Whitney U test and the Kruskal-Wallis test. For correlations, Pearson’s test was used for normally distributed data; otherwise, the Spearman rank test was used. All hypothesis tests were two-tailed, with statistical significance assessed at p < 0.05 with 95% confidence intervals. The statistical software used was SPSS 11.5 for Windows (SPSS Inc, Chicago, IL, USA).
Results
Serum testosterone
Among men, there were no significant changes in weight (pre-and post-treatment), and there were no significant changes between the different groups (). In the control group, mean total testosterone increased from 545 ng/dL pretreatment to 687 ng/dL post-treatment (p < 0.03), and free testosterone rose from 107 to 119 pg/mL (nonsignificant). In the Tes group, the corresponding increases in total and free testosterone were 538 to 927 ng/dL (p < 0.002), and 94 to 167 pg/mL (p < 0.002). In the GH group, the increases were 596 to 707 ng/dL (nonsignificant) and 110 to 156 pg/mL (p < 0.006). In the Tes+GH group, the increases were 526 to 814 ng/dL (p < 0.002) and 88 to 126 pg/mL (p < 0.03).
Figure 1 Change in serum testosterone (men). Total and free testosterone increased in all treatment groups. Most of the increases were statistically significant.
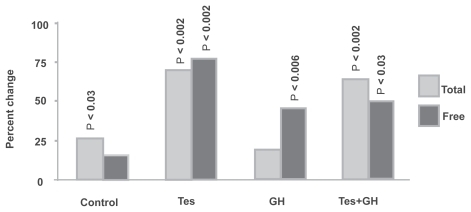
Among women in the Tes, GH, and Tes+GH groups, increases in serum testosterone were significant but of considerably smaller magnitude.
Body weight
Among men, there were no significant within-group or between-group changes in weight. Mean weight across all groups remained stable within the range of 192 to 198 lbs (87–90 kg) over the course of the trial.
However, among women, mean weight between treatment groups was more diverse at baseline (138–155 lbs, or 63–71 kg), and treatment resulted in more notable weight decreases in the control and Tes+GH groups (4.8% and 3.2%, respectively).
Body composition
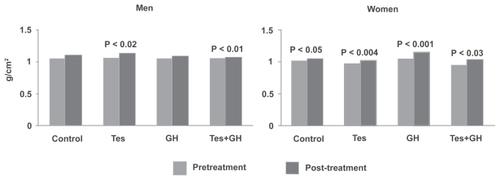
Lean mass increased significantly with Tes (3%) and Tes+GH (6%) in men, and with Tes (2%), GH (13%), and Tes+GH (3%) in women (). Among men, the increase with Tes+GH was significantly greater than with any other regimen.
Figure 2 Lean mass in men and women. Testosterone, alone or in combination with growth hormone (GH), significantly increased lean mass in both men and women.
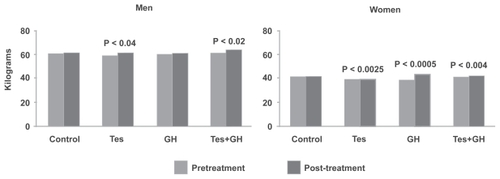
Total body fat was significantly reduced with Tes and Tes+GH in men, and with Tes, GH, and Tes+GH in women; the percentage change was substantially greater in men (). Trunk fat was significantly reduced in the control (p < 0.03) and Tes+GH (p < 0.02) groups in men, and in the Tes (p < 0.01), GH (p < 0.04), and Tes+GH (p < 0.01) groups in women; truncal adiposity increased significantly in the female control group.
Figure 3 Change in total body fat in men and women. Testosterone, alone or in combination with growth hormone (GH), significantly reduced fat mass in both men and women.
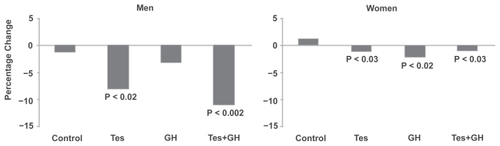
BMDat the hip increased significantly in the Tes (6%) and Tes+GH (3%) groups in men, and in the Tes (5%), GH (11%), Tes+GH (9%), and control (3%) groups in women ().
QoL outcomes
Although none of the groups showed any notable abnormalities at baseline, scores on standard measures of mood, functionality, and quality of life showed improved status over the course of treatment ().
Table 2 Quality-of-life outcomes, mood, functionality, and quality of life showed significant improvements throughout the treatment period
Safety
Physical and laboratory assessments and recording of adverse effects indicated that the study treatments were generally well tolerated. Among the men, mean levels of prostate specific antigen (PSA) showed statistically nonsignificant increases from baseline to post-treatment with all regimens (from 1.05 to 1.40 ng/mLwith Tes, from 1.03 to 1.20 ng/mL with GH, and from 1.04 to 1.14 ng/mL with Tes+GH).
Discussion
This study showed that supplementation with testosterone or GH or both, in conjunction with beneficial lifestyle changes in diet and exercise, produced statistically significant changes in measures of body composition. Treatments were generally well tolerated.
To place these findings in perspective, a systematic review and meta-analysis of 29 randomized controlled trials of testosterone therapy in over 1000 middle-aged and aging men (mean age 64.5 years) found that treatment resulted in a 6.2% reduction in total body fat and a 2.7% increase in lean mass, marginal improvement in strength, significant increase (3.7%) in BMD at the lumbar spine but not at the femoral neck, and reductions in cholesterol, especially in men with lower testosterone concentrations at baseline (CitationIsidori et al 2005). Those findings are generally consistent with the changes noted in the present study, which included men and women spanning a wider age range. However, another systematic review of the literature questioned the benefit of testosterone supplementation in men with normal testosterone levels (CitationKrause et al 2005).
Testosterone supplementation by any route of administration (intramuscular, oral, or transdermal) increases BMD by increasing formation and decreasing resorption of bone, with theoretical but as yet unproven reduction in fracture risk (CitationKöhn 2006). In a 1999 report, individuals with lower pretreatment serum testosterone concentration showed greater changes in lumbar spine BMD during the first 3 years of treatment (CitationSnyder et al 1999). This finding is supported by another study in which men with borderline hypogonadism showed only limited benefit from one year of testosterone therapy (reduction in body fat mass but no significant increase in BMD or lean muscle mass); however, extending the treatment might yield more robust effects (Merza et al 2006).
One of the most important reasons for studying the effects of testosterone treatment is that testosterone therapy also has benefit in terms of cardiovascular health. If changes in body composition measures provide a surrogate measure for decreased cardiovascular morbidity, clinicians would have a valuable tool for determining which patients to treat and guidance for determining treatment end points. A case-control study showed the risk of severe atherosclerotic coronary artery disease in men varied inversely with total testosterone; risk was 5-fold higher among men in lowest quartile than among men in highest quartile (CitationChute et al 1987). A large-scale survey in 1132 men aged 30 to 79 years revealed a significant inverse relationship between blood pressure and levels of testosterone (p < 0.001 for both systolic and diastolic pressure), but no such correlation was seen with other hormones (CitationKhaw et al 1988). The reduction in body fat mass associated with testosterone therapy, along with possible stabilizing effects on blood glucose, has obvious beneficial implications in terms of lowered risk of diabetes, metabolic syndrome, and cardiovascular disease (CitationKöhn 2006). It is noteworthy that the present study showed not only reductions in total body fat with testosterone and GH, but also reductions in truncal fat, which in excess is associated with an increased risk of insulin resistance.
Another potential benefit of testosterone therapy may be decreasing the risk of Alzheimer’s disease. GH is believed to have neuroprotective effects, directly or in conjunction with insulin-like growth factor I (IGF-1; a polypeptide produced mainly in the liver in response to stimulation by GH). In a study of lean elderly subjects, low testosterone availability secondary to high levels of sex hormone binding globulin was associated with a higher incidence of Alzheimer’s disease (CitationPaoletti et al 2004). Although the exclusion of heavier patients may limit the applicability of these findings and the root problem was not absolute testosterone deficiency but excessive binding leaving inadequate free testosterone, there may be a role for testosterone supplementation in patients considered at risk. The QoL measurements used in the present study may provide a useful clinical measurement of cognitive function. Recent reviews on this topic concluded that supplementation may minimize cognitive loss in testosterone-deficient elderly patients at risk (CitationBeauchet 2006) and that supplementation may be more beneficial in elderly men than in elderly women (CitationHogervorst et al 2005).
With reference to GH, a meta-analysis of 10 randomized trials in 458 patients with GH deficiency showed that the mean change in BMD at the lumbar spine was significant at 6 and 12 months, and more strongly significant at 18 and 24 months; however, the magnitude of these changes was small and of uncertain clinical relevance (CitationDavidson et al 2004).
The idea of combining hormonal and bisphosphonate therapy is an attractive approach to improving BMD. A long-term controlled study in 30 adults with GH deficiency showed that the combination of GH and alendronate was highly effective in patients with osteoporosis (CitationBiermasz et al 2004). In contrast, a study in 149 men showed testosterone and alendronate were comparably effective, but the combination offered no additional benefit over monotherapy with either agent (CitationWelch et al 2007).
The role of DHEA supplementation to improve BMD is unclear. In a randomized controlled one-year trial in 140 men and women (aged 60–88 years) with low levels of sulfated DHEA at baseline, supplementation resulted in significant improvements in BMD at the spine among the women and at the hip in both sexes (CitationJankowski et al 2006); however, another double-blind trial in elderly men and women showed limited benefit after two years of treatment (CitationNair et al 2006). In this trial, the only statistically significant changes seen in the control groups receiving DHEA were an increase in total testosterone in men and an increase in BMD at the hip in women.
One special population that merits mention is men at increased risk of prostate cancer, as there may be concern about the safety of testosterone supplementation in these patients. Although castration has been associated with regression or retardation of advanced prostate cancer, it does not automatically follow that restoration of normal testosterone levels in hypogonadal men increases the risk of carcinogenesis. In fact, a detailed review on this subject concludes that increased risk is associated with low rather than high levels of testosterone (CitationRaynaud 2006). In the present study, PSA levels increased with GH and/or testosterone, but the increases were clinically as well as statistically insignificant, as all of the post-treatment values remained well within the normal range (0–2.5 ng/mL). Obviously, men with an established history of prostate cancer would not be considered candidates for treatment.
From the present study, the finding that total and free testosterone increased across all treatment groups in men, including the control group, indicates that the basic regimen of diet and exercise with DHEA and adjunctive hormonal correction as needed was also effective, although the magnitude of the increase was greater in the Tes and Tes+GH groups. Testosterone improved to target ranges with all active treatments except Tes+GH; in this group, the baseline level of free testosterone was unusually low and the post-treatment value, although representing a statistically significant increase, fell just short of the lower limit of the target range.
The study left several questions unanswered. Among the women, weight decreased more in the control group than in the Tes+GH group, which strongly suggests the value of lifestyle change and the need to encourage compliance with diet and exercise; however, it is not clear why this effect was not seen in men.
Another gender issue is the unusually large increase in lean mass seen with GH therapy in women but not in men. Nor is it clear why women but not men on the control regimen showed an unhealthy increase in trunk fat despite the reduction in overall fat mass.
DHEA supplementation changes androgen/estrogen ratios differently in men and women, which may explain our results. There are significant increases in estrogen levels in men, but not much increase in testosterone. For women, DHEA increases androgens but does not have much effect on estrogens (CitationArlt et al 1999, Citation2001; CitationBarnhart et al 1999). In our study, DHEA did affect testosterone levels in men.
The main limitations of this study are its design as a retrospective database survey and the lack of stratification of outcome data by demographic variables other than gender and by concurrent treatment modalities. Moreover, although diet and exercise counseling was provided to patients, it was not feasible to determine the compliance rates. Although the current findings are intriguing, they do not distinguish results in younger versus older patients or in patients with different levels of endogenous hormones and different measures of body composition at baseline. Nevertheless, the results of this investigation are important considering the relative paucity of long-term data (follow-up >1 year) on outcomes with similar treatment strategies. As additional longer-term retrospective data become available for hormone-deficient patients, studies that evaluate correlations between administration of hormones and specific health outcomes will provide clinicians with more precise guidance on which patients to treat and which clinical parameters to use as treatment end points. By using surrogate measures, such as body composition changes, clinicians may be able to more predictably reduce cardiovascular disease and cognitive decline.
Randomized controlled prospective clinical trials are planned, with larger populations followed for even longer periods, which may further clarify the proper role of hormonal supplementation as part of a comprehensive program to preserve vitality throughout life, improve identification of suitable candidates for treatment, and establish optimal individualized regimens.
Conclusions
For patients with clinically documented low androgen levels, testosterone supplementation, alone or in combination with GH (only used in patients who had been diagnosed with adult GH deficiency), produced clinically significant changes in (1) lean body mass, (2) Beck Depression test, (3) change in total body fat, and (4) BMD at the hip in both men and women across a wide age range. These results indicate that hormonal supplementation can augment the benefits of lifestyle change.
Abbreviations
| BMD | = | bone mineral density |
| DHEA | = | dehydroepiandrosterone |
| GH | = | growth hormone |
| PSA | = | prostate specific antigen |
Disclosures
The Cenegenics Education and Research Foundation funded this study. Affiliated with the organization areAlvin Lin, MD, Cenegenics® Medical Institute physician; Denise Olsen, RN, manager of Cenegenics’ Quality Assurance and Research Department; Alan Mintz, MD, cofounder of Cenegenics® Medical Institute.
References
- ArltWCalliesFKoehlerI2001Dehydroepiandrosterone supplementation in healthy men with age-related decline of dehydroepiandrosterone secretionJ Clin Endocrinol Metab8646869211600526
- ArltWHaasJCalliesF1999Biotransformation of oral dehydroepiandrosterone in elderly men: significant increase in circulating estrogensJ Clin Endocrinol Metab842170610372727
- BarnhartKTFreemanEGrissoJA1999The effect of dehydroepiandrosterone supplementation to symptomatic perimenopausal women on serum endocrine profiles, lipid parameters, and health-related quality of lifeJ Clin Endocrinol Metab84389690210566625
- BeauchetO2006Testosterone and cognitive function: current clinical evidence of a relationshipEur J Endocrinol1557738117132744
- BiermaszNRHamdyNAPereiraAM2004Long-term skeletal effects of recombinant human growth hormone (rhGH) alone and rhGH combined with alendronate in GH-deficient adults: a seven-year follow-up studyClin Endocrinol (Oxf)605687515104559
- BravenboerNHolzmannPJter MaatenJC2005Effect of long-term growth hormone treatment on bone mass and bone metabolism in growth hormone-deficient menJ Bone Miner Res2017788416160735
- BrossRCasaburiRStorerTW1998Androgen effects on body composition and muscle function: implications for the use of androgens as anabolic agents in sarcopenic statesBaillieres Clin Endocrinol Metab123657810332559
- ChuteCGBaronJAPlymateSR1987Sex hormones and coronary artery diseaseAm J Med8385393674092
- DavidsonPMilneRChaseD2004Growth hormone replacement in adults and bone mineral density: a systematic review and meta-analysisClin Endocrinol (Oxf)6092814678294
- FrancoCJohannssonGBengtssonBA2006Baseline characteristics and effects of growth hormone therapy over two years in younger and elderly adults with adult onset GH deficiencyJ Clin Endocrinol Metab9144081416940452
- HijaziRACunninghamG2005Andropause: is androgen replacement therapy indicated for the aging male?Annual Rev Med561173715660505
- HoffmanARKuntzeJEBaptistaJ2004Growth hormone (GH) replacement therapy in adult-onset GH deficiency: effects on body composition in men and women in a double-blind, randomized, placebo-controlled trialJ Clin Endocrinol Metab8920485615126520
- HogervorstEBandelowSMoffatSD2005Increasing testosterone levels and effects on cognitive functions in elderly men and women: a reviewCurr Drug Targets CNS Neurol Disord45314016266286
- IsidoriAMGiannettaEGrecoEA2005Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysisClin Endocrinol (Oxf)632809316117815
- JankowskiCMGozanskyWSSchwartzRS2006Effects of dehydroepiandrosterone replacement therapy on bone mineral density in older adults: a randomized, controlled trialJ Clin Endocrinol Metab9129869316735495
- JensenMD2006Adipose tissue as an endocrine organ: implications of its distribution on free fatty acid metabolismEur Heart J8Suppl B B13B19
- KalantaridouSNCalisKA2006Testosterone therapy in premenopausal womenSemin Reprod Med241061416633984
- KhawKTBarrett-ConnorE1988Blood pressure and endogenous testosterone in men: an inverse relationshipJ Hypertens6329323379300
- KöhnFM2006Testosterone and body functionsAging Male9183817178552
- KrauseWMuellerUMazurA2005Testosterone supplementation in the aging male: which questions have been answered?Aging Male831816106921
- LebovitzHEBanerjiMA2005Point: visceral adiposity is causally related to insulin resistanceDiabetes Care282322516123512
- MerzaZBlumsohnAMahPMDouble-blind placebo-controlled study of testosterone patch therapy on bone turnover in men with borderline hypogonadismInt J Androl293819116390499
- NairKSRizzaRAO’BrienP2006DHEA in elderly women and DHEA or testosterone in elderly menN Engl J Med35516475917050889
- NicklasBJPenninxBWCesariM2004Association of visceral adipose tissue with incident of myocardial infarction in older men and womenAm J Epid1607419
- PaolettiAMCongiaSLelloS2004Low androgenization index in elderly women and elderly men with Alzheimer’s diseaseNeurology62301314745075
- RaynaudJP2006Prostate cancer risk in testosterone-treated menJ Steroid Biochem Mol Biol102261617113983
- SnyderPJBillerBMZagarA2007Effect of growth hormone replacement on BMD in adult-onset growth hormone deficiencyJ Bone Miner Res227627017280527
- SnyderPJPeacheyHHannoushP1999Effect of testosterone treatment on bone mineral density in men over 65 years of ageJ Clin Endocrinol Metab8419667210372695
- WelchBJDenkeMAKermaniA2007Comparison of testosterone, alendronate, and a combination of both therapies in men with low bone mineral densityJ Investig Med5516873