Abstract
Hemoglobin variants in which a frameshift results in chain elongation are unusual. Hemoglobin Pakse (Hb Pakse) is an unstable hemoglobin with abnormal elongation, first described in Indochina. An alpha2-globin gene termination codon mutation, TAA → TAT or Term → Tyr, has been described in the pathogenesis of Hb Pakse. This abnormality causes a frameshift that elongates the alpha chain amino acids. Computer-based protein structure modeling was used in a bioinformatics analysis of the tertiary structure of these elongated amino acid sequences. The elongated part of Hb Pakse showed additional helices, which may cause the main alteration in Hb Pakse. Abnormalities in the fold structure of globin in Hb Pakse were identified, and helices additional to the normal alpha globin chains were shown in the elongated part of Hb Pakse.
Introduction
Hemoglobin variants in which a disorder results in chain elongation are unusual (CitationBunn et al 1975; CitationWiwanitkit 2004). The first two well-known disorders to be described are hemoglobin Tak (CitationWiwanitkit 2004) and hemoglobin Cranston (CitationBunn et al 1975). However, other hemoglobinopathies have chain elongation. Hb Pakse is another unstable hemoglobin, first described in Pakse, Laos (CitationSanchaisuriya et al 2002). The Hb Pakse hemoglobinopathy is an unstable variant having an elongated alpha chain due to a DNA mutation, leading to a single amino acid substitution (CitationSanchaisuriya et al 2002; CitationViprakasit et al 2002).
Pathophysiologically, Hb Pakse is caused by an alpha2-globin gene termination codon mutation, TAA → TAT or Term → Tyr, initially described in a Laotian family (CitationSanchaisuriya et al 2002; CitationViprakasit et al 2002). This hemoglobinopathy has alpha chains composed of 173 amino acid residues (CitationSanchaisuriya et al 2002; CitationViprakasit et al 2002). The elongated part of the alpha chain is believed to cause the instability of Hb Pakse (CitationSanchaisuriya et al 2002; CitationViprakasit et al 2002).
Although the primary and secondary structures of Hb Pakse are well known, its tertiary structure is not well documented. Studying the tertiary structures of the elongated part of the alpha chain in Hb Pakse could help explain its pathogenesis.
Materials and methods
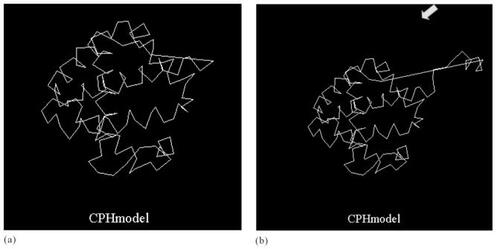
A computer-based bioinformatics analysis was used to model the tertiary structure of the elongated amino acid sequences in Hb Pakse. The protein tertiary structure was predicted from its primary sequence using the CPHmodels 2.0 Server (CitationLund et al 2002).
Results
The tertiary structures of normal alpha globin and alpha globin in the elongated part of Hb Pakse calculated using the Peptide Tertiary Structure Prediction Server (PEPstr) are presented in .
Discussion
Nondeletional gene mutations giving rise to alpha-thalassemia can be found at polymorphic frequency in Southeast Asia (CitationWiwanitkit 2004). Although the most common is hemoglobin Constant Spring (Hb CS), caused by a termination codon mutation (UAA → CAA, Gln) in the alpha globin gene, which reduces synthesis of the elongated alpha globin variant, Hb Pakse (UAA → UAU, Tyr) also has been observed at a significant frequency (CitationSanchaisuriya et al 2002; CitationViprakasit et al 2002).
Hb Pakse results from an aberration in the alpha globin gene. The chain elongation in Hb Pakse can be explained as the result of a mutation in the terminal codon for the alpha globin chain (CitationSanchaisuriya et al 2002; CitationViprakasit et al 2002). The frameshift mutation leads to an abnormal elongation similar to that in Hb CS. Hb Pakse differs from Hb CS by having lysine at 142 instead of glutamine. It also seems to lead to an unstable alpha globin mRNA and slighter higher levels of hemoglobin H (Hb H) disease (CitationTurbpaiboon et al 2004). The clinical significance of this unstable hemoglobin is the relationship with a hypochromic microcytic anemia due to an unstable hemoglobin variant. The main pathogenesis is believed to be due to the nature of this abnormal hemoglobin, resulting from the elongation. The manifestation as Hb H disease has been noted (CitationTurbpaiboon et al 2004).
The structural analysis technique used in the present study is a new approach and has been verified in previous studies on some tropical hemoglobin disorders. CitationWiwanitkit (2005) reported prediction of the tertiary structure of another hemoglobinopathy in Southeast Asia, Hb Suandok; no significant aberration in the tertiary structure of Hb Suandok was reported. In the present study, alterations in the fold structure of globin in Hb Pakse were identified, and helices additional to the normal alpha globin chains were shown in the elongated part of Hb Pakse. Indeed, the structural aberration relating to the helix part of the globin chain shows some possible correlation with hemolysis. CitationColeman et al (1995) studied the molecular basis of transfusion-dependent hemolytic anemia in Hb Medicine Lake and noted that the potentially distorted B helix might provoke further molecular instability, including the presentation of mild hemolytic anemia. The developed molecular structure will be useful in further studies on the molecular structure and molecular action in this disorder.
References
- BunnHFSchmidtGJHaneyDNHemoglobin Cranston, an unstable variant having an elongated alpha chain due to nonhomologous crossover between two normal alpha chain genesProc Natl Acad Sci U S A1975723609131059149
- ColemanMBLuZHSmithCMIITwo missense mutations in the beta-globin gene can cause severe beta thalassemia. Hemoglobin Medicine Lake (beta 32[B14]leucine → glutamine; 98 [FG5] valine → methionine)J Clin Invest19959550397860732
- LundONielsenMLundegaardCCPHmodels 2.0: X3M a computer program to extract 3D models [abstract]CASP5 conference2002 December 1-5Asilomar, CA, USAA102
- PEPstr: Peptide Tertiary Structure Prediction Server [online] URL: http://www.imtech.res.in/raghava/pepstr/
- TurbpaiboonCSiritantikornAThongnoppakhunWHemoglobin Pakse: presence on red blood cell membrane and detection by polymerase chain reaction-single-strand conformational polymorphismInt J Hematol200480136915481441
- SanchaisuriyaKFucharoenGFucharoenSHb Pakse [(alpha2) codon 142 (TAA → TAT or Term → Tyr)J in Thai patients with EAbart’s disease and Hb H diseaseHemoglobin2002262273512403487
- ViprakasitVTanphaichitrVSPung-AmrittPClinical phenotypes and molecular characterization of Hb H-Pakse diseaseHaematologica2002871172511836160
- WiwanitkitVHemoglobin Tak, an unstable hemoglobin from ThailandHaema2004731012
- WiwanitkitVModeling for tertiary structure of globin chain in hemoglobin SuandokHematology200510163516019464