Abstract
Rheumatoid arthritis (RA) is a significant cause of work disability and job loss. The resulting economic burden experienced by patients has received considerable research attention. This research assesses the effect of tumor necrosis factor (TNF) antagonists (infliximab, etanercept) on the ability of RA patients living in Japan to work and participate in society. A total of 42 patients with active RA were enrolled and given biological therapy for 12 months (mo). Of these patients, 14 were employed full-time, 6 were employed part-time, and 22 were not employed. Twenty-six patients were given infliximab, and sixteen were given etanercept. The amount of domestic labor performed before the biologics served as a baseline and was assigned a value of 0%. After treatment with biologics, the productivity was evaluated using the visual analog scale (VAS; −100 to +100 mm). The administration of TNF antagonists to RA patients who exhibited an insufficient response to medical treatment significantly improved the Disease Activity Score 28 (DAS 28) after both 6 mo and 12 mo (P < 0.0001). A significant correlation was found between the improvement in their DAS 28 and improvements in their work situation (Productivity VAS) (P < 0.05). Of particular interest is the significant correlation between the values of baseline mHAQ and the percent changes of Productivity VAS that was observed after 6 mo and 12 mo (P < 0.05). Our findings indicate that medical treatment of RA with TNF antagonists improves the patients' ability to perform their jobs and housekeeping. Because loss of productivity is an important contributor to the indirect costs of RA, our findings are relevant for the pharmacoeconomic assessment of treatments.
Introduction
Rheumatoid arthritis (RA) is a chronic, progressive disease with an estimated worldwide prevalence of 0.1%–1%. In order to minimize morbidity, early diagnosis and aggressive treatment are required.Citation1–Citation3 Joint destruction progresses as a result of continued inflammation. Over the course of RA, there is a relationship among disease activity, joint destruction, and functional capacity.Citation4 In clinical settings, the tumor necrosis factor (TNF) antagonists infliximab (Ifx) and etanercept (Etn) have successfully reduced the TNF-induced production of inflammatory mediators, which reduces the signs and symptoms of RA.Citation5,Citation6
Research has shown that work disability is associated with the disease activity and duration of RA, as well as reduced physical function in patients with longstanding RA.Citation7–Citation9 The loss of productivity associated with RA disability is a significant burden on the patients, their families, and society.Citation10 This negative impact is observed even in patients in the early stages of the disease. Given that the loss of working capacity is a significant outcome of RA, the effectiveness of treatment strategies should be evaluated not only using traditional clinical, functional, and radiographic measures, but also by determining how well the patients receiving these treatments are able to maintain employability Several clinical trials have provided compelling data regarding the clinical efficacy of biologics in both the induction and maintenance of remission in RA. Although biologics often induce rapid clinical improvements in patients with RA, the price of these drugs limits their use.Citation11,Citation12 Thus, estimating the direct and indirect effects of biological agents, such as clinical outcomes, is important.
To assess the impact of the treatment with TNF antagonists in Japanese patients with RA, we examined the influences and correlation of the productivity of daily work and the changes in disease activity.
Patients and methods
Patients
At the baseline visit (Week 0), the patient’s characteristics and employment status (employed [part-time or full-time] or not employed) were assessed. A total of 42 patients with active RA who met the 1987 ACR criteriaCitation13 were enrolled and given biological therapy for 12 months (mo). Among these patients, 14 were employed full-time (laborer: 2, office worker: 12), 6 were employed part-time (all office workers), and 22 were not employed. The patients were given an intravenous dose of Ifx 3 mg/kg at baseline at 2 and 6 weeks, followed by every 8 weeks thereafter. Etn (25 mg twice a week or 50 mg once every week) was subcutaneously injected. The number of patients who received Ifx or Etn were 16 and 26, respectively.
Glucocorticoids (<10 mg/day of prednisolone) and non-steroidal anti-inflammatory drugs had to be given at a stable dose for at least 4 weeks before the enrollment and during the course of treatment. The disease activity and patients' clinical improvement were assessed using the DAS28 (erythrocyte sedimentation rate [ESR] 4) using the European League Against Rheumatism (EULAR) criteria.Citation14,Citation15 According to the EULAR response criteria, patients with moderate to good responses to Ifx therapy were defined as the responsive group. Serum C-reactive protein (CRP) levels and ESR were respectively determined using a latex photometric immunoassay and the Westergren method. The percent improvement in DAS28 from baseline was calculated as follows: (baseline DAS28-post treatment DAS28 [6 or 12 mo]/baseline DAS28) × 100. At baseline, the demographic data and present medication were recorded, a complete history was taken, and a physical examination and routine laboratory examinations were performed, including whole blood count, tests of renal and hepatic function, and urinalysis. Additionally, the modified Health Assessment Questionnaire (mHAQ) score, which is widely used to assess patient health-related quality of life, was calculated at each visit.Citation16
Self-reported productivity in patients with RA
The effects of Biologics in patients with RA on self-reported productivity were prospectively evaluated in RA (referred to as Productivity VAS) and based on a slightly modified protocol described by van der Heijde et al for patients with ankylosing spondylitis.Citation17 At baseline, 6 mo (usually 22 weeks for Ifx and 24 weeks for Etn) and 12 mo (46 weeks for Ifx, 48 weeks for Etn), the patients were asked to indicate how much their disease had affected their productivity at work, school, or home in the past 4 weeks using the VAS scale, with -10 from baseline meaning that they were unable to perform the activity completely, 0 meaning that they did not experience any change in productivity, and 10 meaning that their productivity had improved significantly. A mean change from base 0 in the improvement in self-reported productivity was calculated.
These experiments were conducted in accordance with protocols that were approved by the Human Subjects Research Committee at our institution. Informed consent was obtained from all patients.
Statistical analysis
The data were expressed as the mean ± SD. The differences between groups were evaluated using the Mann-Whitney U-test. Follow-up data were evaluated using Wilcoxon’s test. The relationship between the changes in Productivity VAS and the indicated parameters were evaluated using the Spearman rank correlation. Values of P < 0.05 were considered significant.
Results
Effects of Biologics in patients with RA treated with Ifx: or Etn
The patient’s characteristics are summarized in . At the start of therapy, the mean age of the patients was 53.6 ± 15.7 years (range: 25–82; 47 females, 5 males), the mean disease duration was 4.4 ±3.5 years (0.9–18.9), and the mean baseline DAS28 (ESR) was 5.44 ± 1.25 (2.47–7.71). The methotrexate (MTX) dosage was 5.8 (0–10) mg/week, and the HAQ was 4.3 (0–14). The fact that the dosages of MTX administered to our patients were somewhat lower than those reported in other countries reflects the lower effective and recommended dosages of MTX (6–8 mg per week) in Japanese RA patients. This result is in accordance with the results of a double-blind, placebo-controlled (phase II–III) trial involving Japanese RA patients (unpublished data). Due to increases in RA disease activity or unresponsiveness to the TNF antagonists, the treatment was discontinued in four patients after 48 weeks. Therefore, 42 patients at 6 mo and 38 patients at 12 mo, all of whom had received TNF antagonists, completed the assessments and clinical examinations.
Table I Patient characteristics
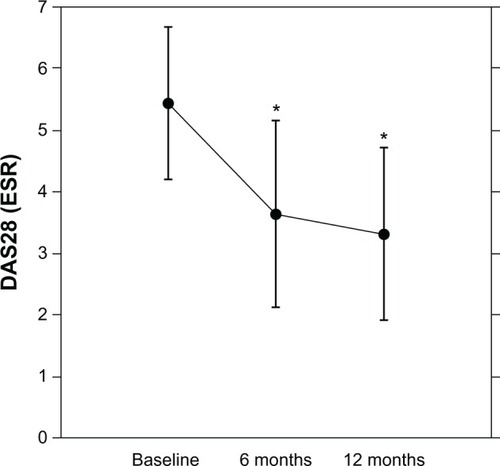
The RA patients who had received the TNF antagonists showed significant improvement in DAS28 after 6 mo (n = 42, baseline 5.44 ± 1.25 to 3.64 ± 1.51, P < 0.0001) and 12 mo (n = 38, baseline 5.38 ± 1.28 to 3.21 ± 1.41, P < 0.0001) (). After 6 mo, 76% of the patients achieved a moderate response in the EULAR response criteria, and 36% of the patients had good responses; after 12 mo, those numbers increased to 84% and 58%, respectively.
Figure 1 Effects of TNF antagonists on DAS28 in patients with RA.
Abbreviations: DAS28, Disease Activity Score 28; ESR, erythrocyte sedimentation rate; mo, months; TNF, tumor necrosis factor; RA, rheumatoid arthritis.
Effects of Biologics on the changes in productivity VAS and the relationship with disease activity
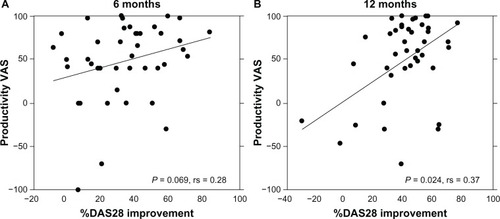
After 6 mo (n = 42, baseline 0 to 48.5 ± 44.5, P < 0.0001) and 12 mo (n = 38, baseline 0 to 47.2 ± 52.6, P < 0.0001), there were significant improvements in the Productivity VAS responses (). Interestingly, there was a significant positive correlation between the percent improvement in DAS28 and the improvement in Productivity VAS after 12 mo but not after 6 mo ().
Figure 2 Effects of TNF antagonists on Productivity VAS in patients with RA.
Abbreviations: VAS, visual analog scale; mo, months; RA, rheumatoid arthritis; TNF, tumor necrosis factor.
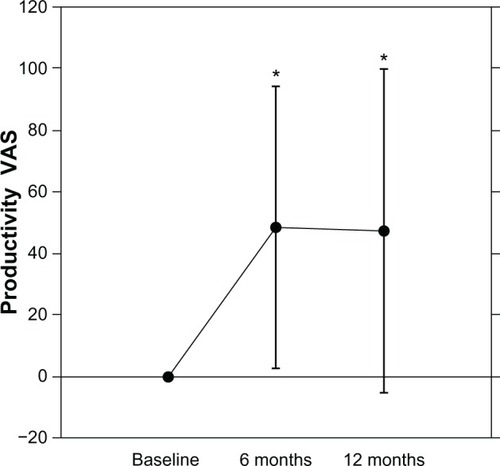
Figure 3 Positive correlation between the changes in DAS28 and Productivity VAS.
Abbreviations: DAS28, Disease Activity Score 28; mo, months; VAS, visual analog scale; RA, rheumatoid arthritis.
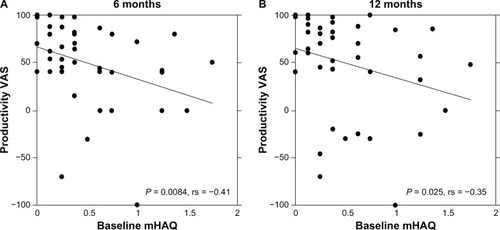
There were significant improvements in the mHAQ responses after both 6 mo (n = 42, baseline 0.54 ± 0.46 to 0.32 ± 0.36, P < 0.001) and 12 mo (n = 38, baseline 0.53 ± 0.46 to 0.30 ± 0.35, P < 0.001). A significant correlation between the values of baseline mHAQ and the percent changes in Productivity VAS was observed after 6 mo and 12 mo (). However, the baseline disease activity (DAS28), patient age, and disease duration were not significantly correlated with the percent change in Productivity VAS at either 6 mo or 12 mo. In addition, one patient was able to start a new job.
Figure 4 Positive correlation between the changes in Productivity VAS and baseline mHAQ in patients with RA.
Abbreviations: VAS, visual analog scale; mHAQ, modified Health Assessment Questionnaire; mo, months; RA, rheumatoid arthritis.
Discussion
The progressive joint destruction as a result of chronic RA often leads to major disabilities. These disabilities severely limit quality of life and social activities. The impact of RA on work activities is a significant problem with profound effects on the individual and society. Furthermore, pain was more correlated with work impairment and activity impairment than was function or fatigue.Citation18 Studies on the effects of TNF antagonists on work limitations in RA patients have had either a retrospective observational design or a clinical primary outcome with work data examined as a secondary outcome.Citation19–Citation24 These studies show an improvement in employability and a reduction in the workday losses in patients with RA and ankylosing spondylitis through the treatment with Biologics (TNF antagonists).Citation17,Citation23,Citation25 In the present study, we examined the correlation between changes in work productivity and disease activity. After 6 mo and 12 mo, TNF antagonists significantly increased productivity in RA patients by 48.6% and 47.2%, respectively. The improvement in the Productivity VAS was correlated significantly with the changes in disease activity (DAS28 changes). Furthermore, changes in the Productivity VAS were significantly correlated with the baseline mHAQ. The most significant identifier of work disability in patients with RA has been shown to be the HAQ functional disability score.Citation26,Citation27 These findings, combined with those of the present report, suggest that functional disability may be closely linked to the improvement of productivity but not to the disease activity of RA at baseline.
The indirect costs of RA, including the economic impact of workday losses, reduced productivity while at work, and the total cost of RA to society, could be much higher.Citation28 For example, the cost of RA has been reported to account for 0.3%) of the gross domestic product in the United States.Citation29 With a larger patient scale, assessing the indirect costs would be supported by objective outcomes, such as the number of lost workdays or reduced working times per day. To validate the efficacy and benefits of Biologics, especially the productivity of daily work, the Work Productivity and Activity Impairment (WPAI) questionnaire is a well-known instrument that measures impairments in both paid and unpaid work and has been validated to quantify work impairments for numerous diseases, including RA.Citation30–Citation32 In our study population, the unemployment rate at baseline was high (52%), which corresponds with the etiology of RA, namely that affected patients with RA are more likely to be older and female, and the reduced employability in patients with RA. In the present study, we simplified this measurement. Further studies are needed to evaluate the economic value of such improvement.
One of the limitations of the present study is that the number of enrolled patients is relatively small. This study is the first to show the impact of the treatment with TNF antagonists in Japanese patients with RA on the productivity of daily work with the changes in disease activity. In order to confirm the relation between the improvements of daily productivity and other patient characteristics or clinical and serological parameters, future studies should examine a larger number of RA patients. In conclusion, we found that the productivity of work in daily life and in society significantly improved during the treatment with TNF antagonists, such as Ifx and Etn, which parallels the reduction in the signs and symptoms of RA. Early and effective treatment of RA may contribute not only to improving the quality of life but also to reducing costs by decreasing lost productivity, surgical procedures and requirements for extended-care facility admission, and social service utilization. Because the loss of productivity is an important contributor to the indirect costs of RA, our findings may be relevant for the pharmacoeconomic assessment of treatments.
Disclosure
The authors report no conflicts of interest in this work.
References
- LeeDMWeinblattMERheumatoid arthritisLancet200135890391111567728
- JenkinsJKHardyKJMcMurrayRWThe pathogenesis of rheumatoid arthritis: a guide to therapyAm J Med Sci2002323417118012003371
- ScottDLWolfeFHuizingaTWRheumatoid arthritisLancet201037697461094110820870100
- WelsingPMvan GestelAMSwinkelsHLKiemeneyLAvan RielPLThe relationship between disease activity, joint destruction, and functional capacity over the course of rheumatoid arthritisArthritis Rheum20014492009201711592361
- HarrimanGHarperLKSchaibleTFSummary of clinical trials in rheumatoid arthritis using infliximab, an anti-TNFalpha treatmentAnn Rheum Dis199958 Suppl 1161164
- GarrisonLMcDonnellNDEtanercept: therapeutic use in patients with rheumatoid arthritisAnn Rheum Dis199958 Suppl 1165169
- PincusTCallahanLFSaleWGBrooksALPayneLEVaughnWKSevere functional declines, work disability, and increased mortality in seventy-five rheumatoid arthritis patients studied over nine yearsArthritis Rheum19842788648726431998
- YelinEHenkeCEpsteinWThe work dynamics of the person with rheumatoid arthritisArthritis Rheum19873055075123593434
- WolfeFHawleyDJThe longterm outcomes of rheumatoid arthritis: Work disability: a prospective 18 year study of 823 patientsJ Rheumatol19982511210821179818651
- StrandVKhannaDThe impact of rheumatoid arthritis and treatment on patients’ livesClin Exp Rheumatol2010283 Suppl 59S32S4020576223
- FautrelBWoronoff-LemsiMCEthgenMImpact of medical practices on the costs of management of rheumatoid arthritis by anti-TNFalpha biological therapy in FranceJoint Bone Spine200572655055615996504
- LaasKPeltomaaRKautiainenHPuolakkaKLeirisalo-RepoMPharmacoeconomic study of patients with chronic inflammatory joint disease before and during infliximab treatmentAnn Rheum Dis200665792492816339293
- ArnettFCEdworthySMBlochDAThe American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritisArthritis Rheum19883133153243358796
- van der HeijdeDMvan‘t HofMAvan RielPLJudging disease activity in clinical practice in rheumatoid arthritis: first step in the development of a disease activity scoreAnn Rheum Dis199049119169202256738
- van GestelAMPrevooMLvan‘t HofMAvan RijswijkMHvan de PutteLBvan RielPLDevelopment and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism CriteriaArthritis Rheum199639134408546736
- PincusTSummeyJASoraciSAJrWallstonKAHummonNPAssessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment QuestionnaireArthritis Rheum19832611134613536639693
- van der HeijdeDHanCDeVlamKInfliximab improves productivity and reduces workday loss in patients with ankylosing spondylitis: results from a randomized, placebo-controlled trialArthritis Rheum200655456957416874778
- GeuskensGAHazesJMBarendregtPJBurdorfAPredictors of sick leave and reduced productivity at work among persons with early inflammatory joint conditionsScand J Work Environ Health200834642042919137203
- WolfeFAllaireSMichaudKThe prevalence and incidence of work disability in rheumatoid arthritis, and the effect of anti-tumor necrosis factor on work disabilityJ Rheumatol200734112211221717787043
- SmolenJSHanCvan der HeijdeDInfliximab treatment maintains employability in patients with early rheumatoid arthritisArthritis Rheum200654371672216508932
- KeatACGaffneyKGilbertAKHarrisCLeederJInfluence of biologic therapy on return to work in people with work disability due to ankylosing spondylitisRheumatology (Oxford)200847448148318281690
- AllaireSWolfeFNiuJZhangYZhangBLaValleyMEvaluation of the effect of anti-tumor necrosis factor agent use on rheumatoid arthritis work disability: the jury is still outArthritis Rheum20085981082108918668597
- BejaranoVQuinnMConaghanPGEffect of the early use of the anti-tumor necrosis factor adalimumab on the prevention of job loss in patients with early rheumatoid arthritisArthritis Rheum200859101467147418821658
- RatermanHGHovingJLNurmohamedMTWork ability: a new outcome measure in rheumatoid arthritis?Scand J Rheumatol201039212713120059369
- KavanaughAAntoniCMeasePEffect of infliximab therapy on employment, time lost from work, and productivity in patients with psoriatic arthritisJ Rheumatol200633112254225916960923
- YelinEMeenanRNevittMEpsteinWWork disability in rheumatoid arthritis: effects of disease, social, and work factorsAnn Intern Med19809345515567436187
- KarassaFBIoannidisJPTouloumiGBokiKAMoutsopoulosHMRisk factors for central nervous system involvement in systemic lupus erythematosusQJM200093316917410751236
- RatACBoissierMCRheumatoid arthritis: direct and indirect costsJoint Bone Spine200471651852415589432
- YelinEThe costs of rheumatoid arthritis: absolute, incremental, and marginal estimatesJ Rheumatol Suppl19964447518833052
- ReillyMCZbrozekASDukesEMThe validity and reproducibility of a work productivity and activity impairment instrumentPharmacoeconomics19934535336510146874
- ReillyMCGoochKLWongRLKupperHvan der HeijdeDValidity, reliability and responsiveness of the Work Productivity and Activity Impairment Questionnaire in ankylosing spondylitisRheumatology (Oxford)201049481281920100797
- ZhangWBansbackNBoonenAYoungASinghAAnisAHValidity of the work productivity and activity impairment questionnaire - general health version in patients with rheumatoid arthritisArthritis Res Ther2010125R17720860837