Abstract
Chitosan is a widely available, mucoadhesive polymer that is able to increase cellular permeability and improve the bioavailability of orally administered protein drugs. It can also be readily formed into nanoparticles able to entrap drugs or condense plasmid DNA. Studies on the formulation and oral delivery of such chitosan nanoparticles have demonstrated their efficacy in enhancing drug uptake and promoting gene expression. This review summarizes some of these findings and highlights the potential of chitosan as a component of oral delivery systems.
Keywords:
Introduction
Effective oral drug administration is desirable but challenging owing to the nature of the gastrointestinal tract. The highly acidic pH in the stomach and the presence of enzymes such as pepsin can cause protein degradation (CitationAllemann et al 1998). Secreted pancreatic enzymes in the lumen of the intestine and membrane-bound brush-border enzymes may also cause substantial loss of drug activity (CitationBernkop-Schnürch and Krajicek 1998). Finally, the physical barrier of the intestinal cells must be crossed before a drug can reach the circulation. This is especially problematic for macromolecular drugs too large to pass between cells through the paracellular pathway and too hydrophilic to be absorbed passively through cell membranes (CitationGoldberg and Gomez-Orellana 2003). These obstacles lead to poor oral bioavailability for many protein and peptide drugs.
Increasingly, nucleic acids are also being applied as drugs, either as components of a vaccine or in gene therapy approaches. Many of the issues facing oral gene delivery are similar to those of oral protein delivery, including protection in the stomach and intestines and transport into or across intestinal epithelial cells. Additional barriers to effective DNA delivery include endosomal escape, nuclear localization, transcription, translation, protein processing, and protein secretion (if necessary) into plasma.
One proposed method to overcome these physical and degradative barriers is formulation of the drug or gene into nanoparticles. Such particles may partially protect the entrapped drug or gene from degradation and improve cellular uptake through endocytosis. While a variety of polymers and lipids have been employed to form drug- or gene-loaded nanoparticles, one biodegradable polymer that has received a good deal of recent attention as a component of oral drug and gene delivery systems is chitosan.
Properties of chitosan
Chitosan is a polysaccharide derived from the partial deacetylation of chitin, primarily from crustacean and insect shells. It consists of repeating units of glucosamine and N-acetyl-glucosamine, the proportions of which determine the degree of deacetylation of the polymer. With a pKa of approximately 6.5 on the amine groups, chitosan is insoluble at neutral pH but is soluble and positively charged at acidic pH (CitationSingla and Chawla 2001; CitationHejazi and Amiji 2003). By affecting the number of protonatable amine groups, the degree of deacetylation fundamentally determines the polymer properties including solubility, hydrophobicity, and the ability to interact electrostatically with polyanions (CitationKiang, Wen, et al 2004; CitationHuang et al 2005). The solubility of chitosan in neutral and basic pH can be improved by quaternization to form trimethyl chitosan derivatives (Citationvan der Merwe et al 2004). The molecular weight of chitosan, which is available over a wide range, is also of fundamental importance. Generally, chitosans having lower molecular weights and lower degrees of deacetylation exhibit greater solubility and faster degradation than their high-molecular-weight counterparts (CitationZhang and Neau 2001, Citation2002; CitationKöping-Höggård et al 2004; CitationMao et al 2004; CitationRen et al 2005).
Positively charged chitosan will bind to cell membranes and is reported to decrease the trans-epithelial electrical resistance (TEER) of cell monolayers as well as to increase paracellular permeability (CitationArtursson et al 1994; CitationDodane et al 1999). Chitosan solutions have been shown to increase trans- and para-cellular permeability in a reversible, dose-dependent manner that also depends on the molecular weight and degree of deacetylation of the chitosan (CitationSchipper et al 1996). The mechanism of action, which appears to be mediated by the positive charges on the chitosan, includes interactions with the tight junction proteins occludin and ZO-1, redistribution of F-actin, and slight destabilization of the plasma membrane (CitationDodane et al 1999; CitationFang et al 2001; CitationThanou, Verhoef, Junginger, 2001). Thus, the ability of chitosan to enhance permeation is influenced by the pH of the environment. As mentioned above, trimethyl chitosan derivatives are soluble at higher pH than unmodified chitosan. For example, a trimethyl derivative with 61.2% quaternization was able to decrease TEER of Caco-2 cells and increase mannitol permeability at pH 7.4, unlike unmodified chitosan hydrochloride or 12.3% quaternized trimethyl chitosan (CitationKotzé et al 1999).
Chitosan is also mucoadhesive (CitationDeacon et al 2000). Mucus is a blend of molecules including salts, lysozyme, and mucins, which are highly hydrated glycoproteins primarily responsible for the viscoelastic properties of mucus. Sialic acid residues on mucin have a pKa of 2.6, making them negatively charged at physiological pH (CitationDeacon et al 2000; CitationWang et al 2000). Therefore, the presence of mucus affects free drug permeability as well as the uptake of particulates by forming both a physical barrier to diffusion as well as by interacting electrostatically with cationic molecules, such as chitosan. Derivatives of chitosan such as trimethyl chitosan retain their mucoadhesive properties, albeit to a lesser extent than unmodified chitosan (CitationSnyman et al 2003). In addition, formation of chitosan into micro- and nano-particles also preserves mucoadhesion (CitationBehrens et al 2002; CitationKockisch et al 2003; CitationDhawan et al 2004).
Chitosan is generally considered nontoxic and biodegradable, with an oral LD50 in mice of over 16 g/kg (CitationHirano 1996). Antimicrobial, antifungal, and wound-healing properties have also been reported (CitationSingla and Chawla 2001). The safety of chitosan, its ability to prolong residence time in the gastrointestinal tract through mucoadhesion, and its ability to enhance absorption by increasing cellular permeability have all been major factors contributing to its widespread evaluation as a component of oral dosage forms.
Chitosan solutions as permeation enhancers
The effects of chitosan solutions on intestinal cells have been extensively investigated (CitationSchipper et al 1996, Citation1997, Citation1999). Absorption enhancement was found to depend on both molecular weight and degree of deacetylation. Polymers with low molecular weight and < 65% deacetylation do not increase transport of mannitol across Caco-2 cell layers. On the other hand, polymers with a high degree of deacetylation exhibit greater cellular toxicity. The optimal combination of absorption enhancement and low toxicity was observed for polymers having a moderate degree of deacetylation and a high molecular weight, particularly a chitosan of 170 kDa and 65% deacetylation (CitationSchipper et al 1996) ().
Figure 1 The mean Papp of mannitol across Caco-2 cell monolayers during 60 minutes’ exposure to 50 μg/ml chitosan. The numbers associated with the bars in the graph show the molecular weight of the studied chitosans in kDa. The Papp of mannitol across untreated monolayers was 2.4 ± 0.2 (×10−7) cm/sec, and is indicated in the figure by the horizontal line. Data are given as the mean of 3–4 experiments. Error bars represent standard deviations. Reprinted from CitationSchipper NG, Vårum KM, Artursson P. 1996. Chitosans as absorption enhancers for poorly absorbable drugs. 1: Influence of molecular weight and degree of acetylation on drug transport across human intestinal epithelial (Caco-2) cells. Pharm Res, 13:1686–92. Copyright © 1996, with permission from Springer Science and Business Media.
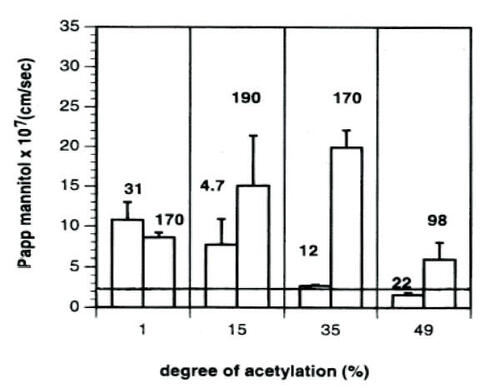
Incubation of Caco-2 cells with 50 μg/mL solutions of chitosan having various molecular weights and degrees of deacetylation (31 kDa, 99% DA and 170 kDa, 65% DA) increased permeation of the drug atenolol across cells (CitationSchipper et al 1999). The fluorescently labeled chitosan was observed to adhere in a layer to cell surfaces. However, the Caco-2 cell model does not include the mucus layer normally present in the gastrointestinal tract. In order to evaluate the behavior of chitosan in the presence of mucus, drug uptake was studied in the mucus-secreting goblet cell line HT29-H. In this instance, uptake of mannitol was enhanced by the presence of chitosan, but this enhancement was less than that observed in Caco-2 cells. Furthermore, binding of chitosan to the cell surface was reduced. Both cell surface binding and drug absorption could be improved by partially removing this mucus layer. These in vitro results were similar to what the authors observed in vivo after perfusion of atenolol through rat ileal sections for 2 hours with or without 250 μg/mL chitosan solutions. A modest increase in effective atenolol permeability was observed. However, chitosan did not appear to increase plasma bioavailability over this time frame, as drug concentrations were not significantly different between chitosan and control samples (CitationSchipper et al 1999). In contrast, intra-duodenal instillation of buserelin to rats led to a significant increase in bioavailability when given in a 1.5% (w/v) chitosan hydrochloride solution, pH 6.7 compared with a control buffer solution (CitationLueBen et al 1996). The absolute bioavailability increased from 0.1% +/− 0.1% to 5.1% +/− 1.5% and peak serum buserelin increased from 6.7 +/− 1.7 ng/mL to 364.0 +/− 140.0 ng/mL for the chitosan solution compared with the control.
Modifications to chitosan have also been tested in efforts to improve mucoadhesion and permeation enhancement. Addition of thiol groups increases mucoadhesion through formation of disulfide bonds with cysteine residues on mucin (CitationBernkop-Schnürch et al 2004) and thiolated polymers in combination with reduced glutathione (GSH) can influence permeability by interfering with the closing of tight junctions (CitationBernkop-Schnürch et al 2003). Chitosan has reportedly been modified with thiol groups to form chitosan–4-thio-butylamidine (chitosan-TBA) and chitosan–thioethylamidine (chitosan–TEA). Incubation with 0.5% thiolated chitosan + 5% GSH resulted in increased permeability of the marker rhodamine through both rat and Guinea pig intestinal segments compared with 0.5% unmodified chitosan (CitationBernkop-Schnürch et al 2004; CitationKafedjiiski et al 2006). Modified trimethyl chitosan derivatives have also been evaluated in vivo for absorption enhancing properties. In rats, intra-jejunal administration of the peptide octreotide with 1% (w/v) N-trimethyl chitosan chloride, pH 7.4 resulted in 5 times greater serum bioavailability, while administration with 1% chitosan hydrochloride, pH 7.4 had no effect (CitationThanou et al 2000). Similar results were reported in pigs, in which 10 mg octreotide administration in 5% or 10% (w/v) N-trimethyl chitosan chloride, pH 7.4 increased bioavailability significantly more than administration in 1.5% chitosan hydrochloride, pH 5.5 (CitationThanou, Verhoef, Verheijden, et al 2001).
Although chitosan is generally considered nontoxic, perfusion with 250 μg/mL chitosan solution caused morphological changes to rat small intestine microvilli, as well as increased secretion of mucin from goblet cells (CitationSchipper et al 1999). Mucus may inhibit the effects of chitosan by acting as a diffusion barrier, by forming electrostatic interactions between positively charged chitosan and negatively charge mucins, and/or by degradation through exposure of chitosan to lysozyme contained in the mucus (CitationSchipper et al 1999). However, the authors speculate that chitosan may ultimately deplete the mucus layer of intestinal cells through enhanced secretion of goblet cell mucus, thereby allowing remaining unbound chitosan to interact directly with cell surfaces. In addition, they speculate that formulation of chitosan in drug delivery systems may increase permeation by locally increasing the effective chitosan concentration (CitationSchipper et al 1999).
Chitosan nanoparticles for oral drug delivery
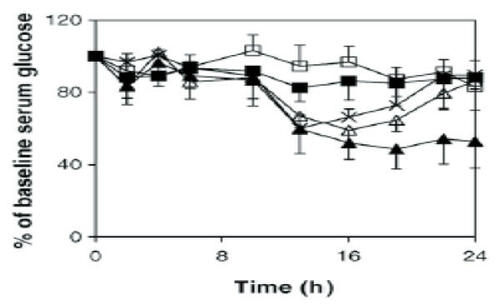
The concept that chitosan in formulations such as nanoparticles may be more efficient than chitosan solution at enhancing protein uptake is supported by several recent studies (CitationFernandez-Urrusuno et al 1999; CitationPan et al 2002; CitationMa and Lim 2003; CitationMa et al 2005). Incubation of Caco-2 cells with chitosan–insulin nanoparticles resulted in greater cell binding and uptake compared with a chitosan–insulin solution (CitationMa and Lim 2003). While most chitosan in solution remained extracellular, a significant amount of fluorescently labeled nanoparticles was localized inside the cells after a 2-hour incubation, principally near the apical surface. Chitosan nanoparticles could also decrease the TEER of the cell monolayers at both pH 5.3 and 6.1, although to a lesser degree than the chitosan solution. Administration of these chitosan–insulin nanoparticles to diabetic rats led to prolonged reductions in serum glucose levels (CitationMa et al 2005). Administration of 50 U insulin/kg as nanoparticles (pH 5.3) decreased glucose levels to about 60% of baseline, while administration of a chitosan–insulin solution was ineffective. Delivery of 100 U/kg chitosan–insulin nanoparticles (pH 5.3) decreased glucose levels to about 50% of baseline starting around 12 hours after delivery and maintained this level until at least 24 hours (). Delivery of 100 U/kg chitosan–insulin nanoparticles (pH 6.1) resulted in a faster onset of action (2 hours after delivery) but less of a decrease in glucose levels (60%–75% of baseline). Fluorescently labeled nanoparticles were also observed in association with rat intestinal epithelia and some particles had been internalized 3 hours after delivery.
Figure 2 Serum glucose levels of streptozotocin-induced diabetic rats (mean ± SD, n = 8) after the oral administration of F5.3np at insulin doses of 50 U/kg (Δ) and 100 U/kg (▲); insulin solution at 50 U/kg (□) and 100 U/kg (■); F5.3np after cross-flow filtration, whose insulin dose was 100 U/kg before cross-flow (×). Reprinted from CitationMa Z, Lim TM, Lim L-Y. 2005. Pharmacological activity of peroral chitosan-insulin nanoparticles in diabetic rats. Int J Pharm, 293:271–80. Copyright © 2005, with permission from Elsevier.
These results confirm prior reports on the effectiveness of chitosan–insulin nanoparticles (CitationFernandez-Urrusuno et al 1999; CitationPan et al 2002). Oral administration of insulin to diabetic rats in the form of chitosan nanoparticles approximately 300 nm in size and positively charged led to reduced plasma glucose levels 10 hours after delivery (CitationPan et al 2002). At a dose of 14 U insulin/kg, rats exhibited a greater drop in glucose than was achieved using a control insulin–chitosan solution. An even greater decrease in glucose levels was observed by increasing the nanoparticle dose to 21 U insulin/kg. The authors theorize that chitosan nanoparticles may protect insulin from gastrointestinal degradation and may enhance uptake through mucoadhesion and/or permeation enhancement.
Similarly, CitationFernandez-Urrusuno et al (1999) reported the formation of 300–400-nm positively charged insulin–chitosan nanoparticles formed using ionic gelation of chitosan hydrochloride with pentasodium tripolyphosphate. Mucosal (intranasal) administration of the insulin-chitosan nanoparticles to rabbits caused a 40% drop in blood glucose level. This drop was significantly greater than was observed following intranasal administration of an insulin–chitosan solution, despite using a greater dose of chitosan in the solution than in the nanoparticles (0.43 mg/kg vs 0.16 mg/kg).
In addition to hydrophilic drugs, lipophilic drugs such as cyclosporine A have also been efficiently encapsulated in chitosan nanoparticles (CitationEl-Shabouri 2002). Oral administration to dogs of cyclosporine A encapsulated in chitosan hydrochloride nanoparticles (~150 nm, + 30 mV) led to peak cyclosporine A plasma levels of 2.8 μg/mL at 3 hours, falling off to less than 1.0 μg/mL at 24 hours. Chitosan nanoparticle delivery led to 73% greater relative bioavailability of drug compared with the commercial microemulsion Neoral®, while delivery of similarly sized cationic nanoparticles formed with gelatin-A led to 18% greater bioavailability. However, delivery of smaller negatively charged sodium glycocholate nanoparticles led to decreased bioavailability, indicating the cationic nature of the particles may be important for efficacy.
Coating lipid nanoparticle formulations with a chitosan layer can also confer beneficial effects. Intra-gastric delivery of chitosan-coated lipid nanoparticles containing calcitonin to rats led to a 27% drop in serum calcium level that was maintained for 24 hours (CitationPrego et al 2005). This drop in calcium was significantly greater than that achieved by delivery of calcitonin solution or by delivery of calcitonin in the un-coated lipid nanoemulsion. Recently, CitationTakeuchi et al (2005) compared the properties of chitosan-coated multi-lamellar liposomes (CS-Lip, ~4–4.6 μm) with chitosan-coated, submicron-sized liposomes (ssCS-Lip, ~300–400 nm) after intra-gastric delivery to rats. They reported improved mucoadhesion and gastrointestinal retention of the smaller particles. In addition, confocal microscopy revealed that the submicron-sized particles were able to penetrate the mucus layer of enterocytes, unlike the multi-lamellar liposomes. Administration of calcitonin (500 IU/rat) in the form of chitosan-coated ss-Lip resulted in a significantly greater drop in blood calcium levels than was achieved with CS-Lip or control calcitonin solution and lasted up to 120 hours.
The ability of chitosan to chelate metal ions has also been exploited to inhibit metallo-proteinase enzymes in the lumen and brush border. Unfortunately, CitationLueBen et al (1997) reported that, despite binding metal cations, chitosan alone was not sufficient to inhibit enzymes such as trypsin and carboxypeptidase B. This led to the development of modified chitosans with greater metal complexing abilities. Chitosan–EDTA conjugates displayed increased binding of divalent cations and inhibition of aminopeptidase N and carboxypeptidase A (zinc-dependent proteases). However, despite binding calcium, these conjugates were not effective against calcium-dependent serine proteases including trypsin, chymotrypsin, and elastase (CitationBernkop-Schnürch and Krajicek 1998).
Competitive enzyme inhibitors such as antipain or chymostatin can also be covalently attached to chitosan through the amine groups, while still allowing chitosan to retain some mucoadhesive properties (CitationBernkop-Schnürch and Scerbe-Saiko 1998). The use of enzyme inhibitors conjugated directly to the chitosan may improve drug bioavailability by localizing the inhibitory effect to the site of drug uptake, as well as reducing toxicity from administration of the inhibitor. A substantial amount of insulin (40%–60%) remained undegraded 4.5 hours after incubation in artificial intestinal fluid when it was encapsulated in a chitosan–EDTA matrix in which 10% of the chitosan–EDTA was substituted with a conjugate containing a Bowman-Birk enzyme inhibitor. In contrast, 90% insulin was degraded from the chitosan–EDTA matrix without the additional inhibitor (CitationBernkop-Schnürch 2000). The use of such chitosan–inhibitor conjugates may represent a valuable approach to improve protection from drug degradation and achieve more effective oral drug delivery.
Chitosan nanoparticles for oral gene delivery
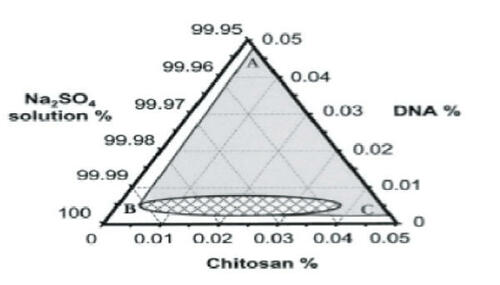
Increasingly, nucleic acids are being applied as drugs, both for vaccination and therapeutic gene expression. Chitosan–DNA nanoparticles may be readily formed by coacervation between the positively charged amine groups on the chitosan and negatively charged phosphate groups on the DNA (CitationLeong et al 1998; CitationMao et al 2001). CitationMao et al (2001) explored the conditions under which chitosan–DNA nanoparticles formed and found that discrete particles formed at chitosan concentrations of 50–400 μg/mL and DNA concentrations of 40–80 μg/mL, where buffer solutions were at pH 5.5 and temperature was 55°C. Sodium sulfate (25 mM) was added as a desolvating agent to enhance the phase separation (). Particle formation was dependent on the N/P ratio (amine groups on chitosan/phosphate groups on DNA) and N/P ratios of 3–8 yielded 150–250-nm particles with surface charges of approximately + 15 mV.
Figure 3 Ternary phase diagram of complex coacervation between pRE-luciferase plasmid and chitosan at 55°C in 50 mM Na2SO4. Sodium sulfate solution was regarded as one component, since the concentration change in the experiment range was minimal. The region to the right of line ABC depicts the conditions under which phase separation occurred. The concentration ranges in the small grid area yielded distinct particles as observed under a phase contrast microscope. Reprinted from CitationMao H-Q, Roy K, Troung-Le VL, et al. 2001. Chitosan-DNA nanoparticles as gene carriers: synthesis, characterization and transfection efficiency. J Control Release, 70:399–421. Copyright © 2001, with permission from Elsevier.
The effect of chitosan molecular weight and charge ratio on particle formation was tested by depolymerizing 102 kDa, 89.4% deacetylated chitosan into smaller oligomers (CitationMacLaughlin et al 1998). At a +/− charge ratio of 6:1, complex size decreased from approximately 500 nm for a 540 kDa, 82.3% deacetylated chitosan to a plateau of around 100 nm for the depolymerized chitosans of 32, 24, and 7 kDa. Incubation of the complexes with saline and 10% serum resulted in dissociation of the particles formed at all charge ratios with 7 kDa chitosan. On the other hand, only high-molecular-weight complexes (540 and 102 kDa chitosans) formed at low charge ratios (0.5:1 and 1:1) dissociated, while 2:1 complexes remained stable and 6:1 complexes aggregated. At a +/− ratio of 2:1, optimal transfection of Cos-1 cells was achieved in the absence of serum with 102 kDa chitosan particles, and in the presence of 10% serum by 540 kDa chitosan particles. However, the levels achieved were still over 200 times lower than were achieved with Lipofectamine. The addition of an endosomolytic peptide increased expression by only a limited extent (about 4 times). However, delivery of the chitosan–endosomolytic nanoparticles by direct instillation into the intestinal lumen of rabbits yielded greater chloramphenicol acetyl transferase (CAT) expression than administration of either a lipid-DNA formulation or naked plasmid DNA.
The effect of degree of deacetylation of chitosan on nanoparticle formation has also been investigated (CitationKiang, Bright, et al 2004). For chitosans of the same molecular weight (390 kDa), decreasing the degree of polymer deacetylation required increasing the amount of chitosan in order to achieve complete DNA complexation. While 90% deacetylated chitosan could fully complex DNA at a +/− ratio of 3.3:1, the required +/− ratio increased to 9:1 for chitosan with a 62% degree of deacetylation. In addition, decreasing the degree of deacetylation of the polymer produced nanoparticles that were less stable in the presence of serum proteins, resulting in lower levels of in vitro transfection. On the other hand, gene expression after intramuscular injection in mice was enhanced for the less stable 62% deacetylated nanoparticles compared with 70% and 90% deacetylated chitosan particles.
Complexation of DNA with low-molecular-weight, highly deacetylated chitosan (5 kDa, 99% DA) has also been reported (CitationLiu et al 2005). The results of circular dichroism suggested that chitosan binds to the minor groove of DNA, although DNA retains its native B conformation. As expected, DNA binding was enhanced at acidic pH owing to protonation of chitosan amines and was substantially reduced at pH 12. A dependence of complex formation on N/P ratio was also observed. At pH 5.4, a chitosan:DNA charge ratio of 1:4 produced only aggregates and free DNA. At a ratio of 2:1 both spherical and irregularly shaped complexes were observed, while an increase in charge ratio to 8:1 produced compact spherical particles of less than 100 nm.
The reactive amine groups on chitosan can serve as functional groups for the attachment of a variety of potential targeting ligands. The transferrin receptor is responsible for iron uptake and is present on many mammalian cells. However, attachment of transferrin to chitosan nanoparticles increased transfection efficiency only modestly (CitationMao et al 2001). Luciferase levels for the modified nanoparticles were only 2–3 times the levels of unmodified particles in HEK293 cells and less than 50% higher in HeLa cells. On the other hand, attachment of the knob domain of the adenovirus capsid fiber protein produced levels about 6–7 times greater than unmodified particles in HEK293 cells and 130 times greater in HeLa cells (CitationMao et al 2001). Incubation with free knob reduced transfection to basal levels, indicating that the uptake was most likely via receptor-mediated endocytosis. However, these levels were still below those achieved with Lipofectamine.
While transferrin or knob conjugations have the potential to increase uptake in a variety of tissues, galactose conjugation has been proposed as a means to increase specific hepatocyte targeting via the asialoglycoprotein receptor (CitationGao et al 2003; CitationKim et al 2004). Conjugation of lactobionic acid to low-molecular-weight chitosan (~21 kDa) produced galactosylated chitosan able to complex DNA into nanoparticles (CitationGao et al 2003). These particles had a mean size of approximately 350 nm while particles from unmodified low-molecular-weight chitosan were around 220 nm. Transfection of galactosylated chitosan nanoparticles into the liver cell lines HepG2, L-02, and SMMC-7721 modestly improved levels of β-galactosidase expression compared with unmodified chitosan nanoparticles. Transfection of HeLa cells lacking the asialoglycoprotein receptor was very low for both modified and unmodified chitosan nanoparticles. Increasing the degree of galactosylation from 0% to 8.3% resulted in about 6 times greater transfection in HepG2 cells, but not in HeLa cells, suggesting that the galactosylated particles were internalized via receptor mediated endocytosis.
Lactobionic acid was also conjugated to a low-molecular-weight, water-soluble chitosan (CitationKim et al 2004). Nanoparticles formed at an N/P ratio of 10 had a size of approximately 100 nm and a charge of + 6 mV, indicating suitability for uptake through fenestrated liver endothelium. Transfection levels were low, however, and were carried out in the presence of calcium, which has been shown to enhance in vitro transfection. Similar to the results reported by CitationGao et al (2003), luciferase expression was the same for both galactosylated and nongalactosylated chitosan nanoparticles in HeLa cells but was substantially enhanced in HepG2 cells. In addition, transfection was inhibited by the presence of free galactose, indicating the uptake was likely through the asialoglycoprotein receptor. Transfection of the galactosylated chitosan nanoparticles could be further enhanced by addition of polyethylenimine (PEI), which may act to disrupt endosomes through pH buffering (CitationKim et al 2005). Addition of PEI to both galactosylated and nongalactosylated chitosan resulted in nanoparticles with increased zeta potentials and improved transfection in both HeLa and HepG2 cells. In HepG2 cells luciferase levels were greater for galactosylated chitosan with 1 or 2 μg added PEI than for unmodified chitosan, while addition of 5 μg of PEI produced similar levels of transfection for both chitosan formulations. Transfection levels for both chitosans were also similar in HeLa cells, which lack the asialoglycoprotein receptor. The results suggest that uptake occurred through the asialoglycoprotein receptor at lower PEI levels but was receptor-independent with higher PEI coating. Interestingly, toxicity of the galactosylated chitosan PEI nanoparticles was less than that of plain PEI nanoparticles, while transfection levels were similar or greater, indicating that such synergistic formulations may be able to take advantage of the desirable properties of several polymers.
Potential endosomolytic agents other than PEI have also been tested in combination with chitosan. Co-encapsulation of chloroquine into chitosan–DNA nanoparticles resulted in the transfection of 3 times more HEK293 cells than control nanoparticles, although fluorescence intensity was 10–20 times lower than cells transfected with Lipofectamine (CitationMao et al 2001). Similarly, there was no significant difference in Luciferase expression between HEK293 cells transfected with chitosan nanoparticles with or without chloroquine, indicating only a modest effect. The pH-sensitive polymer poly(propyl acrylic acid) (PPAA), which can disrupt membranes at acidic pH (CitationCheung et al 2001), has also been combined with chitosan to form DNA nanoparticles that exhibited enhanced transfection in both HEK293 cells and HeLa cells (CitationKiang, Bright, et al 2004). Chitosan, DNA, and PPAA were co-localized at 24 hours, while at later time points DNA and PPAA appeared diffuse in the cell and did not co-localize with lysosomes, indicating escape from the endosomal–lysosomal pathway.
These in vitro studies with reporter genes showed that chitosan could be readily formulated into DNA nanoparticles able to transfect some cell lines better than others. The size and stability of the particles could be influenced by the molecular weight of the chitosan, the degree of deacetylation, and the charge ratio at which the particles were formed. Further modifications to the nanoparticles through ligand conjugation or the addition of endosome-disrupting molecules may further overcome some of the transport barriers to cell transfection and improve expression levels, although results so far have been modest. However, these studies established the basis for using such chitosan particles to deliver genes in vivo both as vaccines and in disease treatment.
Delivery of chitosan–DNA vaccines
One area of oral DNA delivery that has received considerable attention is DNA vaccination. Protein-based subunit vaccines primarily activate humoral immune responses that lead to the production of circulating antibodies against the delivered antigen. However, transfection with antigen-encoding DNA can generate both antibody-based and cell-mediated immune responses (CitationLeitner et al 2000). In addition, unmethylated bacterial CpG motifs in the plasmid DNA act as adjuvants to stimulate the immune response (CitationKrieg 2001). Oral vaccine delivery may be particularly desirable not only for patient preference, but also for the ability to generate immune responses at mucosal surfaces, where many pathogens normally invade (CitationClark et al 2001).
Orally administered particulate vaccines are generally thought to be internalized by antigen-sampling membranous (M) cells in intestinal Peyer’s patches. These M cells have a thinner glycocalyx and less organized microvilli than enterocytes and are known to internalize and transcytose particles to underlying lymphocytes and antigen-presenting cells (CitationNeutra et al 1987; CitationJepson et al 1996; CitationClark et al 2001). Particles up to 10 μm in diameter can be internalized into Peyer’s patches and particles less than 5 μm can be transported to draining lymph nodes and the spleen (CitationEldridge et al 1990).
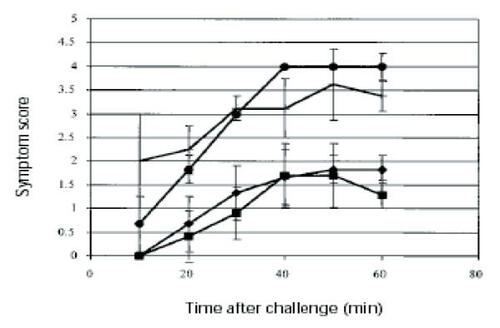
Oral administration to mice of chitosan–DNA nano-particles containing the gene for the dominant peanut allergen Arah2 resulted in the production of secretory IgA and serum IgG2a, as well as a reduced increase in IgE (CitationRoy et al 1999). This immune response was not observed for mice given naked plasmid DNA. Delivery of the chitosan-DNA nanoparticles also mitigated the anaphylactic response to peanut challenge, possibly through redirection of the immune response away from an allergic, IgE-based response to a more TH1-dominated response ().
Figure 4 Anaphylactic response of mice after Arah2 challenge. Mice (n = 6) were immunized with a single dose of chitosan-pArah2 nanoparticles (single dose, G1; ◆); with 2 doses (1 week apart) of chitosan-pArah2 nanoparticles (G2; ■); with “naked” pArah2 (G3; ●); or were not immunized (G4; no symbol). Mice were then sensitized with oral and intraperitoneal doses of crude peanut extract, challenged intraperitoneally with recombinant Arah2 protein, and anaphylaxis was then scored on a scale of 0–5. Results represent average anaphylactic response from 2 separate experiments. Reprinted from CitationRoy K, Mao HQ, Huang SK, et al. 1999. Oral gene delivery with chitosan–DNA nanoparticles generates immunologic protection in a murine model of peanut allergy. Nat Med, 5:387–91. Copyright © 1999, with permission from Nature Publishing Group.
However, in contrast to the assumption that M cells are the primary absorptive cells for particulates, delivery of LacZ-containing chitosan nanoparticles revealed staining in intestinal enterocytes (CitationRoy et al 1999). The ability of nanoparticles to be internalized by non-Peyer’s patch intestinal tissue has been previously reported (CitationDesai et al 1996; CitationDesai et al 1997). PLGA particles up to 10 μm could be internalized by Caco-2 cells, considered a model for intestinal epithelium (CitationDesai et al 1997). PLGA particles (100 nm–10 μm) could also be internalized by both rat Peyer’s patch and nonPeyer’s patch intestinal tissues, although uptake decreased substantially with increasing size (CitationDesai et al 1996). Uptake is likely to depend not only on size, but also on polymer composition. More hydrophobic polystyrene and poly(methyl methacrylate) microparticles were absorbed to a greater extent than PLGA particles, while cellulose-based materials showed poor absorption (CitationEldridge et al 1990). In the study by CitationEldridge et al (1990), uptake by Peyer’s patches did appear to predominate, as microparticles were reportedly not observed in other tissues. However, it may be that the size of the chitosan nanoparticles (~100–200 nm) (CitationRoy et al 1999) coupled with their hydrophobicity and the mucoadhesive properties of chitosan led to increased uptake by the far more abundant enterocytes.
Chitosan–DNA nanoparticles have also been successfully used to generate an immune response to the dust mite allergen Der p 1 (CitationChew et al 2003). Oral delivery of two feedings of chitosan nanoparticles containing 50 μg Der p 1 (114–222) DNA was followed 13 weeks later by an intramuscular boost with 50 μg Der p 1 (1–222) DNA in saline and electroporation. While intramuscular injection alone was unable to generate immune responses to the right domain of the Der p 1 antigen (114–222), oral priming led to detectable levels of IgG2a and low levels of IgA against this domain. IgG1 was not detected, suggesting a shift to a TH1-dominated immune response, similar to what was observed by CitationRoy et al (1999). Oral delivery of naked Der p1 DNA or empty vector DNA in chitosan nanoparticles did not lead to anti-Der p 1 antibody responses.
Interestingly, while CitationRoy et al (1999) reported increased antibody levels by 3–4 weeks, the chitosan-Der p 1 formulation did not result in antibody detection until 8 weeks after delivery. The authors speculate that the delayed appearance of the antibodies may be due to the 10-fold higher concentration of chitosan used (0.2%), which may have retarded DNA release from the particles and delayed the onset of action, highlighting the effect formulation conditions can have on the kinetics of transgene expression.
Oral delivery of chitosan DNA nanoparticles was also tested as a vaccine strategy against the intracellular parasite Toxoplasma gondii (CitationBivas-Benita et al 2003). In this study, both chitosan microparticles loaded with the parasite protein GRA1 and chitosan nanoparticles formed with GRA1 DNA were compared for their ability to generate anti-GRA1 antibodies. Low levels of anti-GRA1 were detected in sera 1 month after intragastric delivery of 3 × 50 μg protein microparticles, although no response was detected for mice given 3 × 50 μg DNA nanoparticles. Boosting with a second round of intragastric micro- or nano-particles increased the antibody response somewhat, although not as well as boosting by intramuscular injection of DNA. In contrast to the studies above, which reported the generation of TH1-type immune responses following oral delivery of chitosan–DNA nanoparticles, the authors reported the IgG2a/IgG1 ratio after oral priming with chitosan-DNA nanoparticles and boosting by intramuscular injection was 0.1, indicating a shift towards a TH2 driven response that would not be protective against T. gondii. In this study, the authors formed the chitosan DNA nanoparticles with an N/P ratio of 6:1 and examined antibody responses at 4 weeks. This ratio is higher than that used by CitationRoy et al (1999). Similar to the finding of CitationChew et al (2003), who did not report antibody generation until 8 weeks, it may be that the GRA1 nanoparticles were too stable to release DNA sufficiently over the time course examined. The GRA1 nanoparticles reportedly did not release any DNA after 8 days incubation in PBS pH 7.2, 25°C, suggesting the particles were fairly stable and likely to release DNA only slowly by enzymatic chitosan degradation (CitationBivas-Benita et al 2003).
Chitosan itself may also have properties that affect immune responses, influencing its use in vaccine systems. Induction of varying levels of TNF-alpha from monocytes was reported for chitosans of 40%–80% deacetylation and 3.5–50 kDa and depended on molecular weight, degree of deacetylation, and neutral solubility (CitationOtterlei et al 1994). In addition, purified influenza surface antigens given intra-nasally to mice with 1% (w/v) chitosan glutamate solution resulted in increased levels of IgG, IgA, and antibody-secreting lymphocytes compared with intra-nasal delivery of the surface antigens alone (CitationBacon et al 2000). Intravenous injection of phagocytosable (1–10 μm) chitosan particles primed alveolar macrophages to release a burst of superoxide anion, although to a lower extent than injection of chitin particles (CitationShibata et al 1997). However, soluble chitosan and chitin oligosaccharides did not have this effect and culturing mouse spleen cells with either chitosan particles or soluble chitosan oligosaccharides did not lead to detectable levels of the macrophage-activating cytokine IFN-gamma. Furthermore, chitosan–DNA nanoparticles formed using the method of CitationMao et al (2001) did not result in the secretion of cytokines TNF-alpha, IL-1 beta, IL-6, or IL-10 after incubation in a macrophage cell line (CitationChellat et al 2005). However, oral feeding of 1–3 mg chitosan solution (80 kDa, 85% deacetylation) to rats resulted in chitosan uptake by macrophages and dendritic cells and increased levels of IL-4 and TGF-beta mRNA in Peyer’s patch cells. Feeding 3 mg (but not 1 mg), harvesting the cells, and restimulating them with 10 μg/mL chitosan also resulted in increased IL-10 secretion by Peyer’s patch, mesenteric lymphocyte, and spleen cells. Therefore it seems that chitosan may have application as a vaccine adjuvant, but that these properties are likely to depend on the type and dose of chitosan used, as well as the delivery method. A fuller understanding of the nature of chitosan-mediated immune modulation, particularly in the context of nanoparticle delivery, will require additional investigation.
Use of chitosan–DNA nanoparticles to deliver therapeutic genes
In addition to oral vaccination, another attractive application is the oral delivery of DNA for therapeutic gene expression as a so-called “gene pill”. The benefits of such a delivery system have been delineated by CitationSheu et al (2003) and include safety, patient compliance, and dose regulation. It is worth noting, however, that one of the arguments proposed for increased safety from an oral nonviral DNA pill is targeting to short-lived gut epithelial cells and lack of systemic cell transfection. However, plasmid DNA can be detected in systemic tissues after oral delivery, albeit at very low copy numbers (CitationBowman et al 2005) and oral delivery of DNA vaccines can produce detectable systemic immune responses, indicating that the effects of an orally delivered formulation may not be locally confined.
We have also explored this oral gene delivery system for gene therapy of hemophilia, by delivering the Factor IX gene to mice through feeding. The DNA nanoparticles were synthesized by complexing chitosan with human factor IX DNA that was driven by a β-actin promoter. The initial dose of 25 μg DNA led to a decline of the hFIX level in plasma of C57bl/6J mice from 37 ng/mL on day 7 to 21 ng/mL on day 28, even with a repeat feeding at day 14 (CitationOkoli et al 2000). The decline appeared to coincide with the rise in anti-hFIX antibody level. At all time points, hFIX levels in control mice, which were fed the same dose of naked DNA, were not significantly different from those of naïve mice.
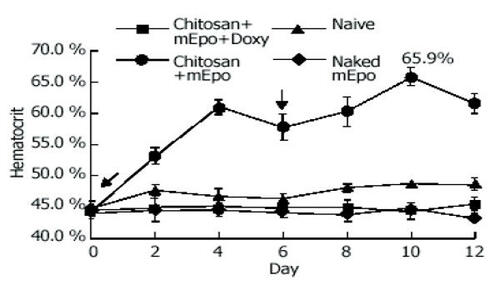
A therapeutic effect following oral administration was also demonstrated for delivery of chitosan nanoparticles containing the gene for murine erythropoietin (mEPO) (CitationChen et al 2004). Using chitosan with a molecular weight of 300 kDa and the method of particle formulation reported by CitationMao et al (2001), the authors formed DNA nanoparticles of approximately 100 nm in diameter with a charge of + 10 mV at pH 5.7. Oral delivery of the chitosan–mEPO nanoparticles at a dose of 50 μg DNA led to increased hematocrit. This rise in hematocrit was not detected in naive mice or in mice given naked mEPO plasmid DNA ().
Figure 5 mEpo expression and its physiological effect test. Hematocrit was measured every 2 days in mice fed with (●) chitosan-mEPO and doxycycline (200 μg/mL), n = 9; chitosan-mEpo alone, n = 3; (◆) doxycycline (200 μg/mL) alone, n = 4; (■) naked mEpo and doxycycline (200 μg/mL), n = 5. ↓ indicates the mice fed with “naked” mEpo DNA or chitosan-Epo. Reprinted from CitationChen J, Yang WL, Li G, et al. 2004. Transfection of mEpo gene to intestinal epithelium in vivo mediated by oral delivery of chitosan-DNA nanoparticles. World J Gastroenterol, 10:112–16. Copyright © 2004, with permission from Elsevier.
The gene for human coagulation factor VIII (FVIII) has also been successfully administered to mice in the form of chitosan-DNA nanoparticles. Our research has focused on using chitosan with a molecular weight of 390 kDa and both 70% and 84% deacetylation. While we have also employed the method of CitationMao et al (2001), in our hands, nanoparticles formed with chitosan and the approximately 10 kb factor VIII plasmid DNA were around 250–300 nm in size, with a zeta potential of + 10 mV at pH 5.7. Delivery of 600 μg FVIII DNA in the form of chitosan nanoparticles led to plasma factor VIII levels equivalent to 0.02–0.04 IU/mL (2%–4% activity) in hemophilia A mice. In addition, delivery of chitosan–FVIII DNA nanoparticles led to significantly greater levels of phenotypic bleeding correction than delivery of naked plasmid DNA, with 13/20 mice fed with high (600 μg) or medium (250 μg) DNA doses exhibiting bleeding correction 1 month after nanoparticle administration. On the other hand, delivery of 50 μg of chitosan–FVIII DNA nanoparticles led to detection of FVIII plasmid DNA in multiple tissues, but did not appear to lead to significant levels of plasma FVIII protein (CitationBowman et al 2005). These are substantially larger DNA doses than those employed by CitationOkoli et al (2000) (25 μg DNA) and CitationChen et al (2004) (50 μg DNA). However, factor VIII is a large protein that undergoes multiple processing and glycosylation events. It also must associate with the carrier protein von Willebrand factor to prolong its plasma half-life. Therefore, it may have greater barriers to functional gene expression than smaller proteins such as erythropoietin or factor IX.
Conclusion
Many of the issues facing effective oral protein and gene delivery are similar. As discussed above, these include the need to protect the protein or gene from the damaging environment of the gastrointestinal tract and to facilitate uptake into cells. In particular, the presence of mucus in the GI tract may be a complicating factor for effective particle delivery. Chitosan mucoadhesion can locally increase the concentration of a drug and thus increase the driving force for drug diffusion into cells, which may be advantageous even if the nanoparticles themselves remain trapped extracellularly in mucus. However, it seems likely that gene expression from chitosan–DNA nanoparticles is mediated by cellular uptake of intact particles followed by intracellular DNA release. In this situation, mucoadhesion may be a double-edged sword, prolonging residence time, but possibly entrapping particles, preventing them from reaching cell surfaces, and causing them to be swept from the intestine. The adsorption of gastrointestinal mucins onto the surfaces of orally administered chitosan nanoparticles may also affect surface charges and interfere with cell binding and internalization, particularly at lower pH where the particles are cationic. The interaction with and diffusion through mucus of many chitosan systems have not been reported. More research needs to be conducted on these topics for the rational design of the next generation of oral chitosan drug and gene delivery systems.
However, the studies above indicate the feasibility of using chitosan nanoparticles to deliver poorly bioavailable drugs or to achieve in vivo gene expression. Mechanistic insight and information such as barriers in the macroscopic transport of these nanoparticles across the mucosal surface, nanoparticle biodistribution in different tissues, types of cells transfected, transgene expression kinetics, and extra- and intracellular release of the drug and DNA from the nanoparticles are needed to advance the chitosan delivery system. Different groups have focused on different molecular weight chitosans, with different degrees of deacetylation, producing nanoparticles of varying sizes and charge ratios. Some of the differences reported in the levels and time-course of protein release or gene expression from these particles may be due to such formulation differences. Further understanding of the parameters influencing nanoparticle formation and uptake may allow researchers to identify the best combination for a particular application. While much work has been done in the last few years to achieve successful oral drug and gene delivery, the field has yet to progress beyond animal models and demonstrate relevant efficacy in humans. However, the many advantages of chitosan, including safety, biodegradability, ease of modification, ease of DNA or protein complex formation, widespread availability, and low cost justify the continuing development of this promising drug and gene delivery system.
Acknowledgements
The funding support of NIH (EB002849) is acknowledged.
References
- AllemannELerouxJ-CGurnyRPolymeric nano- and microparticles for the oral delivery of peptides and peptidomimeticsAdv Drug Deliv Rev1998341718910837677
- ArturssonPLindmarkTDavisSSEffect of chitosan on the permeability of monolayers of intestinal epithelial cells (Caco-2)Pharm Res1994111358617816770
- BaconAMakinJSizerPJCarbohydrate biopolymers enhance antibody responses to mucosally delivered vaccine antigensInfect Immun20006857647010992483
- BehrensIPenaAIAlonsoMJComparative uptake studies of bioadhesive and non-bioadhesive nanoparticles in human intestinal cell lines and rats: the effect of mucus on particle adsorption and transportPharm Res20021911859312240945
- Bernkop-SchnürchAKrajicekMEMucoadhesive polymers as platforms for peroral peptide delivery and absorption: synthesis and evaluation of different chitosan-EDTA conjugatesJ Control Release199850215239685888
- Bernkop-SchnürchAScerbe-SaikoASynthesis and in vitro evaluation of chitosan-EDTA-protease-inhibitor conjugates which might be useful in oral delivery of peptides and proteinsPharm Res19981526399523313
- Bernkop-SchnürchAChitosan and its derivatives: potential excipients for peroral peptide delivery systemsInt J Pharm200019411310601680
- Bernkop-SchnürchAKastCEGuggiDPermeation enhancing polymers in oral delivery of hydrophilic macromolecules: thiomer/GSH systemsJ Control Release20039395103
- Bernkop-SchnürchAGuggiDPinterYThiolated chitosans: development and in vitro evaluation of a mucoadhesive, permeation enhancing oral drug delivery systemJ Control Release2004941778614684281
- Bivas-BenitaMLaloupMVersteyheSGeneration of Toxoplasma gondii GRA1 protein and DNA vaccine loaded chitosan particles: preparation, characterization, and preliminary in vivo studiesInt J Pharm2003266172714559390
- BowmanKSarkarRRautSOral delivery of non-viral DNA nanoparticles for hemophilia A. American Society of Gene Therapy, 8th Annual Meeting [abstract]Mol Ther200511(Suppl 1) Abs 779
- ChellatFGrandjean-LaquerriereALe NaourRMetalloproteinase and cytokine production by THP-1 macrophages following exposure to chitosan-DNA nanoparticlesBiomaterials2005269617015369684
- ChenJYangWLLiGTransfection of mEpo gene to intestinal epithelium in vivo mediated by oral delivery of chitosan-DNA nanoparticlesWorld J Gastroenterol2004101121614695780
- CheungCYMurthyNStaytonPSA pH-sensitive polymer that enhances cationic lipid-mediated gene transferBioconjug Chem2001129061011716680
- ChewJLWolfowiczCBMaoHQChitosan nanoparticles containing plasmid DNA encoding house dust mite allergen, Der p 1 for oral vaccination in miceVaccine2003212720912798609
- ClarkMAJepsonMAHirstBHExploiting M cells for drug and vaccine deliveryAdv Drug Deliv Rev2001508110611489335
- DeaconMPMcGurkSRobertsCJAtomic force microscopy of gastric mucin and chitosan mucoadhesive systemsBiochem J20003485576310839986
- DesaiMPLabhasetwarVAmidonGLGastrointestinal uptake of biodegradable microparticles: effect of particle sizePharm Res1996131838458987081
- DesaiMPLabhasetwarVWalterEThe mechanism of uptake of biodegradable microparticles in Caco-2 cells is size dependentPharm Res1997141568739434276
- DhawanSSinglaAKSinhaVREvaluation of mucoadhesive properties of chitosan microspheres prepared by different methodsAAPS PharmSciTech20046717 5 article
- DodaneVKhanMAMerwinJREffect of chitosan on epithelial permeability and structureInt J Pharm1999182213210332071
- EldridgeJHHammondCJMeulbroekJAControlled vaccine release in the gut-associated lymphoid tissues. I. Orally administered biodegradable microspheres target the Peyer’s patchesJ Control Release19901120514
- El-ShabouriMHPositively charged nanoparticles for improving the oral bioavailability of cyclosporin-AInt J Pharm2002249101812433438
- FangNChanVMaoH-QInteractions of phospholipid bilayer with chitosan: effect of molecular weight and pHBiomacromolecules200121161811777388
- Fernandez-UrrusunoRCalvoPRemunan-LopezCEnhancement of nasal absorption of insulin using chitosan nanoparticlesPharm Res19991615768110554100
- GaoSChenJXuXGalactosylated low molecular weight chitosan as DNA carrier for hepatocyte-targetingInt J Pharm2003255576812672602
- GoldbergMGomez-OrellanaIChallenges for the oral delivery of macromoleculesNat Rev Drug Discov200322899512669028
- HejaziRAmijiMChitosan-based gastrointestinal delivery systemsJ Control Release2003891516512711440
- HiranoSChitin biotechnology applicationsBiotechnol Annu Rev19962237589704098
- HuangMFongCWKhorETransfection efficiency of chitosan vectors: Effect of polymer molecular weight and degree of deacetylationJ Control Release200510639140615967533
- JepsonMAClarkMAFosterNTargeting to intestinal M cellsJ Anat1996189507168982824
- KafedjiiskiKHofferMWerleMImproved synthesis and in vitro characterization of chitosan–thioethylamidine conjugateBiomaterials2006271273516045983
- KiangTWenJLimHWThe effect of the degree of chitosan deacetylation on the efficiency of gene transfectionBiomaterials200425529330115110480
- KiangTBrightCCheungCYFormulation of chitosan-DNA nanoparticles with poly(propyl acrylic acid) enhances gene expressionBiomater Sci Polym Ed200415140521
- KimTHParkIKNahJWGalactosylated chitosan/DNA nanoparticles prepared using water-soluble chitosan as a gene carrierBiomaterials20042537839215020154
- KimTHKimSIAkaikeTSynergistic effect of poly(ethylenimine) on the transfection efficiency of galactosylated chitosan/DNA complexesJ Control Release20051053546615949861
- KockischSReesGDYoungSAPolymeric microspheres for drug delivery to the oral cavity: an in vitro evaluation of mucoadhesive potentialJ Pharm Sci20039216142312884248
- Köping-HöggårdMVårumKMIssaMImproved chitosan-mediated gene delivery based on easily dissociated chitosan polyplexes of highly defined chitosan oligomersGene Ther20041114415215269712
- KotzéAFThanouMMLueBenHLEnhancement of paracellular drug transport with highly quaternized N-trimethyl chitosan chloride in neutral environments: in vitro evaluation in intestinal epithelial cells (Caco-2)J Pharm Sci19998825379950647
- KriegAMImmune effects and mechanisms of action of CpG motifsVaccine2001196182211090712
- LeitnerWWYingHRestifoNPDNA and RNA-based vaccines: principles, progress and prospectsVaccine2000187657710580187
- LeongKWMaoHQTruong-LeVLDNA-polycation nanospheres as non-viral gene delivery vehiclesJ Control Release199853183939741926
- LiuWSunSCaoZAn investigation on the physicochemical properties of chitosan/DNA polyelectrolyte complexesBiomaterials20052627051115585274
- LueBenHLde LeeuwBJLangemeyerMWMucoadhesive polymers in peroral peptide drug delivery. VI. Carbomer and chitosan improve the intestinal absorption of the peptide drug buserelin in vivoPharm Res1996131668728956332
- LueBenHLde LeeuwBJLangemeyerMWMucoadhesive polymers in peroral peptide drug delivery. IV. Polycarbophil and chitosan are potent enhancers of peptide transport across intestinal mucosae in vitroJ Control Release1997451523
- MaZLimL-YUptake of chitosan and associated insulin in Caco-2 cell monolayers: a comparison between chitosan molecules and chitosan nanoparticlesPharm Res20032018121914661926
- MaZLimTMLimL-YPharmacological activity of peroral chitosan-insulin nanoparticles in diabetic ratsInt J Pharm20052932718015778065
- MacLaughlinFCMumperRJWangJChitosan and depolymerized chitosan oligomers as condensing carriers for in vivo plasmid deliveryJ Control Release199856259729801449
- MaoH-QRoyKTroung-LeVLChitosan-DNA nanoparticles as gene carriers: synthesis, characterization and transfection efficiencyJ Control Release20017039942111182210
- MaoSShuaiXUngerFThe depolymerization of chitosan: effects on physicochemical and biological propertiesInt J Pharm2004281455415288342
- NeutraMRPhillipsTLMayerELTransport of membrane-bound macromolecules by M cells in follicle-associated epithelium of rabbit Peyer’s patchCell Tissue Res1987247537463568100
- OkoliGHortelanoGLeongKWOral delivery of plasmid DNA encoding the factor IX gene [abstract]Proceed Intl Symp Control Rel Bioact Mater200027 Abs 234
- OtterleiMVårumKMRyanLCharacterization of binding and TNF-A-inducing ability of chitosans on monocytes: the involvement of CD14Vaccine199412825327526573
- PanYLiYJZhaoHYBioadhesive polysaccharide in protein delivery system: chitosan nanoparticles improve the intestinal absorption of insulin in vivoInt J Pharm20022491394712433442
- PregoCGarciaMTorresDTransmucosal macromolecular drug deliveryJ Control Release20051011516215588901
- RenDYiHWangWThe enzymatic degradation and swelling properties of chitosan matrices with different degrees of N-acetylationCarbohydr Res200534024031016109386
- RoyKMaoHQHuangSKOral gene delivery with chitosan--DNA nanoparticles generates immunologic protection in a murine model of peanut allergyNat Med199953879110202926
- SchipperNGVårumKMArturssonPChitosans as absorption enhancers for poorly absorbable drugs. 1: Influence of molecular weight and degree of acetylation on drug transport across human intestinal epithelial (Caco-2) cellsPharm Res1996131686928956335
- SchipperNGOlssonSHoogstraateJAChitosans as absorption enhancers for poorly absorbable drugs 2: mechanism of absorption enhancementPharm Res19971492399244151
- SchipperNGVårumKMStenbergPChitosans as absorption enhancers of poorly absorbable drugs. 3: Influence of mucus on absorption enhancementEur J Pharm Sci199983354310425384
- SheuERothmanSGermanMThe “Gene Pill” and its therapeutic applicationsCurr Opin Mol Ther20035420714513686
- ShibataYFosterLAMetzgerWJAlveolar macrophage priming by intravenous administration of chitin particles, polymers of N-acetyl-D-glucosamine, in miceInfect Immun1997651734419125555
- SinglaAKChawlaMChitosan: some pharmaceutical and biological aspects- an updateJ Pharm Pharmacol20015310476711518015
- SnymanDHammanJHKotzéAFEvaluation of the mucoadhesive properties of N-trimethyl chitosan chlorideDrug Dev Ind Pharm20032961912602493
- TakeuchiHMatsuiYSugiharaHEffectiveness of submicron-sized, chitosan-coated liposomes in oral administration of peptide drugsInt J Pharm20053031607016125348
- ThanouMVerhoefJCMarbachPIntestinal absorption of octreotide: N-trimethyl chitosan chloride (TMC) ameliorates the permeability and absorption properties of the somatostatin analogue in vitro and in vivoJ Pharm Sci200089951710861597
- ThanouMVerhoefJCJungingerHEChitosan and its derivatives as intestinal absorption enhancersAdv Drug Deliv Rev200150S9110111576697
- ThanouMVerhoefJCVerheijdenJHMIntestinal absorption of octreotide using trimethyl chitosan chloride: studies in pigsPharm Res200118823811474787
- van der MerweSMVerhoefJCVerheijdenJHMTrimethylated chitosan as polymeric absorption enhancer for improved peroral delivery of peptide drugsEur J Pharm Biopharm2004582253515296951
- WangJTauchiYDeguchiYPositively charged gelatin microspheres as gastric mucoadhesive drug delivery system for eradication of H. pyloriDrug Deliv200072374311195431
- ZhangHNeauSHIn vitro degradation of chitosan by a commercial enzyme preparation: effect of molecular weight and degree of deacetylationBiomaterials2001221653811374467
- ZhangHNeauSHIn vitro degradation of chitosan by bacterial enzymes from rat cecal and colonic contentsBiomaterials2002232761612059026