Abstract
Major depressive disorder (MDD) is a common and serious illness of our times, associated with monoamine deficiency in the brain. Moreover, increased levels of cortisol, possibly caused by stress, may be related to depression. In the treatment of MDD, the use of older antidepressants such as monoamine oxidase inhibitors and tricyclic antidepressants is decreasing rapidly, mainly due to their adverse effect profiles. In contrast, the use of serotonin reuptake inhibitors and newer antidepressants, which have dual modes of action such as inhibition of the serotonin and noradrenaline or dopamine reuptake, is increasing. Novel antidepressants have additive modes of action such as agomelatine, a potent agonist of melatonin receptors. Drugs in development for treatment of MDD include triple reuptake inhibitors, dual-acting serotonin reuptake inhibitors and histamine antagonists, and many more. Newer antidepressants have similar efficacy and in general good tolerability profiles. Nevertheless, compliance with treatment for MDD is poor and may contribute to treatment failure. Despite the broad spectrum of available antidepressants, there are still at least 30% of depressive patients who do not benefit from treatment. Therefore, new approaches in drug development are necessary and, according to current research developments, the future of antidepressant treatment may be promising.
Introduction
Major depressive disorder (MDD) is a common and serious illness with the potential of becoming the leading cause of disability worldwide.Citation1 The lifetime prevalence rate is 16.2%, and is expected to increase.Citation2,Citation3 In the elderly, prevalence is about 3% in the general populationCitation4 and 15%–25% among nursing home residents.Citation5 These numbers may be even higher, because it is estimated that clinically significant depression goes untreated in 60% of the elderly.Citation6 The average age of onset of MDD is the mid-20s.Citation3 The lifetime risk in women is twice the risk in men, and is increased during the reproductive years.Citation3
The illness is described by a wide range of symptoms, such as disturbances in sleep, appetite, sexual desire, and constipation. It is also characterized by crying, sadness, and loss of the ability to experience pleasure in work or with friends. Depression is strongly associated with suicidal events, cognitive abnormalities, impaired memory function, and slowing of speech and action.Citation7 Furthermore, patients with MDD often have painful physical symptoms.Citation8 If symptoms which interfere considerably with activities of daily living and domestic relationships persist for more than two weeks, MDD should be considered.Citation7
Mechanisms of disease
MDD is a complex disorder, probably influenced by genetic and environmental factors. Heritability of depression has been estimated to range from 30% to 40%.Citation3 The polymorphisms associated with the serotonin transporter geneCitation9 have been related to more depressive symptoms, diagnosable depression, and tendency to commit suicide.Citation3,Citation10 Nevertheless, the relationship between genetics and depression is probably very complex and not fully elucidated.Citation7
Some environmental factors, such as stress, could pre-dispose to depression by affecting the genome.Citation7,Citation9 Personality characteristics may predict an individual’s susceptibility to depression, but personality may also be modified in the disease. Moreover, personality may alter the clinical presentation of a depressive disorder.Citation11
Monoamine deficiency hypothesis
The monoamine hypothesis of depression postulates a deficiency in monoaminergic neurotransmission in the brain, mediated by serotonin and noradrenaline. Noradrenaline depletion may be due to inhibition of tyrosine hydroxylase (see ), whereas reduced synthesis of serotonin may be due to depletion of dietary tryptophan or mutations of tryptophan hydroxylase.Citation7,Citation12 Given that reduced serotonin levels do not cause depression in all people, it is unclear if decreased serotonin synthesis is a cause or consequence of depression.Citation9
Figure 1 The monoamine deficiency hypothesis.
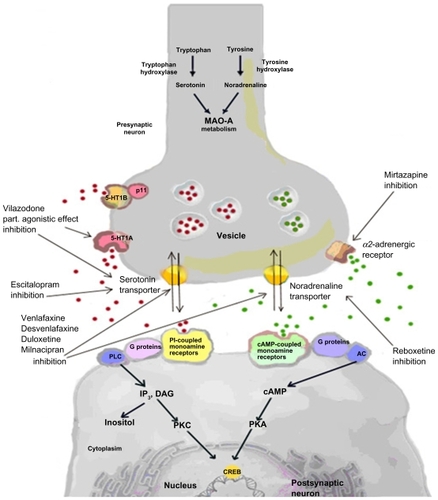
Deficiency in monoaminergic neurotransmission may be caused by disturbed receptor signaling, even with normal monoamine levels. Decreased sensitivity of 5-HT1A and 5-HT1B autoreceptors, which regulate serotonin function, has been associated with depression.Citation13,Citation14 In contrast, the sensitivity of α2-noradrenergic receptors, which modulate noradrenaline release by feedback inhibition, was enhanced in depressed patients.Citation15 Moreover, disturbed receptor signaling could also be a result of malfunction of G-protein or secondary messenger systems, which may impair neurotransmitter function, even without changes in monoamine levels or receptor numbers.Citation7 Decreased levels of secondary messengers, such as inositol,Citation7,Citation16 cAMP,Citation7,Citation17 and cAMP response element-binding protein, have been reported in the brains of patients with MDD at autopsy.Citation7,Citation18
The monoamine deficiency hypothesis is supported by the fact that noradrenaline and serotonin reuptake inhibitors have antidepressant activity. Nevertheless, only 50%–70% of patients respond to these drugs, implicating a more complex mechanism for depression.Citation7,Citation19 Furthermore, dopamine deficiency has been associated with the disease as well. Such a hypothesis is supported by the antidepressant activity of dopamine reuptake inhibitors and dopamine agonists.Citation7
Stress, hypothalamic-pituitary-adrenal axis, and growth factors
Stress is perceived by the brain cortex and transmitted to the hypothalamus, where corticotrophin-releasing hormone is produced and released, leading to further elevation of cortisol plasma levels. The hypothalamic-pituitary-cortisol hypothesis postulates that depression is associated with elevated cortisol levels in response to stress.Citation20,Citation21 However, doubt was cast on this hypothesis by disappointing results in clinical trials with corticotrophin-releasing hormone antagonists.Citation22 It is also difficult to establish the relationship between stress and depression, given that stress may be both the cause and consequence of depressed mood.Citation7
It was suggested that elevated levels of glucocorticoids may reduce neurogenesis and lead to decreased size of the hippocampus in some depressed patients.Citation23 Stress and cortisol may affect and decrease hippocampal levels of brain-derived neurotrophic factor, necessary for axonal growth, neuronal survival, and synaptic plasticity.Citation24–Citation26 Reduced brain-derived neurotrophic factor levels were found in the hippocampi of depressed patients.Citation24
Other possible disease mechanisms
Other theories about the pathophysiology of depression include changes in glutamatergic neurotransmission,Citation25 reduced neurotransmission gamma-butyric acid,Citation26 abnormal circadian rhythms,Citation27 deficient neurosteroid synthesis,Citation28 impaired endogenous opioid function,Citation29 monoamine-acetylcholine imbalance,Citation30 tyroxine abnormalities,Citation31 and dysfunction of specific brain structures and circuits.Citation32 Many of these mechanisms are involved in other psychiatric and neurologic disorders, but the impact on MDD is still unclear.
Traditional therapy
The most common nonpharmacologic approach for treating MDD is psycho-therapy. It is especially helpful in patients with a history of childhood adversity or recent stress.Citation33 Psychotherapy and medication were shown to be comparable for unipolar depression, and it was suggested that psychotherapy may offer a prophylactic advantage compared with medication.Citation34 Other possible approaches include neurostimulation techniques, electroshock, or electroconvulsive therapy, indicated only for treatment of resistant depression.Citation9
Traditional pharmacotherapy includes tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs). However, selective serotonin reuptake inhibitors (SSRIs) and newer antidepressants are considered as “first- line” treatment.
Monoamine oxidase inhibitors
MAOIs inhibit MAO-A and MAO-B and reduce monoamine degradation. Phenelzine, isocarboxazid and tranylcypromine are irreversible nonselective inhibitors, and their effect may persist for weeks until the regeneration of MAO. The use of MAOIs is decreasing due to serious side effects, such as acute hypertensive reactions after consumption of tyramine-rich foods, eg, aged cheese.Citation35 These drugs have severe, potentially life-threatening interactions with many drugs, including meperidine, SSRIs, narcotic medications, and pseudoephedrine.Citation36 Newer MAOIs inhibit the MAO enzyme reversibly. Moclobemide inhibits MAO-A, and does not require strict dietary restrictions.Citation39 Selegiline inhibits MAO-B, and its transdermal formulation provides several advantages compared with orally administered MAOIs, including freedom from dietary tyramine restrictions and a better adverse effect profile.Citation37
Tricyclic antidepressants
The mechanism of action of most TCAs is noradrenaline and serotonin reuptake inhibition.Citation38 They also antagonize post- synaptic histamine H1, α1, 5HT2A, and muscarinic receptors.Citation41 Following oral administration, TCAs are rapidly absorbed. They are highly (90%–95%) bound to plasma albumin, and have large distribution volumes.Citation41 Metabolism occurs primarily by CYP450 (CYP2D6, CYP2C9, CYP2C19, and CYP3A4), and metabolites are renally excreted.Citation41 TCAs may interact with SSRIs by inhibition of CYP450 isoenzymes. Concurrent use of fluoxetine or paroxetine can enhance TCA concentrations.Citation41 Concurrent use of imipramine and clomipramine with MAOIs may cause pharmacodynamic interactions leading to serotonin syndrome.Citation41 Although widely used in clinical practice, combinations of TCAs with MAOIs and SSRIs are generally considered to be unsafe.Citation41 TCAs have a small therapeutic range, and therapeutic drug monitoring is useful.Citation39 Female gender and higher drug doses increase the risk of side effects.Citation40
TCAs were shown to be comparable or more effective than SSRIs, but less well tolerated.Citation41 Their advantage may be efficacy in treatment-resistant depression.Citation42 Nortriptyline, a potent noradrenaline reuptake inhibitor showed superior pharmacologic properties compared with other TCAs.Citation41 Nortriptyline was better tolerated and may be administered concomitantly with MAOIs or SSRIs.Citation41 Clomipramine may be the most efficacious TCA in severe depression.Citation41 Amitriptyline is considered very effective, whereas dothiepin has the highest toxicity among the TCAs.Citation41 The more typical atropinic side effects of TCAsCitation41 are presented in . Enhanced and toxic concentrations of TCA cause serious adverse effects, such as prolonged intracardiac conduction and postural hypotension.Citation41,Citation43
Table 1 Efficacy and adverse effects of triciclyc antidepressants, serotonin reuptake inhibitors and newer antidepressants
Selective serotonin reuptake inhibitors
SSRIs selectively inhibit neuronal reuptake of serotonin, with no significant affinity for histamine, acetylcholine, or adrenergic receptors. The most frequently used SSRIs in the treatment of depression are fluoxetine, fluvoxamine, sertraline, paroxetine, citalopram, and escitalopram.Citation43 These agents have similar efficacy and tolerability.Citation44 However, due to pharmacokinetic differences, they are not interchangeable.Citation45 Sertraline and citalopram show linear pharmacokinetics in contrast with fluoxetine, fluvoxamine, and paroxetine. SSRIs are usually characterized by slow elimination, and it takes time to achieve steady state.Citation47 Fluoxetine has a half-life of 1–4 days and its active metabolite norfluoxetine 7–15 days. Other SSRIs have shorter half-lives of 1–2 days and no clinically significant active metabolites.Citation47 SSRIs are extensively metabolized and show high interindividual variability.Citation47 Fluoxetine and norfluoxetine are inhibitors of CYP2D6Citation46,Citation47 and CYP3A4.Citation46 Paroxetine inhibits CYP2D6,Citation47–Citation49 while fluvoxamine inhibits CYP1A2 and CYP2C19.Citation48,Citation49 As a consequence, their potential to interact with antipsychotics, opioids, and serotonin-norepinephrine reuptake inhibitors is high.Citation48 Clinically significant interactions are more likely to occur with fluvoxamine, fluoxetine, and paroxetine compared with citalopram, escitalopram, or sertraline.Citation48
Drug interactions with MAOIs, TCAs, moclobemide, tryptophan, lithium, and selegiline, as well as SSRI overdoses, may lead to the serotonin syndrome, characterized by change in mental status, myoclonus, restlessness, hyperreflexia, shivering, diaphoresis, tremor, and possibly death.Citation47,Citation48 SSRIs cause fewer side effects, such as dry mouth, constipation, and blurred vision, and have a safer cardiac adverse event profile than the TCAs.Citation47,Citation48 Common adverse effects of SSRIs are listed in .
Paroxetine is a more potent noradrenaline inhibitor compared with the other SSRIs and has the highest affinity for cholinergic receptors causing typical anticholinergic adverse effects.Citation48 Sertraline significantly blocks dopamine reuptake, which may result in cardiovascular and extrapyramidal symptoms.Citation48 Fluoxetine and sertraline have high dopaminergic affinity that may also cause extrapyramidal symptoms. Citalopram has the highest affinity for H1 receptors of all the SSRIs, and may have weak antihistaminic activity at high doses.Citation47,Citation48 Despite these adverse effects, SSRIs remain reasonably well tolerated.Citation44,Citation48
Newer antidepressants
Newer antidepressants are usually characterized by a dual mode of action, such as inhibition of serotonin, noradrenaline, and dopamine reuptake. The pharmacokinetics, efficacy, and adverse effects of the newer antidepressants will be discussed in detail.
Escitalopram
Escitalopram is the most 5-HT transporter-selective compound and the S-(+)-enantiomer of citalopram.Citation47 Both SSRIs share similar pharmacokinetics.Citation22 Following oral administration, escitalopram is rapidly and almost completely absorbed.Citation48,Citation49 The process is not affected by food.Citation55 The pharmacokinetic profiles of the newer antidepressants are summarized in . Escitalopram is widely distributed throughout tissues,Citation55,Citation56 has low protein binding, and is not likely to have interactions with highly protein-bound drugs.Citation55 Escitalopram is extensively metabolized in the liver via oxidative metabolism.Citation50,Citation55 In the brain, metabolism of escitalopram propionate may be mediated by MAO-A, MAO-B, and aldehyde oxidase.Citation51,Citation52 Nevertheless, the metabolites do not contribute appreciably to therapeutic activity.
Table 2 Pharmacokinetic properties of newer antidepressants
Escitalopram is a weak inhibitor of CYP isoenzymes 1A2, 2C9, 2D6, and 3A4, and may have a low potential for clinically significant interactions with substrates for these isoenzymes.Citation55 Ritonavir, a potent CYP3A4 inhibitor, showed no effect on escitalopram pharmacokinetics.Citation53 In contrast, cimetidine and omeprazole increased escitalopram exposure, but the effect is probably not of clinical concern.Citation54 Concomitant administration of escitalopram with MAOIs or other SSRIs should be avoided because of possible serotonin syndrome.Citation55
The elimination half-life is relatively short compared with other SSRIs, and steady-state plasma concentrations are achieved in a week.Citation56 The main elimination route is renal.Citation55
Escitalopram shows linear and dose-proportional pharmacokinetics in the dose range 10–30 mg/day.Citation55,Citation56 No reduction of citalopram dosage seems to be necessary in patients with moderately impaired renal function, but may be appropriate in patients with impaired hepatic function.Citation55 Age and gender showed no clinically significant influence on escitalopram pharmacokinetics.Citation55,Citation56 Risk factors which may necessitate dose adjustment are presented in .
Table 3 Risk factors which may influence the pharmacokinetics of newer antidepressants
Escitalopram may be a suitable first-line antidepressant in moderate to severe major depressionCitation57 and in treatment of depression in adolescents.Citation58 The drug was shown to be more efficacious than placebo and as least as effective or better than citalopram,Citation22,Citation66–Citation69 with an early onset of efficacy.Citation22,Citation59 Differences between the two SSRIs seem to depend on the initial severity of the depressive symptomatology, given that escitalopram has shown superior antidepressive efficacy in severely depressed patients.Citation60,Citation70 Nevertheless, opposite findings were also reported, suggesting methodologic flaws as a cause for the difference in efficacy between the two drugs.Citation60 Efficacy scores for newer antidepressants are presented in .
Escitalopram showed similar efficacy to sertralineCitation61 and superior efficacy to paroxetine, especially in severely depressed patients.Citation62 Furthermore, in short-term studies, superior efficacy of escitalopram compared with citalopram, paroxetine, and duloxetine was observed.Citation63
The efficacy of escitalopram was similar to that of venlafaxine, but there was a trend of higher response and remission rates in the escitalopram group.Citation64,Citation65 The SSRI may be at least as effective as venlafaxine and duloxetine even in severe depression.Citation66
Cipriani et al reported superior efficacy of escitalopram over duloxetine, fluoxetine, fluvoxamine, paroxetine, and reboxetine. Following mirtazapine, escitalopram was the most efficacious drug among 12 antidepressants.Citation67
The prominent side effects of escitalopram are similar to those of other SSRIs (see ).Citation46 Similar tolerability and withdrawal rates for citalopram and escitalopram were reported.Citation22,Citation67–Citation69 In contrast, escitalopram (10–20 mg/day) showed better tolerability in long-term treatment than paroxetine. The most common adverse event with escitalopram was headache, and nausea with paroxetine.Citation74 Moreover, nausea, sweating, and obstipation were significantly less frequent compared with venlafaxine.Citation22,Citation68 Cipriani et al reported better tolerability of escitalopram compared with duloxetine, paroxetine, reboxetine, sertraline, fluvoxamine, and venlafaxine.Citation79
Doses of 10–20 mg/day showed consistent antidepressive efficacy and excellent tolerability in primary care patients with MDD.Citation68 The recommended starting dose of 10 mg/day is appropriate for most patients regardless of age, gender, or mild to moderate renal impairment or hepatic insufficiency.Citation55 A period of at least four weeks is worthwhile before considering further intervention. If 10 mg/day is not effective, an increase to 20 mg/day should be considered.Citation69,Citation70
Mirtazapine
The antidepressant activity of mirtazapine is a result of enhanced serotonergic and noradrenergic neurotransmission through blockade of presynaptic α2-adrenergic autoreceptors and heteroreceptors and postsynaptic 5-HT2 and 5-HT3 receptors.Citation71,Citation72 No influence on serotonin or noradrenaline reuptake was observed.Citation73,Citation84 Mirtazapine has low affinity for central and peripheral dopaminergic and muscarinic receptors, and high affinity for H1 receptors.Citation83,Citation84
Following oral administration, mirtazapine is rapidly absorbed, but the absolute bioavailability is moderate (see ).Citation73 The drug is nonspecifically and reversibly bound to proteins and possess a high distribution volume.Citation75,Citation86 Metabolism is mediated by CYP1A2, CYP2D6, and CYP3A4.Citation75,Citation87 Demethylmirtazapine is the active metabolite, but its exposure in the human body is three times lower compared with the parent drug.Citation74
Low inhibitory effects of mirtazapine on major CYP isoenzymes were reported in vitro.Citation83,Citation88 No significant interactions with the CYP2D6 substrates amitriptyline, clozapine, olanzapine, and risperidone were observed.Citation90–Citation92 In contrast, plasma concentrations of mirtazapine were reduced after concomitant administration of the CYP3A4 inducers carbamazepineCitation87,Citation90 and phenytoin.Citation75 Moreover, mirtazapine disposition was affected by fluvoxamine and, to a lesser extent, by paroxetine.Citation76,Citation77 Coadministration of cimetidine (an inhibitor of CYP3A4, CYP1A2, and CYP2D6) increased mirtazapine plasma concentrations significantly, requiring dose adjustment.Citation78 An additive sedative effect was observed with diazepam. Moreover, patients should be advised to avoid alcohol while taking mirtazapine.Citation83,Citation87
The drug is predominantly excreted in the urine and feces.Citation87,Citation89 The activity is prolonged by the circulation of the parent compound.Citation87,Citation89 High clearance values indicate renal tubular secretion.Citation83,Citation87,Citation89 The elimination rate is strongly affected by CYP2D6 polymorphism.Citation79,Citation80 Steady state is reached in less than a week.Citation81
In the therapeutic range, mirtazapine shows linear pharmacokinetics. Citation99 Nicotine may decrease plasma mirtazapine levels, and smokers may require increased doses.Citation82 In contrast, mirtazapine plasma levels are increased in the elderly,Citation91,Citation100 as well as in patients with hepatic impairment,Citation87 and dose reduction should be considered in both groups. Mirtazapine exposure in patients with severe or moderate renal insufficiency is increased compared with healthy controls.Citation83 Although there are no differences in reported adverse effects,Citation102 the drug should be used with caution in these patients.Citation84 Gender affects mirtazapine plasma levels, but the changes are not clinically important (see ).Citation101
The efficacy of mirtazapine in treatment of patients with moderate to severe MDD was reported in several studies.Citation104–Citation106 Short-term studies revealed similar efficacy for mirtazapine and amitriptyline.Citation107–Citation109 Moreover, mirtazapine had a longer time to relapse than amitriptyline during the first 20 weeks (see ).Citation85
Furthermore, mirtazapine showed similar or greater efficacy than citalopram, fluoxetine, paroxetine, sertraline, duloxetine, fluvoxamine, and reboxetine.Citation79,Citation111–Citation114 In a meta- analysis of 25 randomized, controlled trials, mirtazapine showed a faster onset of action than SSRIs and was superior for short-term (two-week) response and remission rates, but the differences were not significant at the end of acute-phase treatment (6–12 weeks).Citation86 The efficacy of mirtazapine and venlafaxine were similar in patients with severe depression characterized by melancholic features.Citation87
Mirtazapine was generally well tolerated in patients with MDD, with a lower frequency of side effects compared with placebo (see ).Citation84,Citation96,Citation105 Sedation, especially at low dose, and weight increase may be due to H1-receptor blockade.Citation105 In a long-term treatment study, weight gain was the only more frequent side effect with mirtazapine than placebo, whereas blood pressure and heart rate were similar.Citation83,Citation110,Citation117
Compared with amitriptyline, mirtazapine had fewer adverse events and less need for discontinuation of treatment due to an adverse event.Citation83,Citation110 Dry mouth, vertigo, and weight increase were as frequent as with TCAs, but seizures were less frequent.Citation22,Citation84
Discontinuation rates due to adverse events for mirtazapine and SSRIs were similar. Mirtazapine was associated with significantly less insomnia, sexual dysfunction, and nausea than SSRIs, but with significantly more weight gain, dry mouth, fatigue, and excessive somnolence.Citation88 Adverse effects such as increased salivation and weight gain were more frequent with mirtazapine compared with venlafaxine but sweating, constipation, increased sexual desire, and weight loss were more common with venlafaxine.Citation116
Mirtazapine is used as a single agent, or in combination with SSRIs or venlafaxine. The recommended dose is 15–45 mg/day, and it is generally given as a single dose in the evening.Citation46
Bupropion
Bupropion is an atypical antidepressant, probably a selective inhibitor of noradrenaline and dopamine reuptake. Bupropion and its metabolites are slightly more potent inhibitors of dopamine than of noradrenaline reuptake, and do not affect the release or transport of other neurotransmitters, or have appreciable affinity for postsynaptic receptors including histamine, α-adrenergic, serotonin, dopamine, or acetylcholine receptors.Citation89,Citation90
Bupropion is available in three oral formulations, ie, immediate-release (IR), sustained-release (SR), and extended- release (XR).Citation91 Absorption rates vary between the formulations, but there is no significant difference in the extent of absorption.Citation120 Food does not affect absorption, which is at least 87% of an administered dose.Citation120 Pharmacokinetics are linear in the therapeutic range (see ).Citation120,Citation121
Bupropion is extensively distributed and bound to plasma proteins.Citation121 Following hepatic metabolism via CYP2B6, three active metabolites, ie, hydroxybupropion, threohydrobupropion, and erythrohydrobupropion are formed.Citation92,Citation93 Hydroxybupropion and threohydrobupropion possess about 50% of the activity of the parent drugCitation123 and their plasma concentrations are 4–7-fold and ~5-fold higher than bupropion, respectively.
Major effects of CYP2B6 genetic polymorphisms on the pharmacokinetics of bupropion have not been shown.Citation94 However, concomitant administration of CYP2B6 inducers, such as carbamazepine, lopinavir, and ritonavir, decreased bupropion plasma levels.Citation90,Citation95,Citation96
Bupropion and hydroxybupropion may have a low potency for inhibition of CYP2D6.Citation123 Coadministration of bupropion with the CYP2D6 substrates desipramine and venlafaxine resulted in increased levels of the substrates.Citation90,Citation97 A case of severe bradycardia was related to the addition of bupropion to metoprolol.Citation98 Therefore, low doses should be used, and dose monitoring should be considered following concomitant administration of bupropion and CYP2D6 substrates with a narrow therapeutic range.Citation90
The activity of bupropion is prolonged as a result of slow elimination of metabolites.Citation121 Steady-state concentrations are reached after 7–10 days.Citation121 Renal excretion is predominant, but the drug and its active metabolites cross the blood-brain barrier and placenta, and are also excreted in human breast milk.Citation120
The pharmacokinetic properties of bupropion are probably not influenced by nicotine.Citation99,Citation100 However, the effect of gender is unclear due to controversial findings.Citation129–Citation131 Bupropion SR is metabolized more rapidly in children compared with adults,Citation121 and the elderly are at risk of accumulation of the drug and its metabolites.Citation101 Slower elimination of bupropion was observed in patients with renal impairment,Citation102 and high variability in pharmacokinetic parameters was observed in patients with hepatic impairment. Therefore, bupropion should be used with caution in these groups (see ).Citation121
Bupropion was shown to be more efficacious than placebo. Improvement in primary and secondary outcomes were observed after 6–8 weeks with all bupropion formulations in adults with moderate to severe depression.Citation103,Citation104,Citation120
Bupropion IR showed similar efficacy to nortriptyline,Citation105 amitriptyline,Citation106 and fluoxetine.Citation107 No significant differences in efficacy were observed with bupropion SR and sertraline after 8–16 weeksCitation139–Citation141 or fluoxetine.Citation108 In the elderly, bupropion SR and paroxetine showed similar efficacy.Citation109 There were no significant differences between bupropion XR and escitalopramCitation110 or venlafaxine XRCitation111,Citation112 in terms of primary or secondary outcome measures. After switching from citalopram, bupropion SR was as effective as sertraline and venlafaxine XR.Citation113 The drug was as effective as buspirone in augmentation of citalopram (see ).Citation114
Different formulations of bupropion have similar tolerability profiles and are generally well tolerated in adults and the elderly.Citation120 Most adverse events associated with bupropion are mild to moderate in severity (see ).Citation120,Citation134,Citation135
Allergic reactions to bupropion occur rarely but, if symptoms arise, drug discontinuation should be advised.Citation115,Citation120 The risk of seizures is dose- but not formulation-dependent.Citation120 Rate of seizures was 0.1% for doses of 100–300 mg/day, and increased to 0.4% at doses of 300–450 mg/day.Citation120,Citation125,Citation149 Adverse events resulting in discontinuation of therapy were agitation, headache, nausea, and rash, which occurred at a rate of approximately 5%–11% with all bupropion products.Citation149
Compared with nortriptyline, bupropion was associated with significantly fewer adverse events such as dry mouth, somnolence, and tachycardia.Citation136 Generally, the tolerability profiles of bupropion and SSRIs are similar, although bupropion is associated with more headache and dry mouth.Citation115,Citation120 However, sexual dysfunction following SSRIs is not a problem with bupropion,Citation151 and lower rates of somnolence and diarrhea are associated with this agent.Citation151 Similar incidences of adverse events were reported for bupropion and venlafaxine.Citation145
The administration of bupropion has certain advantages, such as a greater reduction in severity of symptoms and fewer adverse events.Citation148 Bupropion is indicated in the treatment of adult patients with major depression but is not approved for use in pediatric patients.Citation149 The recommended initial doses are 100 mg of bupropion IR twice daily, 150 mg of bupropion SR once daily, and 150 mg of bupropion XR once daily.Citation115,Citation120 The maximum recommended dose is 450 mg/day for IR (150 mg three times daily) and XR (450 mg in the morning) formulations, or 400 mg/day of bupropion SR (200 mg twice daily).Citation120,Citation152
Venlafaxine and desvenlafaxine
Venlafaxine probably inhibits serotonin uptake only in low doses, whereas both serotonin and noradrenaline uptake are inhibited following high doses.Citation115 The drug does not possess significant affinity for 5HT1A, 5HT2A, D2, muscarinic, or α1- or α2- receptors, and does not inhibit MAO.Citation116,Citation153 Desvenlafaxine (O-desmethylvenlafaxine), the major metabolite of venlafaxine, has similar potency for the inhibition of serotonin and noradrenaline uptake.Citation153
Following oral administration of venlafaxine, absorption starts after approximately 20 minutes and is completed within three hours for venlafaxine IR and for desvenlafaxine.Citation116,Citation117 Venlafaxine XR is absorbed more slowly, but the extent of absorption is similar between formulations.Citation156
The drug is widely distributed in the body, with low protein binding and a high volume of distribution (see ).Citation118,Citation119 Following oral absorption, venlafaxine undergoes extensive first-pass hepatic metabolism, where conversion to the active metabolite, desvenlafaxine, occurs via demethylation.Citation157 This reaction is mediated by CYP2D6.Citation120 Desvenlafaxine is further metabolized by CYP3A4.Citation122 Other metabolic pathways for venlafaxine include N-demethylation which is probably mediated by CYP3A4.Citation157 CYP2C9 and CYP2C19 isoenzymes may also be involved in the metabolic pathways of both drugs.Citation121
In contrast with desvenlafaxine, the CYP2D6 genetic polymorphism has a significant influence on venlafaxine pharmacokinetics.Citation122 Both drugs may have low potential for drug interactions, because of low protein binding and a relatively weak inhibitory effect on CYP isoenzymes.Citation90,Citation123 Nevertheless, increased plasma levels of imipramine, its metabolite desimipramine,Citation124 and risperidone were associated with concomitant administration of venlafaxine.Citation125 Furthermore, diphenhydramine may alter the disposition of venlafaxine via inhibition of CYP2D6.Citation126 CYP3A4 inducers may enhance the clearance rate of desvenlafaxine.Citation122
Venlafaxine and desvenlafaxine are primarily excreted via the renal route.Citation157,Citation127 About 29% of a venlafaxine dose is excreted as the active metabolite.Citation156,Citation128 Both venlafaxine and desvenlafaxine are rapidly eliminated, and steady-state plasma concentrations are reached within three days. Both drugs show linear pharmacokinetics in the therapeutic range.Citation157
Age and gender differences are not clinically significant and require no dose adjustment for either drug.Citation129 Disposition of venlafaxine and desvenlafaxine may be affected by renal impairment, and a reduction in venlafaxine dose is recommended for patients with creatinine clearance rates <30 mL/min.Citation157,Citation166 Moreover, due to altered metabolism, patients with mild to moderate hepatic impairment require dose adjustment of venlafaxine and desvenlafaxine (see ).Citation157
Superior efficacy of venlafaxine compared with placebo and efficacy similar to that of the TCAs in major depression was reported.Citation22,Citation130,Citation131 However, venlafaxine was superior to TCAs in treatment-resistant depression.Citation170 Controversial reports exist concerning the relative efficacy of venlafaxine and SSRIs. Comparison of venlafaxine with sertraline and escitalopram showed similar efficacy in the treatment of severe depressive disorders.Citation76,Citation80,Citation132 Comparable efficacy has also been reported for venlafaxine, fluoxetine, paroxetine, and fluvoxamine.Citation22,Citation133 However, some authors observed superior efficacy of venlafaxine compared with duloxetine, fluoxetine, fluvoxamine, paroxetine, and reboxetine (see ),Citation79,Citation134,Citation135 while others found only increased efficacy compared with fluoxetine among the second-generation antidepressants.Citation136 Higher remission rates were observed with venlafaxine compared with SSRIs and placebo.Citation172 Long-term venlafaxine treatment was effective in reducing relapse after a major depressive episode.Citation170
Despite the conflicting evidence, venlafaxine may be a cost-effective alternative to fluoxetine and amitriptyline when used as first-line therapy.Citation137 Venlafaxine XR is also probably one of the best alternatives for patients who do not benefit from SSRIs.Citation46,Citation172 Overall response and remission rates in major depression were significantly better with desvenlafaxine 50–100 mg compared with placebo.Citation138
Venlafaxine is better tolerated than TCAs, but may cause a broader array of adverse events, such as dry mouth, constipation, increased pulse, and increased heart rate compared with the SSRIs.Citation46,Citation139 The blood pressure increase seems to be dose-dependent, and ranges from 2% at doses of 75–150 mg/day to 10% for 300 mg/day.Citation22,Citation140,Citation178 Discontinuation syndrome, characterized by nausea, insomnia, chills, irritability, and paresthesias may occur when venlafaxine is stopped abruptly (see ). This syndrome may be suppressed by switching to fluoxetine or tapering venlafaxine prior to withdrawal.Citation140 Furthermore, overdose with venlafaxine may be more serious than with the SSRIs.Citation46,Citation141 Tolerability is dose-dependent and may be improved by slower titration to higher doses.Citation142
Desvenlafaxine has an acceptable safety and tolerability profile.Citation143 A strong dose-response effect on tolerability was reported, but both 50 mg and 100 mg doses were well tolerated.Citation144,Citation177 Discontinuation rates due to adverse events were similar to those with placebo. The most common adverse event was transient mild to moderate nausea. Changes in mean blood pressure were small but statistically significant. Erectile dysfunction in man and anorgasmia in women were the most common sexual adverse events.Citation144
The usual dose of venlafaxine IR is 75–375 mg/day and 75–225 mg/day for venlafaxine XR.Citation46 With rapid venlafaxine dose escalation up to 375 mg/day, onset of efficacy can be achieved after only one week.Citation145 Use of higher doses may also improve response in treatment-resistant depression. However, higher venlafaxine doses (300–375 mg/day) were associated with poorer tolerability.Citation182 The usual dose of desvenlafaxine ranges from 50–100 mg once daily, although doses higher than 50 mg showed no evidence of better efficacy.Citation46,Citation184
Duloxetine
Duloxetine is an inhibitor of serotonin and noradrenaline reuptake, with more than 100-fold greater potency compared with venlafaxine.Citation146 Duloxetine has low affinity for D2, serotonin, α1- and α2-adrenergic, muscarinic, H1, and opioid receptors. Duloxetine does not inhibit gamma-amino butyric acid, choline transporters, MAO-A or MAO-B.Citation187
Duloxetine is absorbed within six hours following oral administration.Citation147 This process may be delayed by food and decreased by evening administration.Citation148 The drug has high protein binding and a high volume of distribution (see ).Citation149,Citation150
Extensive metabolism, predominantly via CYP1A2, to a lesser extent via CYP2D6, and at a very low rate via CYP2C9,Citation151,Citation152 has been reported, but the metabolites have no significant activity.Citation153 Duloxetine is a moderate CYP2D6 inhibitor and may inhibit its own metabolismCitation154,Citation155 as well as the metabolism of CYP2D6 substrates, such as desimipramine.Citation90,Citation195 The inhibition or induction of CYP1A2 is not clinically important, and coadministration of duloxetine with CYP1A2 substrates does not necessitate their dose adjustment.Citation193 However, potent inhibitors of CYP2D6 and CYP1A2 may result in enhanced duloxetine concentrations and a need for dose adjustment.Citation191,Citation193
Due to high protein binding, duloxetine may displace other extensively protein-bound drugs, such as warfarin.Citation191 Elimination of the drug is rapid and primarily via urine and feces.Citation192 Steady state is reached in three days.Citation190 Duloxetine has linear pharmacokinetics in the therapeutic range.Citation194
Female gender and nicotine use have been associated with higher duloxetine plasma levels.Citation196 Hispanic patients had a higher volume of distribution and delayed absorption compared with non-Hispanics.Citation196 Clearance decreases with increasing age, although this effect is small.Citation196 Hepatic impairment decreases the clearance of duloxetine, and dose adjustment is necessary in patients with liver disease (see ).Citation156
At doses of 40–120 mg/day, duloxetine shows superior efficacy compared with placebo in short-term studies (≤15 weeks).Citation198–Citation201 The efficacy of duloxetine in the treatment of painful somatic vegetative symptoms in patients with MDD is questionable.Citation157,Citation158
Duloxetine had better efficacy than paroxetine or fluoxetine only in patients with severe depression (see ).Citation159 The drug showed no significant difference in efficacy compared with venlafaxine,Citation160 but a lower risk of increased blood pressure and fewer discontinuation symptoms when treatment was stopped.Citation204,Citation161 Compared with escitalopram, similar onset and efficacy of duloxetine (60–120 mg/day) has been observed.Citation162,Citation163 In contrast, a meta-analysis reported that escitalopram, mirtazapine, sertraline, and venlafaxine were significantly more efficacious than duloxetine.Citation79
Generally, duloxetine is well tolerated both in short-term and long-term treatment of MDD (see ).Citation179,Citation191,Citation199,Citation201 The incidence of most common side effects may be dose-dependent. Citation164 Long-term treatment has a minimal effect on body weight,Citation165 whereas short-term treatment is associated with modest effects on blood pressure and heart rate, no clinically significant effect on Electrocardiogram profiles,Citation166 an increased incidence of sexual dysfunction,Citation167 and an increased risk of higher serum transaminase levels.Citation209
The safety and tolerability profile of duloxetine 40–120 mg/day is similar to that of paroxetine 20 mg/day.Citation168 However, duloxetine is less well tolerated than escitalopram.Citation207,Citation208 Patients on duloxetine experience higher rates of insomnia and constipation.Citation207 Furthermore, Cipriani et al reported poorer tolerability of duloxetine compared with sertraline.Citation67 Higher discontinuation rates were observed with duloxetine due to adverse events compared with venlafaxine. Nausea and dizziness were more frequent in patients on duloxetine, while patients on venlafaxine experienced significantly greater elevation of systolic blood pressure.Citation204
The usual starting dose is 40 mg/day (20 mg twice daily) to 60 mg/day (30 mg twice daily or 60 mg once daily) in the US and 60 mg once daily in the European Union.Citation191
Milnacipran
Milnacipran inhibits noradrenaline and serotonin uptake at presynaptic sites.Citation169 Despite the high affinity for both serotonin and noradrenaline transporters, noradrenaline reuptake is preferentially blocked.Citation170 Postsynaptic cholinergic, adrenergic, H1, D2, and serotonergic receptors are not affected.Citation171,Citation172
Following oral administration the onset of absorption is delayed.Citation173 Bioavailability is high and not affected by food.Citation174,Citation218 Milnacipran has low protein binding and extensive distribution in the body.Citation219,Citation174 The drug undergoes oxidative biotransformation via CYP3A4 and conjugation.Citation219 Only one of three metabolites has pharmacologic activity, but the concentrations are <1% of the parent compound. The risk of pharmacokinetic drug-drug interactions may be low.Citation175,Citation219 Moreover, induction or inhibition of CYP2D6 or CYP2C19 has no significant effect on milnacipran.Citation175
Due to potential pharmacodynamic interactions, milnacipran is contraindicated in patients receiving MAOIs. Concomitant administration of drugs that may influence serotonin metabolism, such as tramadol, triptanes, and linezolid, is not recommended or requires caution due to potential serotonin syndrome. Coadministration with digoxin may result in potentiation of hemodynamic effects, whereas coadministration with adrenaline and noradrenaline may be associated with paroxysmal hypertension and possibly arrhythmia.Citation218
Milnacipran elimination is rapid and predominantly renal.Citation218,Citation219 Steady-state concentrations are reached within a few days.Citation215,Citation221 The drug shows linear pharmacokinetics over the therapeutic dose range (see ).Citation218
Age and gender influence milnacipran plasma levels but dose adjustment is not necessary.Citation218 Milnacipran should be administered with caution in patients with severe hepatic or moderate to severe renal impairment (see ).Citation176,Citation215,Citation218,Citation220
Milnacipran 50 mg was significantly more effective than placebo in the treatment of MDD.Citation176,Citation177 Comparison of milnacipran with other antidepressants, such as SSRIs and TCAs, demonstrated no significant differences in clinical response or remission rates in the acute phase.Citation178,Citation179 Cipriani et al reported better scores for mirtazapine, escitalopram, venlafaxine, sertraline, and citalopram, than for milnacipran. In contrast, milnacipran scored better than bupropion, duloxetine, fluvoxamine, paroxetine, fluoxetine, and reboxetine.Citation67
Milnacipran is generally well tolerated (see ).Citation180,226 Milnacipran may be superior to TCAs and SSRIs in terms of need for premature treatment withdrawal due to adverse events. Patients who experienced adverse effects from other antidepressants in the acute phase of treatment may benefit from this drug.228
Cipriani et al reported better tolerability scores for escitalopram, sertraline, bupropion, and citalopram compared with milnacipran. In contrast, milnacipran scored better than mirtazapine, fluoxetine, venlafaxine, duloxetine, fluvoxamine, paroxetine, and reboxetine.Citation67
The usual dose range for milnacipran is 100–200 mg/day.Citation46 Titration of the dose is recommended. The initiation dose should be 12.5 mg on the first day and 12.5 mg twice daily on the second and third days, 25 mg twice daily on the fourth to seventh days, and 50 mg twice daily thereafter. Based on individual response, the dose should be increased to 100 mg twice daily.Citation218
Reboxetine
Reboxetine is a potent, selective, and specific noradrenaline reuptake inhibitor, with negligible affinity for muscarinic, H1, α1, and D2 receptors.Citation180
Reboxetine has two chiral centers, but only the (R,R)-(−)- and (S,S)-(+)-enantiomer is present in the marketed product. Some studies suggest that both the therapeutic and adverse effects are related predominantly to (S,S)-(+)- reboxetine.Citation181
Reboxetine is absorbed rapidly and almost completely after oral administration.Citation182,Citation183 Food delays but does not influence the extent of absorption (see ).Citation184 Reboxetine is extensively bound to plasma proteins and has a moderate distribution volume compared with other antidepressants.231–233 Metabolism occurs principally via CYP3A4.231 Each enantiomer is metabolized to the primary metabolite O-desethylreboxetine, and three other metabolites. Citation185 Reboxetine is probably not an inhibitor of CYP isoenzymes.Citation186,231
The drug has a moderate half-life and low clearance.231,232 Reboxetine exhibits linear pharmacokinetics in the therapeutic range.231 After multiple doses, steady state is achieved within four days.231
Ethnicity seems to influence reboxetine pharmacokinetics but dose adjustment is not necessary.Citation186 Plasma levels are higher and more variable in elderly patients, and therefore treatment with reboxetine should be initiated at a lower dose.Citation187,Citation188 The elimination rate of reboxetine decreases as renal function declines.Citation189 Elimination is also slower in patients with hepatic dysfunction, but the degree of dysfunction does not affect reboxetine pharmacokinetics (see ).Citation190
In short-term studies (4–6 weeks) reboxetine showed superior efficacy compared with placebo in primary and secondary outcomes.242–244 Overall, reboxetine scored significantly better in mean responder rate and relapse rates compared with placebo (see ).Citation191,Citation192,242 Compared with imipramine, the efficacy of reboxetine was similar in adultsCitation193 and elderly patients,Citation194 but reboxetine had significant advantages in the treatment of melancholic patients.Citation195 Similar efficacy of reboxetine and fluoxetine was reported, but reboxetine was more effective in a sub-group of severely depressed patients.Citation196,Citation197 Moreover, social functioning was better in patients who achieved remission with reboxetine.250
Nevertheless, Cipriani et al suggested that reboxetine was significantly less effective than bupropion, citalopram, duloxetine, escitalopram, fluoxetine, fluvoxamine, milnacipran, mirtazapine, paroxetine, sertraline, and venlafaxine.Citation79
The drug showed a good safety and tolerability profile (see ).242 Reboxetine and imipramine had similar tolerability in adults and the elderly. Frequency of discontinuation due to adverse events was lower in the reboxetine group, whereas cumulative risk of hypotension, dry mouth, and tremor was significantly higher in the imipramine group.247 Reboxetine patients had a lower risk of serious adverse events, adverse event-related withdrawals, and treatment-related adverse events.248 The overall score of reboxetine for safety and tolerability was better than TCAs.
The adverse event profile of reboxetine is different to that of the SSRIs. Patients on reboxetine experienced less agitation, nervousness, anxiety, and gastrointestinal events compared with those on fluoxetine. Reboxetine was not associated with an increased risk of seizures, orthostatic hypotension, or cardiotoxicity,250 but had poorer tolerability than other antidepressants, including bupropion, citalopram, escitalopram, fluoxetine, and sertraline.Citation79
Nevertheless, reboxetine is considered safe when administered at doses of 8–10 mg/day to adult (18–65 years) and at 4–6 mg/day to elderly (>65 years) patients.242
The recommended therapeutic dose for adults is 4 mg twice daily (8 mg/day). The dose can be increased to 10 mg/day after three weeks if there is an inadequate clinical response. The recommended dose for the elderly (>65 years) is 2 mg bid (4 mg/day) and, if necessary, the dose can be increased to 6 mg/day. The same strategy is used for patients with renal impairment or moderate to severe hepatic insufficiency.242
Agomelatine
Agomelatine is an antagonist of serotonin 5HT2B and 5HT2C receptors and a potent agonist of melatonergic MT1 and MT2 receptors.Citation198,Citation199 Serotonin outflow is not affected, but due to 5HT2C antagonism, overflows of dopamine and noradrenaline are produced in the frontal cortex.Citation200 The drug received marketing authorization for Europe in 2009 and is awaiting Federal Drug Administration approval in the US.
After oral administration, more than 78% of the dose is rapidly absorbed.254 Agomelatine is highly protein-bound and moderately distributed (see ).253
Metabolism to inactive hydroxylated and demethylated metabolites is mediated primarily by CYP1A2 and to a less degree by CYP2C9 and CYP2C19.Citation201 There is a lack of data about potential drug interactions, and this requires further investigation. The metabolites are excreted mainly in urine and feces.253,254 Elimination rate is very fast and steady-state concentrations are reached rapidly.253
The bioavailability of agomelatine may be increased in women and reduced in smokers.255 There are limited data about the pharmacokinetics of agomelatine in the elderly and in patients with renal impairment. Nevertheless, systemic exposure to agomelatine is increased in patients with hepatic impairment (see ).253,255
Agomelatine significantly improved response rates and time to first response compared with placebo in 212 outpatients who received 25 or 50 mg/day.Citation202 Moreover, the onset of response with agomelatine was faster (two weeks) compared with paroxetine (four weeks).Citation203 Higher efficacy than placebo was observed in patients with severe depression and efficacy increased with increasing severity of depression.Citation204 Agomelatine 50 mg/day and venlafaxine 75–150 mg/day had similar response rates after 6–12 weeks.Citation205,Citation206
Agomelatine was generally well tolerated, with a good safety profile (see ).256 Compared with venlafaxine, the agomelatine treatment group experienced less frequent sexual dysfunction and orgasmic dysfunction.259 Safety profile was generally better compared with current standard treatments, including absence of serotonin syndrome, weight gain, and a low incidence of sexual dysfunction and gastrointestinal adverse effects.252 Abrupt cessation of agomelatine was not associated with discontinuation symptoms.Citation207
Despite encouraging results for the safety and tolerability of agomelatine, there is still a lack of data regarding its efficacy which requires further investigation. The usual initiating dose is 25 mg/day which may be increased if necessary to 50 mg/day.253
Aripiprazole
Aripiprazole is an atypical antipsychotic approved as a adjunct treatment for MDD. The probable mechanism of antidepressant action is partial agonism at D2, D3, and 5-HT1A receptors and antagonistic activity at 5-HT2A receptors. Moderate affinity was also found for D4, 5-HT2C, 5-HT7, α1-adrenergic, and H1 receptors, whereas the activity at muscarinic and cholinergic receptors was minimal.Citation208
Aripiprazole is well absorbed following oral administration. Food prolonged the time of absorption for approximately three hours but did not affect extent of absorption (see ). The drug is almost completely bound to plasma proteins. Following metabolism mediated by CYP3A4 and CYP2D6, the active metabolite, dehydroaripiprazole, is formed. Genetic polymorphism of CYP2D6 has a significant influence on aripiprazole plasma levels, and poor metabolizers have an approximately 60% increased exposure to the drug. Aripiprazole has low inhibitory potential for CYP450 isoenzymes. No relevant interactions were observed after coadministration of the drug with SSRIs and venlafaxine. In contrast, concomitant administration of CYP3A4 inducers may require increased doses of aripiprazole whereas concomitant administration of CYP2D6 or CYP3A4 inhibitors may require dose reduction for aripriprazole.262
Aripiprazole is eliminated slowly, therefore takes about two weeks to reach steady state. Urine and feces are the main elimination routes.262 Age, race, gender, smoking status, and hepatic and renal function showed no clinically relevant effects on aripiprazole pharmacokinetics.262
A meta-analysis of clinical efficacy trials of aripiprazole (2–20 mg/day) revealed increased response rates of 8% and increased remission rates of 10% when the drug was used as adjuvant antidepressant medication compared with placebo in patients with MDD (see ).Citation209,Citation210 However, the absolute difference in the efficacy outcome between aripiprazole and placebo was relatively low, and therefore the clinical significance of the findings is debatable.Citation211,Citation212 Because augmentation is used in patients who have failed to respond to monotherapy, evaluation of clinical relevance is difficult and further studies are necessary.
The most common adverse effects of aripiprazole are presented in . Discontinuation of treatment due to adverse effects was rarely observed, and no serious adverse effects were reported.263,264
The starting dose for adjunctive aripiprazole treatment should be 2–5 mg/day. If necessary, a weekly dose increase is recommended up to 15 mg/day. The drug is not approved for the treatment of patients with dementia-related psychosis or depressive pediatric patients.262 Further investigations with aripiprazole are necessary to establish its full potential in the treatment of MDD.
Emergence of new therapeutic agents
Vilazodone is a serotonin reuptake inhibitor and a partial 5-HT1a agonist. This drug is currently under clinical evaluation for the treatment of major depression and awaiting approval by the Federal Drug Administration. So far, results for the clinical efficacy of vilazodone in depressed patients have been conflicting. A large Phase II trial including more than 1000 depressed patients failed to show efficacy of the drug over placebo.Citation213 In contrast, a Phase III trial which included 410 patients with MDD revealed superior efficacy of viladozone (10–40 mg/day) over placebo in primary and secondary outcomes within eight weeks.Citation214 Vilazodone was well tolerated, and adverse effects were mild to moderate, including nausea, somnolence, diarrhea, and dizziness.
Serotonin, noradrenaline, and dopamine (triple) reuptake inhibitors are in process of development,Citation215 and most are now in Phase II clinical trials.Citation216 Some of these drugs (eg, DOV 21947) show significantly higher efficacy compared with placebo and similar efficacy to citalopram.269 In contrast, lack of improved efficacy resulting in discontinued development (NS-2359) was also reported.Citation217
Drugs that antagonize α2-adenoreceptors and suppress reuptake of serotonin or noradrenaline or both (S35966 and R226161) may have a faster onset of effect, and improve cognition and sexual function. However, adverse effects comprising increased arterial pressure and tachycardia were reported.Citation218,Citation219,267
Dual-acting serotonin reuptake inhibitors and H3 antagonists (eg, JNJ-2583867) may improve mood and cognitive impairment in depression and have a low risk of obesity. A possible disadvantage of these substances may be their wake-inducing action.Citation220,267
Some emerging evidence suggests that several families of glutamate receptors may be potential targets for new antidepressants.267,269 CP-10-606, an N-methyl-D-aspartic acid antagonist, significantly improved depressive symptoms compared with placebo.269 There are some suggestions that positive allosteric modulators at α-amino-3-hydroxy- 5-methyl-4-isoxazolepropionic acid receptor (AMPA) receptors may be useful in the clinical management of depression.267,269 However, clinical data on these agents are not yet available.269
Riluzole, a glutamate-modulating agent, approved for treatment of amyotrophic lateral sclerosis, showed antidepressant properties and was well tolerated.Citation221,269 However, its role as monotherapy or augmentation of standard therapy remains to be established.
Metyrapone, an inhibitor of cortisol synthesis, may become a possible adjunctive agent for major depression. 269 Also, a strong antagonist of the Type II glucocorticoid receptor, mifepristone (RU486) was suggested as adjunctive treatment for psychotic depression. 269 However, pivotal Phase III studies of mifepristone in MDD with psychotic features have had discouraging results.269
Compliance
One of the major obstacles to effective management of depression is poor compliance.Citation220 Sawada et al found that only 44.3% patients continued antidepressant treatment after six months. Moreover, 63.1% patients who discontinued therapy did so without consulting their physicians.Citation221
Reasons for treatment discontinuation are multifactorial. Symptoms of depression such as poor concentration and motivation may predispose patients to noncompliance. Early withdrawals are usually due to adverse events or lack of efficacy. However, later dropouts are usually due to patients feeling better or fearing drug addiction. Studies suggest men are more likely to discontinue antidepressant therapy than women following initial treatment efficacy.276
Choice of an adequate, effective, and well tolerated drug with optimal formulation, as well as effective communication between patient and health professionals, are important for successful treatment. The newer, more selective antidepressants may have better tolerability and hence better compliance.277 Moreover, drugs with a longer half-life and once-daily dosing schedules will improve patient compliance.276,277 Patients suggest that information about adverse events and likely duration of treatment may significantly improve compliance. Well informed patients are less likely to discontinue treatment and more likely to switch drug if necessary.Citation222,Citation223
Conclusion
MDD is a complex disease and requires a multifaceted approach for research, diagnosis, and treatment. Modern classes of antidepressants such as SSRIs, serotonin/noradrenaline reuptake inhibitors, and noradrenaline/dopamine reuptake inhibitors offer superior tolerability and safety over older medications like the TCAs and MAOIs. However, the choice among newer antidepressants is difficult, given that all of them showed more or less similar efficacy and good tolerability. Nevertheless, individual patient preferences related to adverse effect profiles and cost of treatment, as well as adjusting the regimen appropriately, may provide the best approach. If a single drug fails, combined treatment with antidepressants having different modes of action may improve treatment efficacy. However, with such approach, the increased risk of interactions should be considered.
It is clear that there are substantial limitations in current antidepressant pharmacotherapy and there is a need for new therapeutic approaches. Advances in understanding the neurobiology of depression have opened up a new era of investigations with novel therapeutic approaches and compounds based on new mechanisms of action. Today, research is focused on a variety of targets such as the L-arginine- nitric oxide-cyclic guanosine monophosphate pathway, the endocannabinoid system, sigma-1 receptors, melatonin, 5-HT6 and 5-HT7 receptor antagonists, β3 adrenergic antagonists, vasopressin receptor antagonists, and NK2 tachykinin receptor antagonists. Although the potential efficacy of these agents remains to be established, the future of antidepressant treatment appears to be promising.
Acknowledgment
We would like to thank the Ministry of Science, Belgrade, Republic of Serbia (Project No 145001) for its support.
Disclosure
The authors report no conflict of interest in this work.
References
- PrattLABrodyDJDepression in the United States household population, 2005–2006NCHS Data Brief200871819389321
- KesslerRCBerglundPDemlerOThe epidemology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R)JAMA2003289233095310512813115
- EbmeierKPDonagheyJCSteeleJDRecent developments and current controversies in depressionLancet2006367950515316716413879
- NIH Consensus ConferenceDiagnosis and treatment of depression in late lifeJAMA19922688101810241501308
- RothschildAJThe diagnosis and treatment of late-life depressionJ Clin Psychiatry19965755118647792
- SteffensDCSkoogINortonMCPrevalence of depression and its treatment in an eldery population: The Cache County studyArch Gen Psychiatry200057660160710839339
- BelmakerRHAgamGMajor depressive disorder: Mechanisms of diseaseN Engl J Med20083581556818172175
- JainRThe implication of pain and physical symptoms in depressionJ Clin Psychiatry2009706e1919573475
- aan het RotMMathewSJCharneyDSNeurobiological mechanisms in major depressive disorderCMAJ2009180330531319188629
- CaspiASugdenKMoffittTEInfluence of life stress on depression: Moderation by a polymorphism in the 5-HTT geneScience2003301563138638912869766
- HansenneMBianchiJEmotional intelligence and personality in major depression: Trait versus state effectsPsychiatry Res20091661636819193446
- ZhangXGainetdinovRRBeaullieuJMLoss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depressionNeuron2005451111615629698
- PitchotWHansenneMPintoEReggersJFuchsSAnsseauM5-Hydroxytryptamine 1A receptor, major depression, and suicidal behaviorBiol Psychiatry2005581185485816139805
- SvenningsonPCherguiKRachleffIAlterations in 5-HT1B receptor function by p11 in depression-like statesScience20063115757778016400147
- OrdwayGASchenkJStockmeierCAMayWKlimekVElevated agonist binding to alpha 2-adrenoceptors in the locus coeruleus in major depressionBiol Psychiatry200353431532312586450
- CouplandNJOgilvieCJHegadorenKMSeresPHanstockCCAllenPSDecreased prefrontal myo-inositol in major depressive disorderBiol Psychiatry200557121526153415953489
- ValdizanEMGutierrezOPazosAAdenylate cyclase activity in post-mortem brain of suicide subjects: Reduced response to beta-adrenergic stimulationBiol Psychiatry200354121457146414675811
- BlendyJAThe role of CREB in depression and antidepressant treatmentBiol Psychiatry200659121144115016457782
- MannJJThe medical management of depressionN Engl J Med2005353171819183416251538
- VreeburgSAHoogendijkWJvan PeltJMajor depressive disorder and hypothalamic-pituitary-adrenal axis activity: Results from a large cohort studyArch Gen Psychiatry200966661762619487626
- BurkeHMDavisMCOtteCMohrDCDepression and cortisol responses to psychological stress: A meta-analysisPsychoneuroendocrinology200530984685615961250
- BaghaiTCVolzHPMollerHJDrug treatment of depression in the 2000s: An overview of achievements in the last 10 years and future possibilitiesWorld J Biol Psychiatry20067419822217071541
- MacQueenGMCampbellSMcEwenBSCourse of illness, hippocampal function, and hippocampal volume in major depressionProc Natl Acad Sci U S A200310031387139212552118
- KaregeFVaudanGSchwaldMPerroudNLa HarpeRNeurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugsBrain Res Mol Brain Res200513612293715893581
- SanacoraGZarateCAKrystalJHManjiHKTargeting the glutamatergic system to develop novel, improved therapeutics for mood disordersNat Rev Drug Discov20087542643718425072
- HaslerGvan der VeenJWTumonisTMayersNShenJDrevetsWCReduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopyArch Gen Psychiatry200764219320017283286
- LewyAJLeflerBJEmensJSBauerVKThe circadian basis of winter depressionProc Natl Acad Sci U S A2006103197414741916648247
- BeasleyCLHonerWGBergmannKFaikaiPLutjohannDBayerTAReductions in cholesterol and synaptic markers in association cortex in mood disordersBipol Disord200575449455
- KennedySEKoeppeRAYoungEAZubeitaJKDysregulation of endogenous opoid emotion regulation circuitry in major depression in womenArch Gen Psychiatry200663111199120817088500
- JanowskyDSOverstreetDHSoaresJCYoungAHCholinergic-muscarinic dysfunction in mood disordersBipolar Disorders: Basic Mechanisms and Therapeutic Implications2nd edNew York, NYInforma Healthcare2007
- SullivanGMMannJJOquendoMALoESCooperTBGormanJMLow cerebrospinal fluid transthyretin levels in depression: Correlations with suicidal ideation and serotonin functionBiol Psychiatry200660550050616487493
- MilakMSParseyRVKeilpJOquendoMAMaloneKMMannJJNeuroanatomic correlates of psychopathologic components of major depressive disorderArch Gen Psychiatry200562439740815809407
- NemeroffCBHeimCMThaseMEDifferential response to psycho-therapy versus pharmacotherapy in patients with chronic forms of major depression and childhood traumaProc Natl Acad Sci U S A200310024142931429614615578
- ImelZEMaltererMBMcKayKMA meta-analysis of psychotherapy and medication in unipolar depression and dysthymiaJ Affect Disord2008110319720618456340
- KrishanKRRevisiting monoamine oxidase inhibitorsJ Clin Psychiatry200768Suppl 8S35S41
- FiedorowiczJGSwartzKLThe role of monoamine oxidase inhibitors in current psychiatric practiceJ Psychiatr Pract200410423924815552546
- JessenLKovalickLJAzzaroAJThe selegiline transdermal system (Emsam): A therapeutic option for the treatment of major depressive disorderP T200833421224619750165
- GillmanPKTricyclic antidepressant pharmacology and therapeutic drug interactions updatedBr J Pharmacol2007151673774817471183
- BurkeMJPreskornSHTherapeutic drug monitoring of antidepressants: Cost implications and relevance to clinical practiceClin Pharmacokinet199937214716510496302
- BillupsSJDelateTDuganDEvaluation of risk factors for elevated tricyclic antidepressant plasma concentrationsPharmacoepidemiol Drug Saf200918325325719148878
- AndersonIMSSRI versus tricyclic antidepressants in depressed inpatients: A meta-analysis of efficacy and tolerabilityJ Affect Disord2000581193610760555
- GervasoniNAurbyJMGex-FarbyMBertschyGBondolfiGIs there a place for tricyclic antidepressant and subsequent augmentation strategies in obtaining remission for patients with treatment resistant depression?Pharmacol Res200959320220619073260
- KoenigAMThaseMEFirst-line pharmacotherapies for depression – what is the best choicePol Arch Med Wewn200911978478486
- HiemkeCHartterSPharmacokinetics of selective serotonin reuptake inhibitorsPharmacol Ther2000851112810674711
- DraperBBermenKTolerability of selective serotonin reuptake inhibitorsDrugs Aging200825650151918540689
- GreenblattDJvon MoltkeLLSchmiderJHarmatzJSShaderRIInhibition of human cytochrome P4503A isoforms by fluoxetine and norfluoxetine: In vitro and in vivo studiesJ Clin Pharmacol19963697927988889899
- OwensMJKnightDLNemeroffCBSecond-generation SSRIs: Human monoamine transporter binding profile of escitalopram and R-fluoxetineBiol Psychiatry200150534535011543737
- RaoNThe clinical pharmacokinetics of escitalopramClin Pharmacokinet200746428129017375980
- SøgaardBMengelHRaoNLarsenFThe pharmacokinetics of escitalopram after oral and intravenous administration of single and multiple doses to healthy subjectsJ Clin Pharmacol200545121400140616291715
- SidhuJPriskornMPoulsenMSegonzacAGrollierGLarsenFSteady-state pharmacokinetics of the enantiomers of citalopram and its metabolites in humansChirality1997976866929366029
- RochatBKoselMBossGTestaBGilletMBaumannPStereoselective biotransformation of the selective serotonin reuptake inhibitor citalopram and its demethylated metabolites by monoamine oxidase in human in human liverBiochem Pharmacol199856115239698084
- KoselMGnerreCVoirolPIn vitro biotransformation of the selective serotonin reuptake inhibitor citalopram, its enantiomers and demethylated metabolites by monoamine oxidase in rat and human brain preparationsMol Psychiatry20027218118811840311
- GutierrezMMRosenbergJAbramowitzWAn evaluation of the potential for pharmacokinetic interaction between escitalopram and the cytochrome P450 3A4 inhibitor ritonavirClin Ther20032541200121012809966
- MallingDPoulsenMNSogaardBThe effect of cimetidine or omeprazole on the pharmacokinetics of escitalopram in healthy subjectsBr J Clin Pharmacol200560328729016120067
- JoffePLarsenFSPedersenVLarsen-RingHJørgensen-AaesTSidhuJSingle-dose pharmacokinetics of citalopram in patients with moderate renal insufficiency or hepatic cirrhosis compared with healthy subjectsEur J Clin Pharmacol199854392372429681666
- PericlouARaoNShermanTVenturaDAbramowitzWSingle-dose pharmacokinetics study of escitalopram in adolescents and adultsPharmacotherapy2003231013611362
- CiprianiASantilliCFurukawaTAEscitalopram versus other antidepressive agents for depressionCochrane Database Syst Rev2009CD00653219370639
- EmslieGJVenturaDKorotzerATourkodimitrisSEscitalopram in the treatment of adolescent depression: A randomized placebo-controlled multisite trialJ Am Acad Child Adolesc Psychiatry200948772172919465881
- LlorcaPMAzorinJMDespiegelNVerpillatPEfficacy of escitalopram in patients with severe depression: A pooled analysisInt J Clin Pract200559326827515857321
- SvenssonSMansfieldPREscitalopram: Superior to citalopram or a chiral chimera?Psychother Psychosom2004731101614665791
- AlexopoulosGSPriviteraWVenturaDBoseAWangQDouble-blind comparison of escitalopram 10 mg/day and optimally-dosed sertraline 50–200 mg/day in the treatment of major depressive disorderPoster presented at the 42nd annual meeting of the American College of NeuropsychopharmacologySan Juan, Puerto RicoDecember 7–11, 2003
- KasperSBaldwinDSLonnSLBoulengerJPSuperiority of escitalopram to paroxetine in the treatment of depressionEur Neuropsychopharmacol200919422923719185467
- MontgomerySAMollerHJIs the significiant superiority of escitalopram compared with other antidepressants clinically relevant?Int Clin Psychopharmacol200924311111819357527
- BielskiRJVenturaDChangDDA double-blind comparison of escitalopram and venlafaxine extended release in the treatment of major depressive disorderJ Clin Psychiatry20046591190119615367045
- KennedySHAndersenHFThaseMEEscitalopram in the treatment of major depressive disorder: A meta-analysisCurr Med Res Opin200925116117519210149
- KornsteinSGLiDMaoYLarssonSAndersenHFPapakostasGIEscitalopram versus SNRI antidepressants in the acute treatment of major depressive disorder: Integrative analysis of four double-blind, randomized clinical trialsCNS Spectr200914632633319668123
- CiprianiAFurukawaATSalantiGComparative efficacy and acceptability of 12 new-generation antidepressants: A multiple-treatments meta-analysisLancet2009373966574675819185342
- MontgomerySAHuusomAKTBothmerJA randomized study comparing escitalopram with venlafaxine XR in primary care patients with major depressive disorderNeuropsychobiology2004501576415179022
- BechPAndersenHFWadeAEffective dose of escitalopram in moderate versus severe DSM-IV major depressionPharmacopsychiatry200639412813416871468
- BaldwinDSSteinDJDolbergOTBandelowBHow long should a trial of escitalopram treatment be in patients with major depressive disorder? An exploration of a randomized controlled trial databaseHum Psychopharmacol200924426927519334042
- CroomKFPerryCMPloskerGLMirtazapine: A review of its use in major depression and other psychiatric disordersCNS Drugs200923542745219453203
- AnttilaSAKLeinonenEVJA review of the pharmacological and clinical profile of mirtazapineCNS Drugs Rev 200173249264
- VoortmanGPaanakkerJEBioavability of mirtazapine from Remeron® tablets after single and multiple oral dosingHum Psychopharmacol199510Suppl 2S83S96
- DelbressineLPCMoonenMEGKaspersenFMPharmacokinetics and biotransformation of mirtazapine in human volunteersClin Drug Invest19981514555
- SpaansEvan den HeuvelMWSchnabelPGConcomitant use of mirtazapine and phenytoin: A drug-drug interaction study in healthy male subjectsEur J Clin Pharmacol200258642342912242602
- RuweFJSmuldersRAKleijnHJMirtazapine and paroxetine: A drug-drug interaction study in healthy subjectsHum Psychopharmacol200116644945912404553
- AnttilaAKRasanenLLeinonenEVFluvoxamine augmentation increases serum mirtazapine concentrations three- to four-foldAnn Pharmacother200135101221122311675851
- SitsenJMMarisFATimmerCJConcomitant use of mirtazapine and cimetidine: A drug-drug interaction study in healthy male subjectsEur J Clin Pharmacol200056538939411009047
- GrasmäderKVerwohltPLKühnKUPopulation pharmacokinetic analysis of mirtazapineEur J Clin Pharmacol200460747348015289959
- BrockmöllerJMeinekeIKirchheinerJPharmacokinetics of mirtazapine: Enantioselective effects of the CYP2D6 ultra rapid metabolizer genotype and correlation with adverse effectsClin Pharmacol Ther200781569970717329996
- TimmerCJLohmannAAMMinkCPAPharmacokinetic dose-proportionality study at steady state of mirtazapine from Remeron® tabletsHum Psychopharmacol199510Suppl 2S97S106
- LindABReisBengtssonFSteady-state concetration of mirtazapine, N- desmethylmirtazapine, 8-hydroxymirtazapine and their enantiomers in relation to cytochrome P450 2D6 genotype, age and smoking behaviourClin Pharmacokinet2009481637019071885
- BengtssonFHöglundPTimmerCHegbrantJMirtazapine oral single dose kinetics in patients with different degrees of renal failureHum Psychopharmacol1998135357365
- Organon USA IncRemeron (mirtazapine) tablets: US prescribing information (online) Avaliable from URL:http://www.organon.comAccessed May 5, 2010
- MontgomerySAReimitzPEZivkovMMirtazapine versus amitriptyline in long-term treatment of depression: A double-blind placebo-controlled studyInt Clin Psychopharmacol199813263739669186
- WatanabeNOmoriIMNakagawaAMirtazapine versus other antidepressants in the acute-phase treatment of adults with major depression: Systematic review and meta-analysisJ Clin Psychiatry20086991404141519193341
- GuelfiJDAnsseauMTimmermanLKørsgaardSMirtazapine versus venlafaxine in hospitalized severely depressed patients with melancholic featuresJ Clin Psychopharmacol200121442543111476127
- PapakostasGIHombergerCHFavaMA meta-analysis of clinical trails comparing mirtazapine with selective serotonin reuptake inhibitors for the treatment of major depressive disorderJ Psychopharmacol200822884384818308801
- StahlSMPradkoJFHaightBRModellJGRockettCBLearned- CoughlinSA review of the neuropharmacology of bupropion, a dual norepinephrine and dopamine reuptake inhibitorPrim Care Companion J Clin Psychiatry20046415916615361919
- DhillonSYangLPHCurranMPBupropion: A review of its use in the managment of major depressive disorderDrugs200868565368918370448
- JeffersonJWPradkoJFMuirKTBupropion for major depressive disorder: Pharmacokinetic and formulation considerationsClin Ther200527111685169516368442
- RotzingerSBourinMAkimotoYCouttsRTBakerGBMetabolism of some “second” and “fourth” generation antidepressants: Iprindole, viloxazine, bupropion, mianserin, maprotilin, trazodone, nefazodone, and venlafaxineCell Mol Neurobiol199919442744210379419
- HesseLMVenkatakrishnanKCourtMHCYP2B6 mediates the in vitro hydroxylation of bupropion: Potential drug interactions with other antidepressantsDrug Metab Dispos20028101176118310997936
- KirchheinerJKleinCMeinekeIBupropion and 4-O-Hbupropion pharmacokinetics in relation to genetic polymorphisms in CYP2B6Pharmacogenetics2003131061962614515060
- KetterTAJenkinsJBSchroederDHCarbamazepine but not valproate induces bupropion metabolismJ Clin Psychopharmacol19951553273338830063
- HogelandGWSwindellsSMcNabbJCKashubaADYeeGCLindleyCMLopinavir/ritonavir reduces bupropion plasma concentrations in healthy subjectsClin Pharmacol Ther2007811697517186001
- KennedySHMcCannSMMasellisMCombining bupropion SR with venlafaxine, paroxetine, or fluoxetine: A preliminary report on pharmacokinetic, therapeutic, and sexual dysfunction effectsJ Clin Psychiatry200263318118611926715
- McCollumDLGreeneJLMcGuireDKSevere sinus bradycardia after initiation of bupropion therapy: A probable drug-drug interaction with metoprololCardiovasc Drugs Ther200418432933015367832
- StewartJJBerkelHJParishRCSingle-dose pharmacokinetics of bupropion in adolescents: Effects of smoking status and genderJ Clin Pharmacol200141777077811452710
- HsyuPHSinghAGiargiariTDDunnJAAscherJAJohnstonJAPharmacokinetics of bupropion and its metabolites in cigarette smokers versus nonsmokersJ Clin Pharmacol19973787377439378846
- SweetRAPollockBGKirshnerMWrightBAltieriLPDeVaneCLPharmacokinetics of single- and multiple-dose bupropion in elderly patients with depressionJ Clin Pharmacol19953598768848786247
- TurpeinenMKoivuviitaNTolonenAEffect of renal impairment on the pharmacokinetics of bupropion and its metabolitesBr J Clin Pharmacol200764216517317335546
- ReimherrFWCunninghamLABateySRJohnstonJAAscherJAA multicenter evaluation of the efficacy and safety of 150 and 300 mg/d sustained-release bupropion tablets versus placebo in depressed outpatientsClin Ther19982035055169663366
- JeffersonJWRushAJNelsonJCExtended-release bupropion for patients with major depressive disorder presenting with symptoms of reduced energy, pleasure, and interest: Findings from a randomized, double-blind, placebo-controlled studyJ Clin Psychiatry200667686587316848645
- MascoHLKievAHollomanLCBateySRJohnstonJALineberryCGSafety and efficacy of bupropion and nortriptyline in outpatients with depressionCurr Ther Res1994557851863
- MendelsJAminMMChouinardGA comparative study of bupropion and amitriptyline in depressed outpatientsJ Clin Psychiatry1983445 Pt 21181206406439
- FeighnerJPGardnerEAJohnstonJADouble-blind comparison of bupropion and fluoxetine in depressedoutpatients J Clin Psychiatry 1991528329335
- ColemanCCKingBRBolden-WatsonCA placebo-controlled comparison of the effects on sexual functioning of bupropion sustained release and fluoxetineClin Ther20012371040105811519769
- WeihsKLSettleEJrBateySRHouserTLDonahueRMAscherJABupropion sustained release versus paroxetine for the treatment of depression in the elderlyJ Clin Psychiatry200061319620210817105
- ClaytonAHCroftHAHorriganJPBupropion extended release compared with escitalopram: Effects on sexual functioning and antidepressant efficacy in two randomized, double-blind, placebo-controlled studiesJ Clin Psychiatry200667573674616841623
- HewettKChrzanowskiWSchmitzMEight-week, placebo-controlled, double-blind comparison of the antidepressant efficacy and tolerability of bupropion XR and venlafaxine XRJ Psychopharmacol200923553153818635695
- ThaseMEClaytonAHHaightBRThompsonAHModellJGJohnstonJAA double-blind comparison between bupropion XR and venlafaxine XR: Sexual functioning, antidepressant efficacy, and tolerabilityJ Clin Psychopharmacol200626548248816974189
- RushAJTrivediMHWisniewskiSRBupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depressionN Engl J Med2006354121231124216554525
- TrivediMHFavaMWisniewskiSRMedication augmentation after the failure of SSRIs for depressionN Eng J Med20063541212431252
- RoseboomPHKalinNHNeuropharmacology of venlafaxineDepress Anxiety200012Suppl 1S20S29
- TroySMParkerVDFruncilloRJChiangSTThe pharmacokinetics of venlafaxine when given in twice-daily regimenJ Clin Pharmacol19953544044097650231
- TroySMDileaCMartinPTLeisterCAFruncilloRJChiangSTPharmacokinetics of once-daily venlafaxine extended release in healthy volunteersCurr Ther Res1997568504514
- EreshefskyLDuganDReview of the pharmacokinetics, pharmacogenetics, and drug interaction potential of antidepressants: Focus on venlafaxineDepress Anxiety200012Suppl 1S30S44
- IlettKFKristensenJHHackettPPaechMKohanRRamponoJDistribution of venlafaxine and its O-desmethyl metabolite in human milk and their effects in breastfed infantsBr J Clin Pharmacol2002531172211849190
- OttonSVBallSECheungSWInabaTRudolphRLSellersEMVenlafaxine oxidation in vitro is catalysed by CYP2D6Br J Clin Pharmacol19964121491568838442
- FogelmanSMSchmiderJVenkatakrishnanKO- and N- demethylation of venlafaxine in vitro by human liver microsomes and by microsomes from cDNA-transfected cells: Effect of metabolic inhibitors and SSRI antidepressantsNeuropsychopharmacology199920548049010192828
- PreskornSPatronevaASilmanHComparation of the pharmacokinetics of venlafaxine extended release and desvenlafaxine in extensive and poor cytochrome P450 2D6 metabolizersJ Clin Psychopharmacol2009291394319142106
- PreskornSHNicholsAIPaulJPatranevaALHelznerECGuico-PabiaCJEffect of desvenlafaxine on the cytochrome P450 2D6 enzyme systemJ Psychiatr Pract200814636837819057238
- AlbersLJReistCVuRLEffect of venlafaxine on imipramine metabolismPsychiatry Res200096323524311084219
- AmchinJZarycranskiWTaylorKPAlbanoDKlockowskiPMEffect of venlafaxine on the pharmacokinetics of risperidoneJ Clin Pharmacol199939329730910073330
- LessardEYessineMAHamelienBADiphenhydramine alters the disposition of venlafaxine through inhibition of CYP2D6 activity in humansJ Clin Psychopharmacol200121217518411270914
- TroySMSchultzRWParkerVDChiangSTBlumRAThe effect of renal diseaase on the disposition of venlafaxineClin Pharmacol Ther199456114218033490
- HowellSRHusbandsGEMScatinaJASisenwineSFMetabolic disposition of 14C-venlafaxine in mouse, rat, dog, rhesus monkey and manXenobiotica1993234349598337893
- KlamerusKJParkerVDRudolphRLDerivanATChiangSTEffects of age and gender on venlafaxine pharmacokineticsPharmacotherapy1996165915238888087
- ThaseMEEfficacy and tolerability of once-daily venlafaxine extended release (XR) in outpatients with major depression. The Venlafaxine XR 209 Study GroupJ Clin Psychiatry19975893933989378690
- BauerMTharmanathanPVolzHPMoellerHJFreemantleNThe effect of venlafaxine compared with other antidepressants and placebo in the treatment of major depressionEur Arch Psychiatry Clin Neurosci2009259317218519165525
- SirAD’SouzaRFUguzSRandomized trial of sertraline versus venlafaxine XR in major depression: Efficacy and discontinuation symptomsJ Clin Psychiatry200566101312132016259546
- ThaseMEntsuahARudolphRRemission rates during treatment with venlafaxine or selective serotonine reuptake inhibitorsBr J Psychiatry2001178323424111230034
- ThaseMEEntsuahRAhmedSSloanDMNemeroffCBMeta-analysis of randomized controlled trials comparing venlafaxine and SSRIs: The evidence revisitedPresented at the 2005 annual meeting of the American Psychiatric AssociationAtlanta, GeorgiaMay 21–26, 2005
- AndersonIMMeta-analytical studies of new antidepressantsBr Med Bull200157116117811719915
- HansenRAGartlehnerGLohrKNGaynesBNCareyTSEfficacy and safety of second-generation antidepressants in the treatment of major depressive disorderAnn Intern Med2005143641542616172440
- Lenox-SmithAGreenstreetLBurslemKKnightCCost effectivness of venlafaxine compared with generic fluoxetine or generic amitriptyline in major depressive disorder in the UKClin Drug Investig2009293173184
- LiebowitzMRManleyALPadmanabhanSKGangulyRTummalaRTourianKAEfficacy, safety, and tolerability of desvenlafaxine 50 mg/ day and 100 mg/day in outpatients with major depressive disorderCurr Med Res Opin20082471877189018507895
- FeighnerJPThe role of venlafaxine in rational antidepressant therapyJ Clin Psychiatry199455Suppl A62687961545
- HaddadPMAntidepressant discontinuation syndromesDrug Saf200124318319711347722
- BuckleyNAMcManusPRFatal toxicity of serotoninergic and other antidepressant drugs: Analysis of United Kingdom mortality dataBMJ200232573761332133312468481
- ThaseMESheltonRCKhanATreatment with venlafaxine extended- release after SSRI nonresponse or intolerance: A randomized comparison of standard- and higher-dosing strategiesJ Clin Psychopharmacol200626325025816702889
- PaeCUDesvenlafaxine: A new antidepressant or just another oneExpert Opin Pharmacother200910587588719351235
- ClaytoneAHKornsteinSGRosasGGuico-PabiaTourianKAAn integrated analysis of the safety and tolerability of desvenlafaxine compared with placebo in the treatment of major depressive disorderCNS Spectr200914418319519407730
- GuelfiJDWhiteCHackettDGuichouxJYMagniGEffectiveness of venlafaxine in patients hospitalized for major depression and melancholiaJ Clin Psychiatry199556104504587559370
- BymasterFPDreshfield-AhmadLJThrelkeldPGComparative affinity of duloxetine and venlafaxine for serotonin and norepinephrine transporters in vitro and in vivo, human serotonin receptor subtypes, and other neuronal receptorsNeuropsychopharmacology200125687188011750180
- ZhaoRKChengGTangJSongJPengWXPharmacokinetics of duloxetine hydrocloride enteric-coated tablets in healthy Chinese volunteers: A randomized, open-label, single- and multiple-dose studyClin Ther20093151022103619539103
- SkinnerMHSkerjanecASegerMHewittRThe effect of food and bedtime administration on duloxetine pharmacokineticsClin Pharmacol Ther2000672129 Abstract
- SharmaAGoldbergMJCerimeleBJPharmacokinetics and safety of duloxetine, a dual-serotonin and norepinephrine reuptake inhibitorJ Clin Pharmacol200040216116710664922
- FramptonJEPloskerGLDuloxetine: A review of its use in the treatment of major depressive disorderCNS Drugs200721758160917579500
- LantzRJGillespieTARashTJMetabolism, exretion, and pharmacokinetics of duloxetine in healthy human subjectsDrug Metab Dispos 20033191142115012920170
- LoboEDBergstromRFReddySIn vitro and in vivo evaluations of cytochrome P450 1A2 interactions with duloxetineClin Pharmacokinet200847319120218307373
- HunzikerMESuehsBTBettingerTLCrismonMLDuloxetine hydrochloride: A new dual-acting medication for the treatment of major depressive disorderClin Ther20052781126114316199241
- SkinnerMHKuanHYPanADuloxetine is both an inhibitor and a substrate of cytochrome P450 2D6 in healthy volunteersClin Pharmacol Ther200373317017712621382
- LoboEDQuinlanTO’BrienLPat KnadlerMHeathmanMPopulation pharmacokinetics of orally administered duloxetine in patientsClin Pharmacokinet200948318919719385712
- SuriAReddySGonzalesDRKnadlerMPSkinnerMDuloxetine pharmacokinetics in cirrhotics compared with healthy subjectsInt J Clin Pharmacol Ther2005432788415726876
- BrannanSKMallinckrodtCHBrownEBWohlreichMMWatkinJJGSchatzbergAFDuloxetine 60 mg once daily in the treatment of painful physical symptoms in patients with major depressive disorderJ Psychiatr Res2005391435315504423
- SpielmansGIDuloxetine does not relieve painful physical symptoms in depression: A meta-analysisPsychother Psychosom2008771121618087203
- ThaseMEPritchettYLOssannaMJSwindleRWXuJDetkeMJEfficacy of duloxetine and selective serotonin reuptake inhibitors: Comparisons as assessed by remission rates in patients with major depressive disorderJ Clin Psychopharmacol200727667267618004135
- PerahiaDGPritchettYLKajdaszDKA randomized, double blind comparison of duloxetine and venlafaxine in the treatment of patients with major depressive disorderJ Psychiatr Res2008421223417445831
- GuptaSNihalaniNMasandPDuloxetine: Review of its pharmacology, and therapeutic use in depression and other psychiatric disordersAnn Clin Psychiatry200719212513217612852
- WadeAGembertKFloreaIA comparative study of the efficacy of acute and continuation treatment with escitalopram versus duloxetine in patients with major depressive disorderCurr Med Res Opin20072371605161417559755
- NierenbergAAGreistJHMallinckrodtCHDuloxetine versus escitalopram and placebo in the treatment of patients with major depressive disorder: Onset of antidepressant action, a non-inferiority studyCurr Med Res Opin200723240141617288694
- MallinckrodtCHPrakashAAndornACWatkinJGWohlreichMMDuloxetine for the treatment of major depressive disorder: A closer look at efficacy and safety data across the approved dose rangeJ Psychiatr Res200640433734816271726
- WiseTNPerahiaDGPangalloBALosinWGWiltseCGEffects of the antidepressants duloxetine on body weight: Analysis of 10 clinical studiesPrim Care Companion J Clin Psychiatry20068526927817235383
- ThaseMETranPVWiltseCPangalloBAMallinckrodtCDetkeMJCardiovascular profile of duloxetine, a dual reuptake inhibitor of serotonin and norepinephrineJ Clin Psychopharmacol200525213214015738744
- DelgadoPLBrannanSKMallinckrodtCHSexual functioning assessed in 4 double-blind placebo- and paroxetine- controlled trials of duloxetine for major depressive disorderJ Clin Psychiatry200566668669215960560
- NelsonJCLu PritchettYMartynovOYuJYMallinckrodtCHDetkeMJThe safety and tolerability of duloxetine compared with paroxetine and placebo: A pooled analysis of 4 clinical trialsPrim Care Companion J Clin Psychiatry20068421221916964316
- SpencerCMWildeMIMilnacipran: A review of its use in depressionDrugs19985634054279777315
- VaishnaviSNNemeroffCBPlottSJRaoSGKranzlerJOwensMJMilnacipran: A comparative analysis of human monoamine uptake and transporter binding affinityBiol Psychiatry200455332032214744476
- HindmarchIRigneyUStanleyNBrileyMPharmacodynamics of milnacipran in young and elderly volunteersBr J Clin Pharmacol200049211812510671905
- CadaDJLevienTLBakerDEMilnacipranHosp Pharm2009447604615
- PuozzoCLensSRehCLack of interactions of milnacipran with the cytochrome P450 isoenzymes frequently involved in the metabolism of antidepressantsClin Pharmacokinet200544997798816122284
- PuozzoCLeonardBEPharmacokinetics of milnacipran in comparison with other antidepressantsInt Clin Psychopharmacol199611Suppl 4S15S27
- PuozzoCHermannPChassardDLack of pharmacokinetic interaction when switching from fluoxetine to milnacipranInt Clin Psychopharmacol200621315315816528137
- LecrubierYPletanYSollesATournouxAMagneVClinical efficacy of milnacipran: Placebo-controlled trialsInt Clin Psychopharmacol199611Suppl 4S29S33
- MacherJPSichelJPSerreCVon FrenckellRHuckJCDemarezJPDouble-blind placebo-controlled study of milnacipran in hospitalized patients with major depressive disordersNeuropsychobiology198922277822701744
- PapakostasGIFavaMA meta-analysis of clinical trials comparing milnacipran, a serotonin-norepinephrine reuptake inhibitor for the treatment of major depressive disorderEur Neuropsychopharmacol2007171323616762534
- NakagawaAWatanabeNOmoriIMEfficacy and tolerability of milnacipran in the treatment of major depression in comparison with other antidepressants: A systemic review and meta-analysisCNS Drugs200822758760218547127
- WongEHFSondersMSAmaraSGReboxetine: A pharmacologically potent, selective, and specific norepinephrine reuptake inhibitorBiol Psychiatry200047981882910812041
- FleishakerJCClinical pharmacokinetics of reboxetine, a selective norepinephrine reuptake inhibitor for the treatment of patients with depressionClin Pharmacokinet200039641342711192474
- EdwardsDMPellizzioniCBreuelHPPharmacokinetics of reboxetine in healthy volunteers. Single oral doses, linearity and plasma protein bindingBiopharm Drug Dispos19951664434607579027
- FleishakerJCMucciMPellizzioniCPoggesiIAbsolute bioavailability of reboxetine enantiomers and effects of gender on pharmacokineticsBiopharm Drug Dispos1999201535710086838
- PellizzioniCBenedetti StrolinMPoggesiIFrigerioEToonSLangleySJPharmacokinetics of reboxetine in healthy volunteers: Relative bioavailability and food effectPharmacol Res199531Suppl 1S41
- WienkersLCAllieviCHauerMJWynaldaMACytochrome P450- mediated metabolism of the individual enantiomers of the antidepressant agent reboxetine in human liver microsomesDrug Metab Dispos199927111334134010534319
- HendershotPEFleishakerJCLinKMNuccioIDPolandREPharmacokinetics of reboxetine in healthy volunteers with different ethnic descentsPsychopharmacology2001155214815311401003
- PoggesiIPellizzoniCFleishakerJCPharmacokinetics of reboxetine in elderly patients with depressive disordersInt J Clin Pharmacol Ther200038525425910839469
- BergmannJFLaneuryJPDuchenePFleishakerJCHouinGSégrestaaJMPharmacokinetics of reboxetine in healthy, elderly volunteersEur J Drug Metab Pharmacokinet20002534195198
- CoulombFDucretFLaneuryJPPharmacokinetics of single-dose reboxetine in volunteers with renal insufficiencyJ Clin Pharmacol200040548248710806601
- TranALaneuryJPDuchênePPharmacokinetics of reboxetine in volunteers with hepatic impairmentClin Drug Invest2000196473477
- MontgomerySFergusonJMSchwartzGEThe antidepressant efficacy of reboxetine in patients with severe depressionJ Clin Psychopharmacol2003231455012544375
- VersianiMMehilaneLGasznerPArnaud-CastiglioniRReboxetine, a unique selective NRI, prevents relapse and recurrence in long-term treatment of major depressive disorderJ Clin Psychiatry199960640040610401920
- BerzewskiHVan MoffaertMGagianoCAEfficacy and tolerability of reboxetine compared with imipramine in a double-blind study in patients suffering from major depressive offsodesEur Neuropsychopharmacol19977Suppl 1S37S479169309
- KatonaCBercoffEChiuETackPVersianiMWoelkHReboxetine versus imipramine in the treatment of elderly patients with depressive disorders: A double-blind randomized trialJ Affect Disord19995523203213
- MontgomerySAChairman’s overview. The place of reboxetine in antidepressant therapyJ Clin Psychiatry199859Suppl 14S26S29
- MassanaJMollerHJBurrowsSGMontenegroRMReboxetine: A double-blind comparison with fluoxetine in major depressive disorderInt Clin Psychopharmacol1999142738010220121
- AndreoliVCaillardVDeoRSRybakowskiJKVersianiMReboxetine, a new noradrenaline selective antidepressant, is at least as effective as fluoxetine in treatment of depressionJ Clin Psychopharmacol200222439339912172339
- KasperSHamonMBeyond the monoaminergic hypothesis: Agomelatine, a new antidepressant with an innovative mechanism of actionWorld J Biol Psychiatry200910211712619255935
- ZupancicMGuilleminaultCAgomelatine: A preliminary review of a new antidepressantCNS Drugs2006201298199217140278
- MillanMJGobertALejeuneFThe novel melatonin agonist agomelatine (S20098) is an antagonist at 5-hydroxytryptamine (2C) receptors, blockade of which enhances the activity of frontocortical dopaminergic and adrenergic pathwaysJ Pharmacol Exp Ther2003306395496412750432
- ServierValdoxanSummary of product characteristics Available from: http://www.servier.co.uk/pdfs/Valdoxan_SPC.pdfAccessed on 2010 May 5
- KennedySHEmsleyRPlacebo-controlled trial of agomelatine in the treatment of major depressive disorderEur Neuropsychopharmacol20061629310016249073
- LooHHaleAD’haenenHDetermination of the dose of agomelatine, a melatoninergic agonist and selective 5-HT2C antagonist, in the treatment of major depressive disorder: A placebocontrolled dose range studyInt Clin Psychopharmacol200217523924712177586
- MontgomerySAKasperSSevere depression and antidepressants: Focus on a pooled analysis of placebo-controlled studies on agomelatineInt Clin Psychopharmacol200722528329117690597
- KennedySHSexual function in remitted depressed patients following agomelatine and venlafaxine XR treatmentEur Neuropsychopharmacol200515Suppl 3 Abstr S440
- GuilleminaultCEfficacy of agomelatine versus venlafaxine on subjective sleep of patients with major depressive disorderEur Neuropsychopharmacol200515Suppl 3S419S420 Abstract
- MongomerySAKennedySHBurrowsGDLejoyeuxMHindmarchIAbsence of discontinuation symptoms with agomelatine and occurrence of discontinuation symptoms with paroxetine: A randomized, double-blind, placebo-controlled discontinuation studyInt Clin Psychopharmacol200419527128015289700
- WeberJLyseng-WilliamsonKAScottLJAripiprazole: In major depressive disorderCNS Drugs2008221080781318788833
- BermanRMMarcusRNSwaninkRThe efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: A multicenter, randomized, double-blind, placebo-controlled studyJ Clin Psychiatry200768684385317592907
- MarcusRNMcQuadeRDCarsonWHThe efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: A second multicenter, randomized, double-blind, placebo-controlled studyJ Clin Psychopharmacol200828215616518344725
- CarrollBJAripiprazole in refractory depression?J Clin Psychopharmacol2009291909119142117
- TsaiACUnclear clinical significance of findings in adjunctive aripiprazole for major depressive disorder. Comments on article by Marcus et alJ Clin Psychopharmacol2009291919219142118
- DawsonLAWatsonJMVilazodone: A 5-HT1A receptor agonist/ serotonin transporter inhibitor for the treatment of affective disordersCNS Neurosci Ther200915210711719499624
- RickelsKAthanasiouMRobinsonDSGibertiniMWhalenHReedCREvidence for efficacy and tolerability of vilazodone in the treatment of major depressive disorder: A randomized, doubleblind, placebo-controlled trialJ Clin Psychiatry200970332633319284933
- MillanMJDual- and triple-acting agents for treating core and comorbid symptoms of major depression: Novel concepts, new drugsNeurotherapeutics200961537719110199
- SenSSanacoraGMajor depression: Emerging therapeuticsMt Sinai J Med200875320422518704976
- NeuroSearch announces the results of Phase II proof of concept studies with NS2359 in depression Available from: https://newsclient.omxgroup.com/cdsPublic/viewDisclosure.action?disclosureId=313018&messageId=372903Accessed on 2010 May 5
- AndrésJIAlcazarJAlonsoJMTricyclic isoxazolines: Identification of R226161 as a new antidepressant that combines potent serotonin reuptake inhibition and α2-adrenoceptor antagonismBioorg Med Chem200715113649366017407815
- GobertACussacDLejeuneFThe novel antidepressant, S35966, is a mixed serotonin and noradrenaline reuptake inhibitor and an antagonist at α2-adrenoceptorsEur Neuropsychopharmacol200212Suppl 3S248
- DemyttenaereKCompliance and acceptance in antidepressant treatmentInt J Psych Clin Pract20015Suppl 1S29S35
- SawadaNUchidaHSuzukiTPersistance and compliance to antidepressant treatment in patients with depression: A chart reviewBMC Psychiatry 200993819531229
- RooseSPCompliance: The impact of adverse events and tolerability on the physician’s treatment decisionsEur Neuropsychopharm200313Suppl 3S85S92
- HaslamCBrownSAtkinsonSHaslamRPatients’ experiences of medication for anxiety and depression: Effects on working lifeFamily Practice200421220421215020393