Abstract
Fungal infections and leishmaniasis are an important cause of morbidity and mortality in immunocompromised patients. The macrolide polyene antibiotic amphotericin B (AmB) has long been recognized as a powerful fungicidal and leishmanicidal drug. A conventional intravenous dosage form of AmB, AmB- deoxycholate (Fungizone or D-AmB), is the most effective clinically available for treating fungal and parasitic (leishmaniasis) infections. However, the clinical efficacy of AmB is limited by its adverse effects mainly nephrotoxicity. Efforts to lower the toxicity are based on synthesis of AmB analogues such as AmB esters or preparation of AmB-lipid associations in the forms of liposomal AmB (L-AmB or AmBisome), AmB lipid complex (Abelcet or ABLC), AmB colloidal dispersion (Amphocil or ABCD), and intralipid AmB. These newer formulations are substantially more expensive, but allow patients to receive higher doses for longer periods of time with decreased renal toxicity than conventional AmB. Modifications of liposomal surface in order to avoid RES uptake, thus increased targetability has been attempted. Emulsomes and other nanoparticles are special carrier systems for intracellular localization in macrophage rich organs like liver and spleen. Injectable nanocarriers have important potential applications as in site-specific drug delivery.
Introduction
Systemic fungal infections may randomly be divided into two wide categories: endemic diseases such as histoplasmosis, coccidioidomyosis, and blastomycosis; and opportunistic diseases such as cryptococcosis, aspergillosis, and candidosis, which occur almost entirely in patient with impaired host defenses. Antifungal therapy is based on several factors, such as the causative agent, the succession or incursion of the disease, and so on. Antifungal therapy may have to be administered empirically in febrile neutropenic patients who do not respond to treatment with antibacterial drugs (CitationMedoff et al 1992). Antifungal agents are considerably fewer in number because of emergence of newer pathogenic fungi causing deep-seated mycosis. Clinically used major groups of antifungal agents are polyene antibiotics, azole derivatives, allylamines-thiocarbamates, morpholines and miscellaneous compounds such as 5-fluorocytosine and griseofulvin. Polyenes and azoles are most commonly used. Polyene antifungal agents used for the treatment of human diseases are amphotericin B (AmB) nystatin and natamycin. The only parenteral preparation with broad range of antifungal activity is AmB. Over the past several years, augmented efforts in both basic and clinical antifungal pharmacology have resulted in a number of exclusively new, reengineered or reconsidered compounds, which are at various stages of preclinical and early clinical development (CitationHay 1994; CitationGeorgopapadakou and Walsh 1996; CitationMaesaki 2002).
Similarly, leishmaniasis causes high morbidity and mortality worldwide which is escalating due to its spread as a HIV-associated infection (CitationAlvar et al 1997; CitationHerwaldt 1999; CitationMurray 1999). Due to serious side-effects of pentavalent antimonials (the first-line treatment) such as cardiac and renal toxicity and failures, and development of resistant strains in prevalent areas many practitioners have turned to conventional AmB, a very active antifungal agent, for first-line therapy, which remains almost 100% effective (CitationPearson and Sousa 1996; CitationAlvar et al 1997; CitationSereno et al 2000). Since the parasites are found only within reticulo-endotelial macrophages, the disease is preferably suited for drug delivery therapy. Therefore, the new AmB lipid-based formulations (AmBisome, Abelcet, and Amphocil) have been proposed for the treatment of visceral leishmaniasis (VL) (CitationDavidson et al 1991; CitationBerman et al 1992; CitationPaul et al 1997). The results indicated that these AmB carriers were effective at lower doses with abridged toxicity as compared to the conventional AmB formulation. The US Food and Drug Administration approved L-AmB for the treatment of VL and higly recommended their use for resistant VL in immunocompromised patients (CitationMeyerhoff 1999; CitationEspuelas et al 2002).
Recently, drug delivery systems (DDS) have received substantial attention in the field of drug development. In DDS, pharmacological techniques are used to control pharmacokinetic properties (absorption, distribution, metabolism, and excretion) and to improve the efficacy and safety of a drug. Lipid formulations, such as liposomes and emulsion based carriers, are very highly predictable and are now explored in numerous directions, and several products have already been made commercially available (CitationTomii 2002).
In this review the enhancement of the efficacy of AmB is addressed using lipid-based nanocarriers and paying particular attention towards current commercial liposomal formulations.
Parent amphotericin B
AmB, a lipophilic polyene antifungal agent, was initially secluded from a strain of Streptomyces nodosus in 1956 by Gold et al (CitationGold et al 1956). It is an amphoteric compound composed of a hydrophilic polyhydroxyl chain along one side and a lipophilic polyene hydrocarbon chain on the other (CitationHoeprich 1992). AmB is poorly soluble in water (CitationStorm and van Etten 1997). The drug became available commercially as Fungizone (Bristol-Myers-Squibb, USA) in 1960 as a colloidal suspension of AmB in which the bile salt deoxycholate was used as the solubilizing agent (CitationArikan and Rex 2001).
Role and mechanisms
The interaction of AmB with membrane sterol changes the membrane permeability, which in turn leads to cellular dysfunction and eventually to cell destruction and death (CitationBolard et al 1991; CitationLegrand et al 1992). AmB inhibits membrane enzymes like proton ATPase in fungal cells (CitationSurarit and Shepherd 1987) and Na+/K+-ATPase in mammalian cells (CitationVertut-Doi et al 1988) and this inhibitory activity depletes cellular energy reserves and reduces proliferative ability (CitationSchindler et al 1993). Another possible mechanism by which membrane permeability changes occur is AmB-induced lipid peroxidation of cell membranes (CitationBrajtburg et al 1985). Likewise, binding of AmB to low-density lipoprotein (LDL) and its consequent internalization modulate its toxicity (CitationBrajtburg and Bolard 1996).
Pharmacology and adverse effects
Klepser et al obtained the time-kill curves for AmB against Candida albicans (CitationKlepser et al 1997) and showed that AmB displays concentration-dependent fungicidal activity. Andes (CitationAndes 1999) investigated the pharmacodynamics of AmB in neutropenic mice with disseminated candidiasis and showed non-linear kinetics, in vivo concentration-dependent killing, and prolonged concentration-dependent post-antibiotic effects (PAEs) of AmB. The efficacy of AmB is compromised by a high frequency of adverse effects, including fever, chills, nausea, vomiting, headache, and renal dysfuntion with associated anemia, hypokalemia, and hypomagnesemia (CitationHiemenz and Walsh 1996).
Role of lipid formulations
There are three ways by which the therapeutic index of AmB might be improved: (i) increasing the selectivity of polyene-induced damage to fungal, as opposed to mammalian, cells; (ii) decreasing toxicity to host cells bearing LDL receptors; and (iii) decreasing toxicity for cells of the immune system, thereby protecting the immunostimulatory activity. Approaches designed to address these three issues involve the preparation of AmB-lipid associations. Therefore, there has been substantial exploration into the development of less toxic preparations of AmB. For the past decade, investigators have evaluated the use of colloidal dispersions and phospholipids vesicles known as liposomes as a targeted drug delivery systems for AmB. These efforts have led to the expansion of commercial preparations of phospholipid vesicles for therapeutic use such as AmBisome, ABLC, and ABCD (CitationHiemenz and Walsh 1996; CitationWong-Beringer et al. 1998). These preparations have been shown to be less toxic than AmB and to display altered pharmacokinetic properties because they are concentrated in the organs of the reticulo-endothelial system, but not in the kidney where only low concentrations are achieved (CitationKretschmar et al 2001).
Selected characteristics of lipid formulations that have been studied thoroughly and are in clinical trials are summarized in .
Table 1 Characteristics of some lipid formulations under clinical trials.
Intermediaries of antifungal and antiparasitic activity
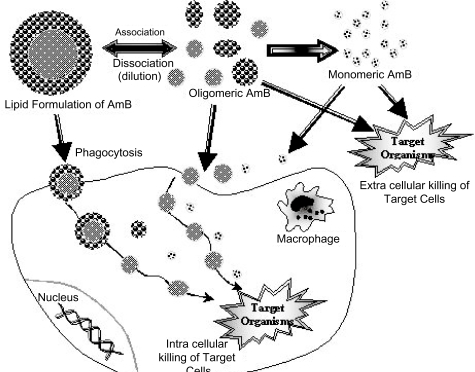
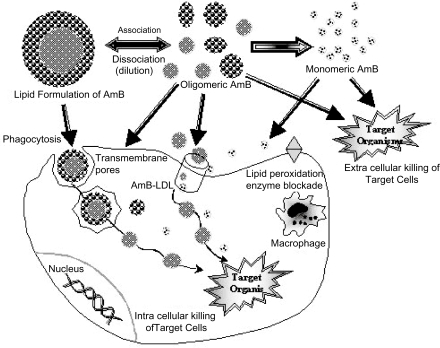
Macrophages may function as a reservoir of AmB for intracellular and extracellular antimicrobial action. CitationMehta et al (1997) conducted a study to investigate the role of macrophages in candidacidal activity of L-AmB. The results suggested that the improved candidacidal activity of L-AmB was possibly not due to activation of the macrophages. Instead, higher uptake and retention of L-AmB and its slow release from the macrophages led to its improved candidacidal activity. When lipids associated AmB is taken into macrophages (CitationLegrand et al 1996; CitationMehta et al 1994) or monocytes, it may function to inhibit fungal or parasitic cells also present inside these cells or it may dissociate from the complex inside the phagocytic cell and exit as free AmB to inhibit extracellular microbes (). If the AmB-lipid bond is strong, AmB will dissociate slowly as a monomer. The monomer, then, would be active against fungal and parasitic cells and not toxic to mammalian cells.
Effects of lipid-based carrier constructs on AmB Binding to lipoproteins and its internalization
AmB binding to lipoproteins may persuade the ability of mammalian cells to internalize the drug. If the AmB-carrier bond is weak and labile, as is presented in then when the complex is diluted in blood, AmB will dissociate from the lipid carrier and bind to LDL, just as AmB in Fungizone does when it dissociates from deoxycholate. The LDL-AmB complex can be internalized into cells bearing LDL receptors, and toxic effects comparable to those observed with Fungizone will occur.
When the AmB-carrier complex is strong and inert, it remains intact after introduction into the bloodstream but can still bind lipoproteins. ABLC may bind to highdensity lipoproteins (HDL) and remain in the bloodstream, lacking toxicity (CitationWasan et al 1994). On the other hand, neither ABCD (CitationGuo and Working 1993) nor AmB incorporated into egg lecithin-bile salts mixed micelles (CitationBrajtburg et al 1994) binds to lipoproteins, and both are relatively nontoxic (CitationBrajtburg and Bolard 1996).
Liposomal amphotericin B [L-AmB]
In 1965, CitationBangham et al (1965) reported that a small closed vesicular structure, consisted of lipid bilayers could be formed when phospholipids are hydrated by the addition of water. These structures were first named as “smectic mesophases” by Bangham and later called ‘liposomes’ by Gerald Weissman (CitationOstro and Cullis 1989; CitationBangham 1992). In 1981, New et al. (CitationNew et al 1981) first examined the effects of L-AmB, using leishmania model and reported that L-AmB had a lower toxicity than AmB itself and the treatment with a higher dose of L-AmB could be feasible. Afterward, the validity of the L-AmB for mice histoplasmosis (CitationTaylor et al 1982), cryptococcosis (CitationGraybill et al 1982), and candidiasis (CitationLopez-Berestein et al 1983; CitationTremblay et al 1984) was assessed. In all cases, the L-AmB showed a lower toxicity than AmB to the host animals and thus could be administered at higher doses. Drugs incorporated in liposome were also shown to distribute mainly to reticuloendothelial tissues including liver, spleen, and lung (CitationAbra and Hunt 1981). Later, a clinical trial performed in cancer patients who co-developed fungal infection confirmed that the L-AmB showed a higher tolerance than AmB even in human (CitationLopez-Berestein et al 1985).
AmBisome
Early evaluations were performed using the MLV-type agents. In 1987, Szoka et al prepared the Small unilamellar vesicles (SCV) containing sterol and explored the effects of component substances of liposome and a size of the particle on the expression of toxicity (CitationSzoka et al 1987). They concluded that the sterol including L-AmB was less toxic than that without sterol. They also reported that, when sterol was integrated, the smaller liposome was less toxic than the larger liposome and that, when sterol was excluded in contrast, the larger particle was less toxic than the smaller particle. Based on these findings, NeXtar Inc. succeeded in formulating the SUV type L-AmB (AmBisome). AmBisome has been licensed for use in Europe for over 5 years. It received FDA approval on 11th August 1997 for the treatment of patients with aspergillosis, candidiasis, and/or cryptococcal infections refractory or intolerant to AmB.
General Properties
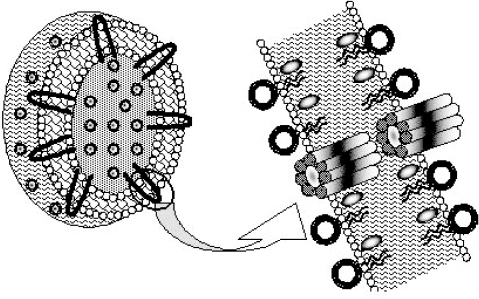
Among the new generation lipid-associated AmB formulations being developed throughout the world, the only true liposomal form of AmB is AmBisome. AmBisome is a suspension of small unilamellar liposomes in buffered 9% sucrose whose composition is HSPC (hydrogenated soya phosphatidyl choline)/Chol/DSPG/AmB (2:1:0.8:0.4 mole ratio). AmB is anchored tightly in the AmBisome bilayer due to favorable interactions of the macrolide with the surrounding lipids. DSPG probably interacts directly with AmB; cholesterol may also play a role. The exact nature of these interactions is not known, but the data are consistent with a barrel like structure formed by AmB molecules. Two barrels fit together tail to tail to span the lipid bilyer and form a pore that is permeable to ions and other solutes (). The product is stored as a lyophilized powder that is reconstituted with the addition of water for injection followed by the few seconds of shaking to produce a slightly opalescent, yellow solution. In its lyophilized presentation, stored at 4°C, AmBisome has a shelf life in excess of 30 months (CitationSchmidt et al 1998).
Figure 3 Proposed arrangement of AmB molecules (black) in the AmBisome bilayer. This structure accounts for the observation of rapid ion fluxes across the AmBisome bilayer in response to imposing a pH gradient from inside to outside. The individual AmB molecules form a “barrel” two of which fit tail-to-tail to form a pore spanning the bilayer. This structure is believed to contribute to the exceptional stability of AmBisome to loss of drug in buffer or plasma.
Mechanism of action
AmBisome has been tested in mammalian cell toxicity assay and has proved to be remarkable benign. Rat cell lysis assays are a measure of free (or readily available) AmB. Fungizone produced 92% lysis of rat cells in two hours at 37°C at a drug concentration of 1 μg/ml. AmBisome produced only 5% lysis under the same conditions and time of incubation even at high concentration of 100 μg/ml (AmB equivalent). These data suggest that AmB is retained sufficiently tightly inside the AmBisome so that less than 1% of the drug is free (or loose enough to be transferred to mammalian cells) in buffer. Potentially the association of AmB with AmBisome is dependent on the concentration of liposomes, if there exist equilibrium between free and liposome bound drug. But, in buffer, even as low as 1 μg/ml, the drug remains exclusively with the liposome as evidenced by circular dichroism studies over a range of concentrations (CitationFujii 1996). In vitro studies in human and mouse serum show complete retention of AmB by AmBisome for 6–24 hours. For AmBisome in vivo there is evidence that AmB is largely retained by the liposome over several hours of circulation in mice (Citationvan Etten et al 1995). Certainly the drug is not available in a free or toxic form since the LD50 of AmBisome is greater than 160 mg/kg in this species, as compared to 2.3 mg/kg for D-AmB.
There is evidence that AmBisome (and liposomes of the same composition without drug) can gain direct access to sites of fungal infection as intact structures probably because of leaky vasculature. The assumption has been made that with the prolong circulation life time seen for AmBisome, uptake into infected tissue and direct action of the liposomal drug may contribute to therapy (CitationAdler-Moore et al 1993). Indeed, AmBisome is highly active against cultured fungal species (CitationAnaissie et al 1991), although the liposomal drug may be somewhat slower acting than D-AmB (Citationvan Etten et al 1995). The liposomes, with or without drug bind to fungal cells, and AmBisome (but not drug free liposomes) disintegrates slowly. Gold labeled lipids incorporated in AmBi-some like liposomes can be located by electron microscopy (after silver enhancement). Initially intact liposomes are seen gathered around and bound to the cell wall of Candida glabrata. After 14 hours, incubation, gold labeled lipid is seen inside the cell membrane. The cell structure appears disrupted at this point, presumably due to action of AmB that accompanies breakup of the liposome (CitationAdler-Moore 1994). While it appears feasible for AmBisome to act directly on systemic fungal infections, the quantitative contribution of intact liposomes to the success of systemic treatment with AmBisome needs further study. Macrophages, including kupffer cells of the liver and stationary macrophage in the spleen, are a major cellular site for uptake of AmBisome and other lipid-associated AmB preparations (CitationHartsel and Bolard 1996). It is likely that macrophages and possibly neutrophils play key roles as depots for AmB, although the details have not been elucidated (CitationSchmidt et al 1998).
Pharmacology, efficacy- and toxicity during preclinical trials
Boswell et al (CitationBoswell et al 1998) examined the single- and multiple-dose pharmacokinetics and toxicological profile of AmBisome in rats. Rats were administered AmBisome at doses of 1, 3, 9, and 20 mg/kg/day. Substantial plasma concentrations (380 and 500 μg/mL in females and males, respectively) were attained after AmBisome therapy of 20 mg/kg for 30 days. The results suggested that, 100-fold higher plasma concentrations of AmB could be attained with AmBisome at doses up to 20 mg/kg/day as compared to conventional AmB. Unlike the conventional preparation, AmBisome at high doses resulted in slight nephrotoxicity but moderate hepatotoxicity. Another study showed that in brain tissue of noninfected rabbits, AmBisome attained mean tissue levels 4–7 times higher than that with D-AmB, ABCD, or ABLC. Conversely, none of the lipid formulations nor the conventional AmB can attain detectable levels in cerebrospinal fluid (CSF) in the absence of meningocerebral inflammation. Nevertheless, the high level of AmBisome attained in brain tissue is potentially promising for its future use in fungal infections of central nervous system (CitationGroll et al 1997). The efficacy of D-AmB compared to those of the lipid formulations in murine cryptococcosis also showed that AmBisome was one of the most efficacious formulations (CitationClemons and Stevens 1998).
The results of in vitro experiments against common pathogens including Candida, Cryptococcus, Aspergillus, and Fusarium species from both tube macrodilution and plate microdilution test methods confirmed that the MIC and minimal fungicidal concentration (MFC) profile for AmBisome is similar to that of AmB (CitationAdler-Moore and Proffitt 1998; CitationAnaissie et al 1991). The MIC of AmBisome ranged from 0.05 to 2.5 mg/L as compared with 0.1 to 2.5 mg/L for AmB. Thus the integration of AmB into the liposome bilayer of AmBisome has little or no inhibitory effect on its MICs in vitro. The in vivo study conducted by CitationFrancis et al (1994) on neutropenic rabbits with pulmonary aspergillosis designed to compare the clinical outcome with AmBisome at doses of 1, 5, and 10 mg/kg and conventional AmB at a dose of 1 mg/kg. All doses of AmBisome showed better survival than was seen with conventional AmB. Pulmonary haemorrhage was also reduced significantly in all treatment groups, but the lesions were smaller and less striking in rabbits treated with AmBisome at 5 mg/kg (P < 0.001) or 10 mg/kg (P < 0.0001) compared to AmB (P < 0.01). In conclusion, AmBisome at 5 mg/kg was more efficacious than D-AmB. The antifungal effectiveness of AmBisome was also compared with AmB in cultured Langerhans cells infected with C. glabrata (CitationSperry et al 1998). The Candida infected cells were incubated with AmB or AmBisome at 12.5 mg/L for up to 48 hours. Both AmBisome and AmB were found to be equally effective after 48 hours, reducing the amount of viable fungus by 5 logs. Nevertheless, AmBisome was much less cytotoxic to the cultured Langerhans cells then AmB at this concentration. Effectiveness of increasing doses of AmBisome (8 to 30 mg/kg/day) vs D-AmB (1 or 2 mg/kg/day) was also examined in neutropenic mice with hematogenous C. lusitaniae and C. krusei infection. Two of the infecting C. lusitaniae strains were resistant to AmB. Despite the fact that high doses of AmBisome were significantly more effective in infections due to AmB-susceptible isolates, there was no advantage of using AmBisome over the conventional preparation for infections due to AmB–resistant isolates (CitationKaryotakis and Anaissie 1994).
AmBisome have proved to be an effective treatment for VL. In vitro, free AmB was 3–6 times more active than AmBisome against both Leishmania major promastigotes in culture and amastigotes in murine macrophages. In a BALB/c L. major model of cutaneous infection, AmBisome administered once a day on 6 alternate days by the intravenous route produced a dose-response effect between 6.25 and 50 mg/kg (CitationYardley and Croft 1997). The intracellular fungus that has been found to be highly susceptible to AmBisome therapy in an immunosuppressed mouse model is Histoplasma capsulatum (CitationAdler-Moore 1994). Low doses of Fungizone or AmBisome (4 doses of 0.3 or 0.6 mg/kg) were compared, and a higher dose of AmBisome (4 doses of 6 mg/kg) was also tested to resolve whether a higher dose gave a better therapeutic response. At the lower doses neither Fungizone nor AmBisome were predominantly effective. Twenty-four hours after the last lower dose treatments, colony forming units (cfu)/g of spleen were abridged by c. 2 logs compared with untreated controls, but regrowth was evident after 14 days in all cases. Conversely the higher dose of AmBisome (6 mg/kg) considerably reduced the cfu by 5 logs at 24 hours post- treatment compared with control.
CitationGroll et al (2000) evaluated groups of uninfected and C. albicans-infected rabbits that were treated daily for 7 days with each of the three commercially available lipid formulations of AmB as well as with D-AmB and showed that the AmBisome treated animals attained considerably higher drug concentration in the plasma of both the infected and uninfected groups compared with other formulations. Practically no drug (<0.1 mg/L) was found in the cerebrospinal fluid (CSF) of any of the treatment groups. Nevertheless, there was a considerably higher concentration of AmB in the brain tissue itself in the AmBisome-treated groups than in groups receiving any of the other formulations, which in turn could be a reason for increased efficacy. The tolerance and efficacy of Fungizone (6 doses of 0.8 mg/kg, i.v.) were compared with those of AmBisome (6 doses of 0.8, 5 and 50 mg/kg, i.v.) and meglumine antimoniate (11 doses of 200 mg/kg i.p.) in a BALB/c mice model of VL induced by Leishmania infantum. A dose range study showed that administration of AmBisome at the well-tolerated doses of 5 or 50 mg/kg of body weight completely eradicated the parasites from the liver, spleen and lungs. At 0.8 mg/kg, AmBisome proved more efficacious than Fungizone administered at the same dose and was capable to decrease the parasitic burden by at least 4–6 logs in the spleen and liver compared with untreated controls (CitationGangneux et al 1996).
CitationAlbert et al (1995) treated a mouse model of meningitis (caused by Cryptococcus neoformans) with multiple doses of AmB (0.3 mg/kg i.v. or 0.3 mg/kg i.p.) or AmBisome (1, 3, 20, or 30 mg/kg i.v.). Some animals were killed during the therapy, and culture results showed that 3 mg/kg AmB was more effective than 3 mg/kg of AmBisome for lowering fungal cfu in the brain. Nevertheless, when the animals were killed two weeks after the full six treatment regimen there was a 6 log increase in the number of C.neoformans cfu in the brains of mice treated with AmB. In contrast, in the AmBi-some 3 mg/kg group, the cfu dropped by 1 log showing that AmBisome therapy was continue to kill the fungi even after treatment was stopped. In an efficacy study of AmBisome by CitationBerman et al (1986), 99% of Leishmania donovani parasites were eliminated from the liver and spleen of infected hamsters by one administration of 1.5–11 mg of AmBisome per kg. A total of 98%–99% of hepatosplenic parasites were eliminated from squirrel monkeys by three administrations of 4 mg of AmBisome per kg. AmBisome was 170–750 times as active as antimony in hamsters, and approximately 60 times as active as antimony in monkeys. Recently CitationClemons et al (2000) challenged the immunosuppressed rabbits intracisternally with Coccidiodes immitis. Five days post-infection, groups of rabbits were treated with either fluconazole (19 doses of 80 mg/kg/day, p.o.), AmBisome (15 mg/kg i.v. 3 times a week for 3 weeks), AmB (1 mg/kg i.v. three times a week for 3 weeks), or 5% glucose (control). All animals treated with fluconazole, AmB and AmBisome were survived, whereas 75% of the controls were died (P < 0.0005). The AmBisome-treated group had 3- and 11- fold lower cfu in the brain and in the spinal cord, respectively, compared with the fluconazole group, and 6- and 35- fold lower cfu, respectively, compared with the AmB treated group and AmBisome was found to be superior to either fluconazole or AmB for the treatment of experimental coccidiodal meningitis.
In another study the efficacy of AmBisome (5 doses: 0.05, 0.1, 0.5, 0.8, and 3 mg/kg of body weight) was compared to that of Fungizone (4 doses: 0.05, 0.1, 0.5, and 0.8 mg/kg) in a BALB/c mice model of VL induced by Leishmania infantum. AmBisome was about 3 times more active than the conventional drug against both strains (strain 1 was obtained from an untreated patient, and strain 2 was obtained from a patient who had received 12.5 g of AmB over 3 years). Median effective doses (ED50) of AmBisome were 0.054 (strain 1) and 0.194 (strain 2) mg/kg. ED50 of conventional AmB were 0.171 (strain 1) and 0.406 (strain 2) mg/kg. Determination of drug tissue levels, 3 days after the last drug administration, showed the drug accumulation in hepatic and splenic tissues much higher after administration of AmBisome than after conventional AmB. A lack of toxicity was noted in all groups treated with AmBisome (CitationPaul et al 1997).
In a pulmonary aspergillosis model in mice, immuno-suppressed mice were challenged intranasally with 8 × 104 A. fumigatus conidia (CitationOlson et al 2000). Groups of seven infected mice were treated intravenously with AmBisome 15 mg/kg, Abelcet 15 mg/kg, AmB 1 mg/kg, or 5% glucose daily for 4 days beginning 2 hours after challenge. All of the control mice were dead by day 5. The survival rate for groups treated with either Abelcet or AmB (Fungizone) was 29% on day 9 post-infection. However, the AmBisome treatment group had 86% rate of survival. Leenders et al (CitationLeenders et al 1996) compared the efficacy of AmBisome and the AmB in an unusual rat aspergillosis model. The rats were infected only in the left lung, and 40 h later they were treated with either AmB 1 mg/kg/day or AmBisome 1 or 10 mg/kg/day for 10 consecutive days. Both AmB 1 mg/kg/day and AmBisome 10 mg/kg/day increased survival; nevertheless, only AmBisome 10 mg/kg/day was able to cause a significant diminution in cfu in the left lung (P = 0.003). Interestingly, distribution to the right lung was abridged in both of the AmBisome treatment groups, while conventional AmB was ineffective to prevent lung dissemination. Distribution to the liver and spleen was reduced by all treatments, but statistically significant reductions were only observed in the AmBisome treatment groups (1 or 10 mg/kg/day). AmBisome 10 mg/kg/day completely prevented the distribution to the liver and spleen. Animal studies have revealed that AmBisome is also very effective in both treating and preventing fungal infections in the kidneys (CitationAdler-Moore et al 1991; Citationvan Etten et al 1993; CitationGarcia et al 2000). In the prophylactic study, AmB levels in the kidneys of AmBisome-treated mice (5, 10, or 20 mg/kg) were ranged from 0.63 to 8.08 mg/kg 7 days after treatment (CitationAdler-Moore and Proffitt 2002).
Clinical efficacy and safety
AmBisome is much better endured than conventional AmB and is specified in the treatment of severe systemic fungal infections where patients fail to respond to AmB, are intolerant to its side-effect, or who have renal impairement prohibiting the use of conventional drug. AmBisome was first used clinically in 1987 when a heart transplant patient developed pulmonary aspergillosis, which due to nephrotoxicity could not be treated with conventional AmB (CitationKatz et al 1990). After 34 days of treatment with AmBisome at 1 mg/kg/day, the infection was exterminated and no proof of reccurence was reported during a 16-month follow up period. Kidney function was also improved and acute side-effects such as fever and chills were not seen during therapy. Since then, AmBisome has been developed throughout the world and is currently licensed in more than 30 countries, including the US where it has sanctioned for empiric use (fever of unknown origin). In a controlled randomized trial, a short antifungal prophylaxis course of AmBisome was found to reduce the incidence of proven invasive fungal infections considerably during the first month following liver transplantation surgery. AmBisome was well tolerated, although backache, thrombocytopenia and renal function impairment were reported in a few patients (CitationTollemar et al 1995). Clinical studies on immunocompromised adult and pediatric patients with invasive fungal infections, primarily candidiasis and aspergillosis, were designed to evaluate the efficacy of AmBisome. The results obtained for AmBisome in these studies were promising and complete or partial response was seen. Specifically, the use of AmBisome in febrile neutropenic patients with suspected or confirmed invasive mycoses resolved the fungal infection in 61% of the episodes. Treatment efficacy was 77% for aspergillosis (CitationMills et al 1994). Ringden et al (CitationRingden et al 1991) also reported a favorable eradication rate of 83% for Candida and 41% for Aspergillus infections in immunocompromised patients treated with AmBisome. AmBisome was also effective in pediatric patients with similar disorders. A report on the serum and pulmonary concentrations of AmBisome in a patient with acute liver transplant failure is also noteworthy. During follow-up of a patient with liver transplant failure and pulmonary aspergillosis, it was observed that peak and trough serum concentrations of AmB were increased, as were pulmonary concentrations of the drug (CitationHeinemann et al 1997). The authors hypothesized that, in absence of a normally functioning liver tissue as a component of RES, the clearance function of the liver was diminished and that the clearance by the lung began to be important (CitationSchmidt et al 1998).
The multicenter study by CitationMeunier et al (1991) included 126 patients receiving 133 episodes of AmBisome treatment. The majority of these patients had failed previous conventional AmB therapy due to toxicity. AmBisome was administered for 21 days at an average daily dose of 2.1 mg/kg (range = 0.45–5 mg/kg). Hypokalaemia was the most common side-effect observed in 24 cases. In 17 episodes, creatinine was initially high, but returned to normal. Glutamyloxaloacetate transaminase became elevated in 19 instances, and elevation in alkaline phosphatase was observed in 22 instances. Nevertheless there was no report of discontinuation of AmBisome therapy due to adverse side-effects. Thus, AmBisome was well tolerated even in severely ill patients. CitationWalsh et al (1998) administered AmBisome to 36 febrile neutropenic patients for empirical antifungal therapy at doses of 1, 2.5, 5, or 7.5 mg/kg. No fungal infections were observed, suggesting that AmBisome was effective in preventing breakthrough fungal infections. A more recent report including 687 febrile neutropenic patients and comparing D-AmB with AmBisome as empirical therapeutic agents validated the previous data. It was again shown that AmBisome was as effective as the conventional drug and was associated with fewer breakthrough fungal infections and fewer toxic reactions (CitationWalsh et al 1999).
Recently AmBisome safety was judged in a series of 187 transplant recipients. AmBisome was administered daily at dose levels between 1 and 4 mg/kg for a median of 11 days (range of 1–112 days). Side effects including allergic reaction, low back pain during infusion, dyspnea, low serum potassium, and nausea and vomiting ascribed to AmBisome therapy were observed in only 7% of the cases and resulted in discontinuation of therapy in 6 cases. In this context, with patients receiving a variety of potentially toxic drugs, the AmBisome side-effect profile was mild and controllable in the vast majority of patients (CitationRingden et al 1994). Recent multicenter randomized trials compared D-AmB at 1 mg/kg/day to AmBisome at 1 and 3 mg/kg/day in adults (CitationPrentice et al 1997) and children (CitationHann et al 1995) with febrile neutropenia unresponsive to broad spectrum antibiotics. A group of 193 adult patients was prospectively randomized into the three treatment groups. Fifty-two patients had confirmed mycosis, seven were not classifiable and the rest were stratified as having fever of unknown origin (FUO). The adult study showed significantly lower adverse events for the AmBisome groups. D-AmB showed 50% nephrotoxicity compared with 16% and 18% showed by AmBisome 1 and 3 mg/kg/day groups (p = 0.001). Also hypokalemia was considerably less in the AmBisome cohorts. A paediatric study created a similar picture, but differed in detail. Nephrotoxicity was lower in the AmBisome compared with D-AmB but the differences were not statistically significant. Considerable advantages were seen for AmBisome therapy in incidences of hypokalemia, treatment delay, and resolution of fever. Davidson et al measured the optimum dose and schedule for AmBisome treatment of VL. A group of 88 patients, mostly children was treated with 4 different dose regimens. Eighty-four patients were completely cured of their disease by the initial treatment course lasting 10 days (4 or 5 days daily treatment at 3 or 4 mg/kg/day and 1 follow up on day 10). Four relapsing children received an additional 10-day course of treatment at 3 mg/kg/day which cured them all (CitationDavidson et al 1996). This study is outstanding not only for the short course treatment and high cure rate of VL patients, but also for the favorable safety profile (no significant adverse events) (CitationSchmidt et al 1998).
The efficacy and safety of 3 regimens of AmBisome in the treatment of Indian VL were compared in a prospective open randomized trial. Thirty parasitologically confirmed patients were randomly divided into 3 equal treatment groups; group 1 received AmBisome 2 mg/kg on days 1, 2, 3, 4, 5, 6, and 10 (total dose 14 mg/kg); group 2 received AmBisome 2 mg/kg on days 1, 2, 3, 4, and 10 (total dose 10 mg/kg); group 3 received the same dosage on days 1, 5 and 10 (total dose 6 mg/kg). Clinical cure resulted in all patients by day 24. Haemoglobin, white blood cell count, body weight and serum albumin level improved on day 24 and became normal by day 180. No patient relapsed within 12 months of follow up (CitationThakur et al 1996).
Immunoliposomes
The current encouraging progress regarding lipid-based formulations of AmB is the development of novel liposomes with specific properties. One of these, “immunoliposomes”, contains fungus-specific antibodies on their surface which target them directly to the fungal cells. AmB coated with immunoliposomes abridged mortality appreciably in mice with invasive pulmonary aspergillosis as compared conventional L-AmB (100% vs 16.7% survival rate). AmB coated with immunoliposomes was also more effective than AmB integrated with long-circulating liposomes (100% vs 83.3% survival rate) (CitationOtsubo et al 1998). Likewise, treatment of murine candidiasis and cryptococcosis with AmB integrated with immunoliposomes proved enhanced activity compared to that with conventional L-AmB (CitationBelay et al 1991; CitationDromer et al 1990).
Long-circulating liposomes
The other novel delivery system, ‘long circulating liposomes’ are coated with polyethylene glycol (PEG), resulting in a sterically stabilized surface. Since the time period to reside in circulation is prolonged by the structural nature of long-circulating liposomes, more intact liposomes can get localized at the site of infection, thus enhancing the in vivo efficacy (CitationStorm and van Etten 1997). In an experimental murine model of systemic candidiasis, AmB integrated with long-circulating liposomes (PEG-L-AmB) proved to be more effective than the conventional L-AmB (Citationvan Etten et al 1995; Citationvan Etten et al 1998). Nevertheless, intracellular antifungal activity of PEG-L-AmB assessed in C. albicans infected murine peritoneal macrophages was as low as that of conventional L-AmB, while it was higher for D-AmB (Citationvan Etten et al 1998).
Other lipid based nanomodifications
Lipid nanospheres
Studies on efficacies of NS-718, AmB encapsulated in lipid nanosphere are in progress. Lipid nanosphere is composed of equal amounts of egg lecithin and soybean oil. The carrier potentials of lipid nanosphere are characterized by lower uptake by the reticuloendothelial system and good distribution to the sites of inflammation. When equivalent dose of NS-718 or Fungizone were injected intravenously into rats, the plasma AmB level yielded by NS-718 was higher than Fungizone at all time up to 2 hours. In a tissue distribution study, the concentration in the liver after the injection of NS-718 was lower than that of Fungizone. This characteristic of NS-718 to avoid uptaking by reticuloendothelial system (RES) is related to high plasma concentration of AmB. These results suggest that NS-718 have several unique characteristics different from other lipid formulations for the treatment of fungal infections (CitationSeki et al 1994; CitationTomii 2002). In another study NS-718 was found to be more effective than D-AmB or L-AmB against clinical isolates of C. albicans and Aspergillus fumigatus. NS-718 was well tolerated and showed improved survival markedly at equivalent doses in treating pulmonary aspergillosis in rat. Increased activity was also supported by pharmacokinetic study (CitationKohno et al 1995).
Fukui et al investigated whether AmB retained its antifungal activity in NS-718 (CitationFukui et al 1996). Antifungal activity of NS-718 against Candida albicans was similar to that of AmB and Fungizone. However, the antifungal activity of L-AmB was decreased. Thus, NS-718 maintained the potent activity of AmB against fungal cell even though the AmB was incorporated into LNS particles. Hossain et al compared the direct cytotoxicity of NS-718 with that of Fungizone in human proximal tubule cells in vitro and showed decreased cytotoxicity of NS-718 (CitationHossain et al 2000). These results showed an increased selectivity between toxicity of NS-718 against mammalian cells and antifungal activity.
In vitro and In vivo antifungal efficacy of NS-718 was also studied in pulmonary cryptococcosis in mice. NS-718 was found to have better in vitro efficacy against clinical isolates of Cryptococcus neoformans than other AmB formulations, was well tolerated, and efficacy was much higher than that of D-AmB or L-AmB in treating pulmonary cryptococcosis in mice (CitationHossain et al 1998). In vivo anti-fungal efficacy of NS-718 was also studied in invasive pulmonary aspergillosis in rats (CitationOtsubo et al 1999). The results showed that NS-718 was effective in treating pulmonary aspergillosis in rats, but equivalent doses of Fungizone and L-AmB were either lethally toxic or ineffective.
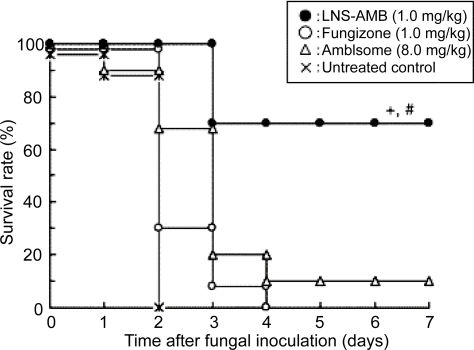
In a rat model of localized candidiasis, LNS-AmB significantly inhibited the growth of C. albicans in the pouch, whereas AmBisome did not, even though the AmB concentrations in the pouch were similar. This difference in anti-fungal activity between LNS-AmB and AmBisome was also found in vitro. That is, the antifungal activity of LNS-AmB against C. albicans was similar to that of Fungizone and dimethyl sulfoxide-solubilized AmB, while AmBisome showed weaker antifungal activity than did other formulations (). In a mouse model of systemic candidiasis, LNS-AmB (1.0 mg/kg) greatly improved the survival rate () and was therefore much more effective than AmBisome (8.0 mg/kg) (P<0.05) or Fungizone (1.0 mg/kg) (P<0.01) (CitationFukui et al 2003).
Figure 4 Antifungal activity of LNS-AmB, Fungizone, AmBisome, and DMSO-solubilized AmB in vitro. The growth inhibition of C. albicans was measured by the change in optical density at 540 nm in SD-MOPS broth after a 24-h incubation at 35°C. Results are the mean of two experiments.
Adapted from CitationFukui et al (2003).
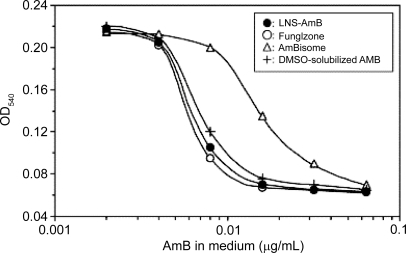
Figure 5 Survival of mice infected with C. albicans and treated with LNS-AmB, Fungizone, or AmBisome. Treatment was started 4 hours after fungal inoculation. +, P<0.05 compared with AmBisome; #, P<0.01 compared with Fungizone.
Adapted from CitationFukui et al (2003).
Cochleates
Cochleates are stable phospholipid-calcium precipitates comprised mainly of phosphatidylserine. The in vivo therapeutic efficacy of cochleates containing AmB (CAmB) administered orally was evaluated in a mouse model of systemic candidiasis. The fungal tissue burden in kidneys and lungs was assessed, and a dose-dependent reduction in C. albicans from the kidneys was observed, with a maximum 3.5-log reduction in total cell counts at 2.5 mg/kg/day. However, complete clearance of the organism from the lungs, resulting in more than a 4-log reduction, was observed at the same dose. (CitationSantangelo et al 2000).
In the study by Zarif et al (CitationZarif et al 2000) CAmB protect ICR mice infected with C. albicans when the agent is administered intraperitoneally at doses of as low as 0.1 mg/kg/day. In a tissue burden study, CAmB, Fungizone, and AmBisome were effective in the kidneys, but in the spleen CAmB was more potent than Fungizone at 1 mg/kg/day and was equivalent to AmBisome at 10 mg/kg/day.
Emulsome: a novel nano lipid particle
Emulsomes are a new generation colloidal carrier system in which the internal core is made of solid fats and triglycerides which is stabilized by high concentration of lecithins in the form of o/w emulsion (CitationAmselem et al 1994). The effects of emulsomes, nanosize range lipid particles containing AmB (EAmB) were compared with the reference formulation Fungizone and with the commercial preparation AmBisome. Both Fungizone and EAmB had a minimum inhibitory concentration (MIC) of 0.039 μg/ml against C. albicans ATCC10231, whereas the MIC for AmBisome was considerably higher (0.156 μg/ml). However, the yeasts were more rapidly killed by Fungizone than by EAmB in spite of similar MIC values. The killing of C. albicans was delayed when EAmB was used. In a tissue culture model and in mice, the incorporation of AmB into emulsomes resulted in a considerable reduction of toxicity in comparison with Fungizone. For comparison of the in vivo effect of the preparations, a mouse model of systemic infection with C. albicans was used. All preparations were able to reduce the fungal burden in the liver and kidneys in comparison with control animals treated with isotonic saline. AmBisome was more efficient in the reduction of the fungal burden of the liver than EAmB and Fungizone, even when applied in a similar dosage of 1 mg/kg. In the kidneys, EAmB and Fungizone were slightly more effective than AmBisome. Therefore the incorporation of AmB into nanosize lipid particles was able to reduce toxicity without loss of efficiency (CitationKretschmar et al 2001).
In our laboratory we have developed and evaluated AmB loaded emulsomes for the treatment of VL. By virtue of solidified or semisolidified internal oily core it provided a better opportunity to load AmB in high concentration. In vivo studies on L. donovani infected hamsters showed better results for AmB emulsomes as compared to control (D-AmB, Mycol) ().
Figure 6 Photographs showing geimsa stained splenic smears of hamster treated with emulsomes and control formulations. A-untreated control group; B-Mycol (AmB for injection) treated group; C-TLEs orTrilaurin based emulsomes treated group; D-TSEs or Tristearin based emulsomes treated group.
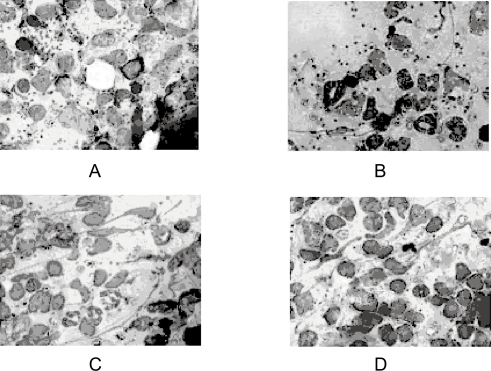
The maximal percentage of parasite suppression (55.7%) was obtained with 0.5 mg/kg of AmB loaded trilaurin emulsomes (TLEs). Tristearin emulsomes (TSEs) showed 40.7% parasite suppression at the same dose whereas only 33.6% of parasite suppression was observed with relatively higher dose (1 mg/kg) of D-AmB or Mycol ().
Table 2 Activity of emulsome formulations against L. donovani in hamsters infected for 30 days
Future directions
Fungal infections are on the rise worldwide, particularly as the population of immunocompromised patients continues to grow. By itself, AmB is an effective antifungal and anti-leishmanial agent, though it is highly toxic, particularly to the kidney. The goal of these lipid formulations of the AmB is to transport the drug throughout the body without exposing it to sensitive organs and tissues and then to deliver it in concentrated dosage to the target site. To a certain extent all the lipid formulations accomplish this goal. The maximum tolerable dose of AmB is about 1 mg/kg/day. However these lipid formulations allow physician to go up to 5 times the dose of AmB without increasing infusion related toxicities. All the lipid formulations of AmB demonstrate improved efficacy, primarily because of the higher administered dose, and reduced kidney toxicity, compared to AmB. As such the future of these lipid formulations is bright and it is apparent that these lipid based products will replace AmB as the mainstays in the treatment of systemic fungal infections and leishmaniasis.
Targeting AmB using the colloidal carrier systems, ie, liposomes, emulsomes, or nanospheres etc to the sites of infection could readily be utilized in terms of their industrial application as this can provide a better therapy mode for treatment of systemic fungal infections and leishmaniasis in comparison with currently available drug regimen in the market for these respective diseases. High loading efficiency and protracted release profile may further reduce the dose size and dose frequency. Further the easier ligation of surface specific ligands could enhance the target specificity and performance efficiency. Thus the drug AmB, which is well known for its effectiveness however, compromised due to its contraindicated manifestations, can safely be administered for effective cure of infective diseases. Nevertheless, these nanocarriers may provide curable disposition of systemic microbial infections. Moreover, the colloidal nature of these nanocarriers leads to their passive accumulation in pathogen harbouring or infected macrophages. More advances in nanotechnology will hopefully result in more efficient and less toxic AmB therapeutic regimens.
References
- AbraRMHuntCA1981Liposome disposition in vivo. III. Dose and vesicle–size effectsBiocim Biophys Acta666493503
- Adler-MooreJPChiangSSatoriusA1991Treatment of murine Candidosis and cryptococcosis with a unilamellar liposomal amphotericin B formulation (AmBisome)J Antimicrob Chemother28Suppl B63711778893
- Adler-MooreJPFujiiGLeeMJA1993In vitro and in vivo interactions of AmBisome with pathogenic fungiJ Liposome Res31516
- Adler-MooreJPProffittRT1998AmBisome: long circulating formulation of Amphotericin BWoodleMCStormGLong Circulating Liposomes: Old drugs, New TherapeuticsNew YorkSpringer-Verlag185206
- Adler-MooreJProffittRT2002AmBisome: liposomal formulation, structure, mechanism of action and preclinical experienceJ Antimicrob Chemother49Suppl S1213011801577
- Adler-MooreJP1994AmBisome targeting to fungal infectionsBone marrow transplantation14Suppl 5S3S77703928
- AlbertMMStahl-CarrollLLutherMF1995Comparison of liposomal amphotericin B to amphotericin B for treatment of murine cryptococcal meningitisJ Myco Med516
- AlvarJCanavateCGutierrez-SolarB1997Leishmania and human immunodeficiency virus co-infection: the first 10 yearsClin Microbiol Rev102983199105756
- AmselemSYogevAZawoznikE1994Emulsomes, a novel drug delivery technologyProceedings of the International Symposium on Controlled Release of Bioactive Materials21136869
- AnaissieEPaetznickVProffitR1991Comparison of the in vitro antifungal activity of free and liposome-encapsulated Amphotericin BEur J Clin Microb Infect Dis1066568
- AndesD1999In 39th Interscience Conference on Antimicrobial Agents and ChemotherapySan Franciscoabstract no. 100228
- ArikanSRexJH2001Lipid-based antifungal agents: current statusCurr Pharm Des739341511254895
- BanghamADStandishMMWatkinsJC1965Diffusion of univalent ions across the lamellae of swollen phospholipidsJ Mol Biol13238525859039
- BanghamAD1992Liposomes: realizing their promiseHosp Pract (Off Ed)275161452605
- BelayTHospenthalDRRogersAL1991Evaluation of antibody-bearing liposomal amphotericin B in the treatment of systemic candidiasis in a neutropenic murine modelJ Med Vet Mycol294194211815035
- BermanJDHansonWLChapmanWL1986Antileishmanial activity of liposome–encapsulated amphotericin B in hamsters and monkeysAntimicrob Agents Chemother30847513813512
- BermanJDKsionskiGChapmanWL1992Activity of amphotericin B cholesterol dispersion (Amphocil) in experimental visceral leishmaniasisAntimicrob Agents Chemother361978801416890
- BolardJLegrandPHeitzF1991One–sided action of amphotericin B on cholesterol–containing membranes is determined by its self–association in the mediumBiochemistry305707152043613
- BoswellGWBekerskyIBuellD1998Toxicological profile and pharmacokinetics of a unilamellar liposomal vesicle formulation of amphotericin B in ratsAntimicrob Agents Chemother42263689527770
- BrajtburgJBolardJ1996Carrier effects on biological activity of amphotericin BClin Microb Rev951231
- BrajtburgJElbergSKobayashiGS1994Amphotericin B incorporated into egg lecithin–bile salt mixed micelles: molecular and cellular aspects relevant to therapeutic efficacy in experimental mycosesAntimicrob Agents Chemother38300068192456
- BrajtburgJElbergSSchwartzDR1985Involvement of oxidative damage in erythrocyte lysis induced by amphotericin BAntimicrob Agents Chemother27172763985601
- ClemonsKVHowellKJCalderonL2000Efficacy of intravenous AmBisome against coccidioidal meningitis in rabbitsIn Abstracts of the Fortieth Interscience Conference on Antimicrobial Agents and ChemotherapyToronto, CanadaAbstract 2120. Washington, DC. American Society for Microbiology.396
- ClemonsKVStevensDA1998Comparison of fungizone, Amphotec, AmBisome, and Abelcet for treatment of systemic murine cryptococcosisAntimicrob Agents Chemother428999029559804
- DavidsonRNCroftSLScottA1991Liposomal amphotericin B in drug–resistant visceral leishmaniasisLancet3371061621673494
- DavidsonRNdi MartinoLGradoniL1996Short–course treatment of visceral leishmaniasis with liposomal amphotericin B (AmBisome)Clin Infect Dis6938438783690
- de MarieSJanknegtRBakker-WoudenbergIAJ1994Clinical use of liposomal and lipid–complexed amphotericin BJ Antimicrob Chemother33907168089064
- DromerFBarbetJBolardJ1990Improvement of amphotericin B activity during experimental cryptococcosis by incorporation into specific immunoliposomesAntimicrob Agents Chemother342055602073097
- EmmingerWGraningerWEmminger-SchmidmeierW1994Tolerance of high doses of amphotericin B by infusion of a liposomal formulation in children with cancerAnn Hematol6827318110875
- EspuelasMSLegrandPLoiseauPM2002In vitro antileishmanial activity of amphotericin B loaded in poly(epsilon-caprolactone) nanospheresJ Drug Target105939912683663
- FrancisPLeeJWHoffmanA1994Efficacy of unilamellar liposomal amphotericin B in treatment of pulmonary aspergillosis in persistently granulocytopenic rabbits: the potential role of bronchoalveolar D–mannitol and serum galactomannan as markers of infectionJ Infect Dis169356688106769
- FromtlingRA1993Amphotericin B cholesterol sulfate complex (colloidal dispersion)Drugs Future1830306
- FromtlingRA1995Amphotericin B lipid complexDrugs Future2012934
- FujiiG1996Liposomal Amphotericin B (AmBisome): Realization of the drug delivery conceptVesicles12491526
- FukuiHKoikeTNakagawaT2003Comparison of LNS–AmB, a novel low-dose formulation of amphotericin B with lipid nano–sphere (LNS), with commercial lipid-based formulationsInt J Pharm2671011214602388
- FukuiHKoikeTSahekiA1996In 23rd International Symposium on Controlled Release of Bioactive Materials
- GangneuxJPSulahianAGarinYJ1996Therapy of visceral leishmaniasis due to Leishmania infantum: experimental assessment of efficacy of AmBisomeAntimicrob Agents Chemother401214188723469
- GarciaAAdler-MooreJPProffittRT2000Single dose AmBisome (liposomal amphotericin B) as prophylaxis for murine systemic candidiasis and histoplasmosisAntimicrob Agents Chemother4423273210952575
- GeorgopapadakouNHWalshTJ1996Antifungal agents: chemotherapeutic targets and immunologic strategiesAntimicrob Agents Chemother40279918834867
- GokhalePCBarapatreRJAdvaniSH1993Pharmacokinetics and tolerance of liposomal amphotericin B in patientsJ Antimicrob Chemother321331398226404
- GokhalePCBarapatreRJAdvaniSH1993Successful treatment of disseminated candidiasis resistant to amphotericin B by liposomal amphotericin B: a case reportJ Cancer Res Clin Oncol1195695718335676
- GoldWStoutHAPaganoJF1956Antibiotics Annual 1955–1956WelchHMarti-IbanezFMedical EncyclopediaNew York57986
- GraybillJRCravenPCTaylorRL1982Treatment of murine cryptococcosis with liposome–associated amphotericin BJ Infect Dis145748527077097
- GrollAGiriNGonzalezC1997In 37th Interscience Conference on Antimicrobial Agents and ChemotherapyToronta, Canadaabstract A– 90.
- GrollAHGiriNPetraitisV2000Comparative efficacy and distribution of lipid formulations of amphotericin B in experimental Candida albicans infections of the central nervous systemJ Infect Dis1822748210882607
- GuoLSWorkingPK1993Complexes of amphotericin B and cholesteryl sulfateJ Liposome Res3473490
- HannINStevensRFPinkertanCR19952nd International Symposium on Febrile NeutropeniaBrussels
- HartselSBolardJ1996Amphotericin B: new life for an old drugTrends Pharmcol Sci1744549
- HayRJ1994Antifungal drugs on the horizonJ Am Acad Dermatol31S82858077515
- HeinemannVBosseDJehnU1997Enhanced pulmonary accumulation of liposomal amphotericin B (AmBisome) in acute liver transplant failureJ Antimicrob Chemother40295979302000
- HerwaldtBL1999LeishmaniasisLancet35411919910513726
- HiemenzJWWalshTJ1996Lipid formulations of amphotericin B: recent progress and future directionsClin Infect Dis22Suppl 2S133448722841
- HoeprichPD1992Clinical use of amphotericin B and derivatives: lore, mystique, and factClin Infect Dis14Suppl 1S114S91562682
- HossainMAMaesakiSKakeyaH1998Efficacy of NS-718, a novel lipid nanosphere-encapsulated amphotericin B, against Cryptococcus neoformansAntimicrob Agents Chemother421722259661011
- HossainMAMaesakiSRazzaqueMS2000Attenuation of nephrotoxicity by a novel lipid nanosphere (NS-718) incorporating amphotericin BJ Antimicrob Chemother46263810933650
- JanoffASPerkinsWRSaletanSL1993Amphotericin B lipid complex (ABLC). A molecular rationale for the attenuation of amphotericin B related toxicitiesJ Liposome Res345171
- KaryotakisNCAnaissieEJ1994Efficacy of escalating doses of liposomal amphotericin B (AmBisome) against hematogenous Candida lusitaniae and Candida krusei infection in neutropenic miceAntimicrob Agents Chemother382660627872764
- KatzNMPiercePFAnzeckRA1990Liposomal amphotericin B for treatment of pulmonary aspergillosis in a heart transplant patientJ Heart Transplant914172313415
- KlepserMEWolfeEJJonesRN1997Antifungal pharmacodynamic characteristics of fluconazole and amphotericin B tested against Candida albicansAntimicrob Agents Chemother411392959174207
- KohnoSOtsuboTHaraK1995American Society for Microbiology, Washington D.CPrograms and abstracts of the 35th Interscience Conference on Antimicrobial agents and Chemotherapy131Abstr F109.
- KretschmarMAmselemSZawoznikE2001Efficient treatment of murine systemic infection with Candida albicans using amphotericin B incorporated in nanosize range particles (emulsomes)Mycoses442818611714063
- LeendersACAPde MarieSten KateMT1996Liposomal amphotericin B (AmBisome) reduces dissemination of infection as compared with amphotericin B deoxycholate (Fungizone) in a rat model of pulmonary aspergillosisJ Antimicrob Chemother38215258877535
- LegrandPRomeroEACohenBE1992Effects of aggregation and solvent on the toxicity of amphotericin B to human erythrocytes. Antimicrob.Agents Chemother36251822
- LegrandPVertut–DoiABolardJ1996Comparative internalization and recycling of different amphotericin B formulations by a macrophage–like cell lineJ Antimicrob Chemother37519339182109
- Lopez–BeresteinGBodeyGPFrankelLS1987Treatment of hepatosplenic candidiasis with liposomal-amphotericin. BJ Clin Oncol5310173806172
- Lopez–BeresteinGFainsteinVHopferR1985Liposomal amphotericin B for the treatment of systemic fungal infections in patients with cancer: a preliminary studyJ Infect Dis151704103973417
- Lopez-BeresteinGJulianoRL1987Application of liposomes to the delivery of antifungal agentsOstroMJLiposomesNew YorkMarcel Dekker253276
- Lopez-BeresteinGMethaRHopferRL1983Treatment and pro-phylaxis of disseminated infection due to Candida albicans in mice with liposome–encapsulated amphotericin BJ Infect Dis147939456842027
- Lopez-BeresteinG1987Liposomes as carriers of antimicrobial agentsAntimicrob Agents Chemother3167583300535
- MaesakiS2002Drug delivery system of anti–fungal and parasitic agentsCurr Pharm Des84334012069380
- MedoffGDismukesWEPappagianisD1992Evaluation of new antifungal drugs for the treatment of systemic fungal infections. Infectious Diseases Society of America and the Food and Drug AdministrationClin Infect Dis15Suppl 1S2742811477243
- MehtaRTMcQueenTJKeyhaniA1994Phagocyte transport as mechanism for enhanced therapeutic activity of liposomal amphotericin BChemotherapy40256648082414
- MehtaRTPoddarSKalidasM1997Role of macrophages in the candidacidal activity of liposomal amphotericin BJ Infect Dis175214178985224
- MeunierFPrenticeHGRingdenO1991Liposomal amphotericin B (AmBisome): safety data from a phase II/III clinical trialJ Antimicrob chemother28Suppl B83911778895
- MeunierFSculierJPCouneA1988Amphotericin B encapsulated in liposomes administered to cancer patientsAnn N Y Acad Sci5445986102850759
- MeyerhoffA1999US Food and Drug administration approval of AmBi-some (liposomal amphotericin B) for treatment of visceral leishmaniasisClin Infect Dis28495110391695
- MillsWChopraRLinchDC1994Liposomal amphotericin B in the treatment of fungal infections in neutropenic patients: a single–centre experience of 133 episodes in 116 patientsBr J Haematol86754607918068
- MurrayHW1999Kala-azar as an AIDS related opportunistic infectionAids Patient care STDS134596510800524
- NewRRChanceMLHeathS1981Antileishmanial activity of amphotericin and other antifungal agents entrapped in liposomesJ Antimicrob Chemother8371817319979
- OlsonJHuynhVBunchT2000Differences in efficacy and tissue drug concentrations following treatment of murine pulmonary aspergillosis with AmBisome or AbelcetIn Abstracts of the Fourteenth International Society for Human and Animal Mycoses, Buenos AiresArgentinaAbstract 217, Zurichp.International Society for Human and Animal Mycology228
- OstroMJCullisPR1989Use of liposomes as injectable–drug delivery systemsAm J Hosp Pharm461576872672806
- OtsuboTMaesakiSHossainMA1999In vitro and in vivo activities of NS-718, a new lipid nanosphere incorporating amphotericin B, against Aspergillus fumigatusAntimicrob Agents Chemother43471510049253
- OtsuboTMaruyamaKMaesakiS1998Long–circulating immunoliposomal amphotericin B against invasive pulmonary aspergillosis in miceAntimicrob Agents Chemother414049449258
- PaulMDurandRFessiH1997Activity of a new liposomal formulation of amphotericin B against two strains of Leishmania infantum in a murine modelAntimicrob Agents Chemother411731349257750
- PearsonRDSousaAD1996Clinical spectrum of LeishmaniasisClin Infect Dis221118824958
- PrenticeHGHannIMHerbrechtR1997A randomized comparison of liposomal versus conventional amphotericin B for the treatment of pyrexia of unknown origin in neutropenic patientsBrit J Hematol9871118
- RalphEDBarberKRGrantCWM1993Clinical experience with multilamellar liposomal amphotericin B in patients with proven and suspected fungal infections. ScandJ Infect Dis2348796
- RingdenOAndstromERembergerM1994Safety of liposomal amphotericin B (AmBisome) in 187 transplant recipients treated with cyclosporinBone Marrow Transplant14Suppl BS10S147703925
- RingdenOMeunierFTollemarJ1991Efficacy of amphotericin B encapsulated in liposomes (AmBisome) in the treatment of invasive fungal infections in immunocompromised patientsJ Antimicrob Chemother28Suppl B73821778894
- SantangeloRPaderuPDelmasG2000Efficacy of oral cochleate–amphotericin B in a mouse model of systemic candidiasisAntimicrob Agents Chemother4423566010952579
- SchindlerJJWarrenRPAllenSD1993Immunological effects of amphotericin B and liposomal amphotericin B on splenocytes from immune–normal and immune–compromised miceAntimicrob Agents Chemother37271627218109941
- SchmidtPGAdler-MooreJPForssenEA1998Unilamellar liposomes for anticancer and antifungal therapyLasicDDPapahadjopoulosDMedical Applications of liposomesNew YorkElsevier703731
- SculierJPDelcroixCBrassinneC1989Pharmacokinetics of amphotericin B in patients receiving repeated intravenous high doses of amphotericin B entrapped into sonicated liposomesJ Liposome Res115166
- SekiJSasakiHDoiMYoshikawaH1994Lipid Nano-Sphere (LNS), a protein-free analogue of lipoproteins, as a novel drug carrier for parenteral administration. IVJ Control Release2835253
- SerenoDHolzmullerPLemesreJL2000Efficacy of second line drugs on antimonyl–resistant amastigotes of Leishmania infantumActa Trop74253110643904
- SperryPJCuaDJWetzelSA1998Antimicrobial activity of AmBisome and nonliposomal amphotericin B following uptake of Candida glabrata by murine epidermal Langerhans cellsMed Mycol361351419776826
- StevensDA1994Overview of amphotericin B colloidal dispersion (amphocil)J Infect28S I45498077690
- StormGvan EttenE1997Biopharmaceutical aspects of lipid formulations of amphotericin BEur J Clin Microb and Infect Dis166473
- SuraritRShepherdMG1987The effects of azole and polyene antifungals on the plasma membrane enzymes of Candida albicansJ Med Vet Mycol25403132830394
- SzokaFCMilhollandDBarzaM1987Effect of lipid composition and liposome size on toxicity and in vitro fungicidal activity of liposome–intercalated amphotericin BAntimicrob Agent Chemother314219
- TaylorRLWilliamsDMCravenPC1982Amphotericin B in liposomes: a novel therapy for histoplasmosisAm Rev Resp Dis12561017081822
- ThakurCPPandeyAKSinhaGP1996Comparison of three treatment regimens with liposomal amphotericin B (AmBisome) for visceral leishmaniasis in India: a randomized dose–finding studyTrans Royal Soc Trop Med Hyg9031922
- TollemarJHockerstedtKEriczonB-G1995Liposomal amphotericin B prevents invasive fungal infections in liver transplant recipients. A randomized, placebo–controlled studyTransplantation5945507839427
- TomiiY2002Lipid formulation as a drug carrier for drug deliveryCurr Pharm Des84677412069383
- TremblayCBarzaMFioreC1984Efficacy of liposome-intercalated amphotericin B in the treatment of systemic candidiasis in miceAntimicrob Agent Chemother261703
- van EttenEWOtte–LambillionMvan VianenW1995Biodistribution of liposomal amphotericin B (AmBisome) and amphotericin B–desoxycholate (Fungizone) in uninfected immunocompetent mice and leucopenic mice infected with Candida albicansJ Antimicrob Chemother35509197628985
- van EttenEWSnijdersSVvan VianenW1998Superior efficacy of liposomal amphotericin B with prolonged circulation in blood in the treatment of severe candidiasis in leukopenic miceAntimicrob Agents Chemother422431339736577
- van EttenEWten KateMTStearneLE1995Amphotericin B liposomes with prolonged circulation in blood: in vitro antifungal activity, toxicity, and efficacy in systemic candidiasis in leukopenic miceAntimicrob Agents Chemother391954588540697
- van EttenEWvan den Heuvel-de GrootCBakker-WoudenbergIA1993Efficacies of amphotericin B-desoxycholate (Fungizone), liposomal amphotericin B (AmBisome) and fluconazole in the treatment of systemic candidosis in immunocompetent and leucopenic miceJ Antimicrob Chemother32723398125837
- van EttenEWvan VianenWHakJ1998Activity of liposomal amphotericin B with prolonged circulation in blood versus those of AmBisome and fungizone against intracellular Candida albicans in murine peritoneal macrophagesAntimicrob Agents Chemother422437399736579
- Vertut-DoiAHannaertPBolardJ1988The polyene antibiotic amphotericin B inhibits the Na+/K+ pump of human erythrocytesBiochem Biophys Res Commun1576926972849435
- WalshTJFinbergRWArndtC1999Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. National Institute of Allergy and Infectious Diseases Mycoses Study GroupN Engl J Med3407647110072411
- WalshTJYeldandiVMcEvoyM1998Safety, tolerance, and pharmacokinetics of a small unilamellar liposomal formulation of amphotericin B (AmBisome) in neutropenic patientsAntimicrob Agents Chemother422391989736569
- WasanKMMortonRERosenblumMG1994Decreased toxicity of liposomal amphotericin B due to association of amphotericin B with high–density lipoproteins: role of lipid transfer proteinJ Pharm Sci831006107965656
- Wong-BeringerAJacobsRAGuglielmoBJ1998Lipid formulations of amphotericin B: clinical efficacy and toxicitiesClin Infect Dis27603189770163
- YardleyVCroftSL1997Activity of liposomal amphotericin B against experimental cutaneous leishmaniasisAntimicrob Agents Chemother41752569087483
- ZarifLGraybillJRPerlinD2000Antifungal activity of amphotericin B cochleates against Candida albicans infection in a mouse modelAntimicrob Agents Chemother44146369