Abstract
Carbamazepine (CBZ) has long been a therapeutic option for bipolar disorder. Carbamazepine extended-release capsules (CBZ-ERC) are a recent formulation of CBZ approved by the US Food and Drug Administration in 2004 for the treatment of acute manic and mixed episodes associated with bipolar I disorder. This new formulation was developed to improve dosing convenience and decrease daily fluctuations in serum CBZ concentration, thereby lowering the incidence of adverse events. Two randomized, double-blind, placebo-controlled trials and an open-label extension study have demonstrated that CBZ-ERC monotherapy is efficacious in patients with bipolar I disorder experiencing either manic or mixed episodes. In these trials, CBZ-ERC was shown to be a safe and well-tolerated therapy. Retrospective chart reviews conducted in private practice settings have shown that clinical response to CBZ-ERC is independent of bipolar subtype, as patients with bipolar I depression and bipolar II disorder responded similarly to patients with bipolar I disorder either manic or mixed episodes. CBZ is currently considered a treatment alternative to lithium and valproate according to the American Psychiatric Association’s treatment guidelines for patients with bipolar disorder. Although further study is required, the clinical evidence presented in these studies may change the treatment paradigm.
Introduction
Bipolar disorder is usually a chronic illness with an episodic and variable course (CitationAPA 2002). It is a leading cause of years lived with disability (CitationWHO 2004) and is associated with poor health-related quality of life, high utilization of health care services, work impairment (CitationDean et al 2004), and significant costs to society (CitationWyatt et al 1995).
There are many forms of bipolar disorder, ranging from mild depression and brief hypomania to one of severe depression or mania with psychotic features (CitationMuller-Oerlinghausen et al 2002). To help identify the various forms of the illness, 4 subtypes of bipolar disorder have been defined based on patients’ clinical characteristics: bipolar I disorder, bipolar II disorder, cyclothymia, and bipolar disorder not otherwise specified (CitationAPA 2000). Past estimates of the prevalence of the full spectrum of bipolar disorders have ranged from 3% to 6.5%, with about 1% considered to be the bipolar I subtype (CitationAngst 1998; CitationStimmel 2004). Furthermore, new data on the prevalence of bipolar spectrum disorders have emerged from the recently completed US National Comorbidity Survey Replication, in which trained professionals interviewed a nationally representative population of 9282 English-speaking US residents aged >18 years. National Comorbidity Survey probands were assessed for the presence of Diagnostic and Statistical Manual of Mental Disorders (4th edition) mental disorders in the 12 months prior to the survey using the World Mental Health Survey Initiative version of the World Health Organization Composite International Diagnostic Interview, and it was found that the 12-month prevalence of bipolar disorder (subtype I or II) in the study sample was 2.6% (CitationKessler 2005).
Treatment options
Although psychotherapy is a critical component of intervention in bipolar disorder, pharmacotherapy is essential in treating patients who are acutely symptomatic (CitationSuppes et al 2005). The most recent version of the Texas Implementation of Medical Algorithms (TIMA) calls for the treatment of acute manic, depressive, or mixed bipolar symptoms to enable patients to return to normal psychosocial functioning. According to the TIMA recommendations, acute pharmacotherapy should be initiated for mild to severe bipolar symptoms, with decisions on whether to maintain stable treatment dosing, adjust treatment dosing, or select an alternative pharmacotherapeutic strategy being made on the basis of regular follow-up assessments. Upon symptom remission after treatment of acute bipolar mania, a 4- to 6-month course of continuation therapy and subsequent lifetime maintenance therapy with mood stabilizers are recommended for the prevention of recurrences (CitationSuppes et al 2005).
Despite systematic guidelines for the treatment of bipolar disorder, the selection of appropriate therapeutic agents for use in conjunction with these guidelines can be challenging, because the symptoms of bipolar disorder may be very different in different phases of the illness. Several agents are currently available for the treatment of bipolar disorder, and the initial treatment depends on the type of episode the patient is experiencing at presentation. Lithium has been extensively studied in the treatment of acute mania and as maintenance therapy since the discovery of its mood-stabilizing properties more than 50 years ago (CitationGoodwin et al 1969; CitationBelmaker 2004), and it is currently considered to be a first-line treatment option in acute mania and prophylaxis in bipolar disorder. Nonetheless, despite its first-line status and its demonstrated overall efficacy in these settings, lithium therapy has been shown to be ineffective or poorly tolerated in a significant proportion of patients (CitationBowden et al 1994). In addition, it has a narrow therapeutic range and requires regular blood level monitoring, as severe or toxic effects can occur at twice the therapeutic dose. Its narrow therapeutic range is an especially important consideration in older patients as their renal excretion becomes less efficient, resulting in an increased risk of lithium-associated toxic effects (CitationGoodwin 2003; CitationBelmaker 2004).
Although lithium is widely used globally, the anticonvulsants valproate and carbamazepine (CBZ) have also become established therapeutic options in the treatment of bipolar disorder (CitationMitchell et al 2002; CitationSuppes et al 2005). Extensive clinical research has shown that these agents have mood-stabilizing properties and are therefore effective treatments in the management of bipolar disorder (CitationMitchell et al 2002). The current review will examine data for a twice-daily extended-release capsule formulation of CBZ (CBZ-ERC) (Shire Pharmaceuticals, Wayne, PA, USA) that contains three different types of beads (immediate release, extended release, and enteric release). This particular formulation has been approved by the US Food and Drug Administration (FDA) for the treatment of acute manic and mixed episodes associated with bipolar I disorder.
Rationale for development of CBZ-ERC
Clinical evidence of the efficacy of CBZ in the treatment of bipolar disorder emerged in the early 1970s. At this time, several small studies reported the antimanic effects of CBZ as well as its prophylactic effects against the recurrence of manic and depressive episodes in patients with bipolar disorder (CitationOkuma et al 1973). Over the next several decades, many double-blind, controlled trials demonstrated the efficacy of CBZ in the treatment of acute mania in bipolar disorder, with response rates similar to those of lithium (CitationMcElroy et al 2000). Until recently, all controlled evaluations of CBZ in bipolar disorder have used immediate-release formulations of CBZ that require dosing 3 or 4 times daily to avoid potentially problematic serum drug fluctuations. Studies have shown that the large fluctuations in serum CBZ levels observed with immediate-release CBZ formulations are associated with intermittent adverse effects such as diplopia, drowsiness, and headache in patients with epilepsy (CitationHoppener et al 1980; CitationRiva et al 1984). The established correlation between fluctuations in serum CBZ levels and intermittent side effects helped prompt the development of extended-release formulations of CBZ for use in epilepsy. The subsequent use of extended-release CBZ in patients with epilepsy provided clinical evidence that, compared with immediate-release formulations, extended-release CBZ was associated with lower peak serum CBZ concentrations, decreased circadian toxicity, and decreased central nervous system (CNS) side effects (CitationCanger et al 1990; CitationHaefeli et al 1994).
Non-adherence to medication is common among patients with bipolar disorder (CitationKeck et al 1996; CitationSvarstad et al 2001). Potential factors affecting compliance with medication include adverse effects and the demands of treatment, including frequent dosing regimens (CitationJamison et al 1983; CitationGreenberg 1984). Since extended-release formulations of CBZ have been developed to decrease daily fluctuations in serum CBZ concentrations and improve dosing convenience, several large clinical trials have recently been conducted to assess the efficacy and tolerability of a novel, beaded, extended-release capsule formulation of CBZ in bipolar disorder (CitationWeisler et al 2004c, Citation2005; CitationKetter et al 2004).
Pharmacology
Pharmacodynamics
Although recent neurochemical and neuroimaging studies have been promising, a specific pathophysiological abnormality in bipolar disorder has not been discovered (CitationBelmaker 2004). Clinical and preclinical evidence suggests that second messenger systems and molecular “switches” known as G-proteins are involved in the underlying mechanisms that result in bipolar disorder (CitationGould et al 2002). A variety of medications used to treat bipolar disorder have widely varying mechanisms of action (MOA), which in many cases are also still not well understood. Alterations in postreceptor pathways, intracellular signaling, neural plasticity, and changes in gene expression are believed to play important roles in the therapeutic effects of these agents (CitationGould et al 2002).
The MOA of CBZ in the treatment of bipolar disorder has not been clarified. It has been well established that CBZ exerts its antiepileptic effects by inhibiting the high-frequency firing of sodium channels. This effect produces a functional blockage of voltage-gated sodium channels that could be associated with its mood-stabilizing properties (CitationGould et al 2004). In experimental studies, CBZ acts as an antagonist at adenosine receptors, which are generally G-protein-coupled receptors that modulate neurotransmitter release and numerous behavioral and cognitive functions. Carbamazepine has also been reported to inhibit the enzyme adenylyl cyclase, which attenuates cyclic AMP-mediated signaling and may lead to inhibition of downstream activities with ion channels and transcription factors (CitationGould et al 2004). Additional findings on the MOA of CBZ include reports of its effects on the voltage-gated ion channels in neurons where at therapeutic concentrations it acts as a calcium ion channel blocker (CitationUlrich et al 2003), and it exerts regulatory effects on receptor-mediated excitatory and inhibitory neurotransmission (CitationLi et al 2002).
Pharmacokinetics
The extended-release formulation CBZ-ERC utilizes a drug delivery system consisting of a fixed ratio of specialized beads to extend the release of CBZ beyond what can be achieved with conventional immediate-release formulations. Twenty-five percent of these beads are designed to release drug immediately after swallowing for rapid absorption, 40% of the beads are polymer-coated to dissolve gradually over 8–12 hours to achieve steady-state serum CBZ levels, and the remaining 35% are enteric-release beads with a pH-sensitive coating that releases CBZ slowly in the gut to maintain optimal blood levels. The timed release of CBZ is unaffected by variations in gastrointestinal (GI) transit time. Additionally, the capsule does not have to be taken with food; it can be opened and its contents sprinkled onto soft foods (CitationMcLean et al 2001).
The pharmacokinetics of CBZ-ERC have been determined following single and repeat dose administration. Following a single 200-mg dose of CBZ-ERC, peak plasma CBZ concentration was found to be 1.9 ± 0.3 μg/mL, and time to reach peak was 19 ± 7 hours. After repeated dose administration of CBZ-ERC 800 mg every 12 hours, the peak plasma CBZ concentration was 11.0 ± 2.5 μg/mL and time to reach peak was 5.9 ± 1.8 hours. The pharmacokinetics of CBZ-ERC are linear over a single-dose range of 200–800 mg (CitationShire 2005).
CBZ is 76% bound to plasma proteins and is primarily metabolized in the liver. Cytochrome P450 3A4 was identified as the major isoform responsible for metabolizing CBZ to its active metabolite CBZ-10,11-epoxide. Since CBZ induces its own metabolism, the half-life is variable. The average half-life can range from 35 to 40 hours following a single dose of CBZ-ERC and 12 to 17 hours following repeated dosing of CBZ-ERC.
In a pharmacokinetic study conducted in patients with epilepsy, twice-daily CBZ-ERC was shown to be bioequivalent to immediate-release formulations of CBZ given four times daily (CitationGarnett et al 1998). Minimizing the fluctuations in serum CBZ concentrations inherent in immediate-release formulations was an important factor in the development of CBZ-ERC. Low peak-to-trough blood level variability ensures that blood CBZ levels remain relatively stable (CitationStevens et al 1998). In studies of CBZ in patients with epilepsy, conversion from immediate- to extended-release CBZ resulted in marked reductions in common dose-related CNS side effects. Pharmacokinetic analysis showed marked variability in absorption and blood drug concentrations with immediate-release formulations of CBZ compared with CBZ-ERC. These findings suggest that the improved CNS tolerability observed in patients treated with CBZ-ERC is a result of smoother drug delivery and reduced variability in absorption provided by the extended-release formulation (CitationMiller et al 2004b). These clinical findings, in addition to twice daily dosing with CBZ-ERC, should improve patient compliance by providing a more convenient dosing regimen and improved tolerance with fewer side effects.
Clinical studies
Efficacy
For more than 30 years, CBZ has been used to treat bipolar disorder. Early controlled trials of CBZ in the treatment of acute mania were conducted with conventional immediate-release formulations and confounded by coadministration with lithium or standard antipsychotics. In the first double-blind, placebo-controlled study of CBZ in affectively ill patients (ie, bipolar, unipolar depression, schizoaffective illness), preliminary results showed that 7 of the first 10 patients treated with immediate-release CBZ responded favorably with an antimanic, an antidepressant, or a prophylactic response (CitationBallenger et al 1978). In comparative studies, immediate-release CBZ was found to be as efficacious as lithium in the treatment of acute mania in bipolar disorder (CitationOkuma et al 1990; CitationSmall et al 1991; CitationEmilien et al 1996). In one study, a 4-week, double-blind, multicenter trial involving 105 patients aged 13–65 years with manic or mixed bipolar disorder, 62% of patients in the immediate-release CBZ group showed moderate to marked amelioration of manic symptoms (as judged by the treating physician) at final assessment, compared with 59% in the lithium group (not significant) (CitationOkuma et al 1990). In a separate 4-week, comparative, double-blind study, immediate-release CBZ monotherapy (n = 15) and valproate monotherapy (n = 15) were found to be comparably effective in the treatment of acute mania in patients hospitalized with bipolar disorder (CitationVasudev et al 2000). The primary efficacy analysis in that study revealed that the mean Young Mania Rating Scale (YMRS) score in the valproate group decreased significantly more than in the immediate-release CBZ group, although rates of response (≥50% decrease in total YMRS score from baseline) were not significantly different between the two groups, as favorable clinical responses were attained by 53% of patients in the immediate-release CBZ group and 73% of patients in the valproate group.
While the efficacy of CBZ in treating acute mania associated with bipolar I disorder has been well characterized, evidence suggests that clinical responses to this agent are independent of symptomatology or bipolar subtype. In particular, a 21-day, open-label study involving 36 patients with bipolar disorder revealed that immediate-release CBZ reduced Hamilton Depression Rating Scale (HDRS) scores by an average of 72.7% (from 32.6 to 8.9; p < 0.0001 for change relative to baseline) between baseline and end point in the subset of patients with bipolar depression (n = 27) and by an average of 50.4% (from 35.1 to 17.4; p = 0.0009 for change relative to baseline) in the subset of patients with depressive mania (n = 9) (CitationDilsaver et al 1996). More recently, a retrospective chart review of patients treated in a private practice setting found that CBZ-ERC also appears to be effective in reducing the symptoms of bipolar II disorder (CitationGinsberg 2004a). Of the 111 patients treated with CBZ-ERC in that review (85% of whom were classified as being “markedly ill”, “severely ill”, or “extremely ill” at the time of treatment initiation), 82 (74%) experienced a response to treatment as indicated by the achievement of a Clinical Global Impression-Improvement (CGI-I) score ≤3.
Aside from exhibiting efficacy in the acute treatment of bipolar disorder, CBZ has also been found to be effective as maintenance therapy in ≥10 controlled or partially controlled studies, with a cumulative “marked or excellent” response rate of approximately 61% (CitationDenicoff et al 1997). Several studies have compared the prophylactic efficacy of immediate-release CBZ with that of lithium, which, in a pooled analysis of maintenance studies averaging 1.5 years in length, was found to reduce the likelihood of manic recurrence by a factor of 2.5 and the likelihood of depressive recurrence by a factor of 1.8 (CitationGoodwin and Jamison 1990; CitationBaldessarini et al 1996). Comparison studies involving immediate-release CBZ and lithium have found overall response rates to be similar, with no trends indicating one agent’s superiority to the other in the maintenance setting. A meta-analysis of clinical data from 10 double-blind, randomized, comparative trials of immediate-release CBZ vs lithium as maintenance therapy found a relapse rate of 60% in lithium-treated patients vs 55% of CBZ-treated patients; the difference between the groups was not significant (CitationDavis et al 1999). In one recent randomized, double-blind study comparing the prophylactic efficacy of immediate-release CBZ and lithium in bipolar disorder, lithium was superior to immediate-release CBZ in terms of 2.5-year hospitalization rates (lithium, 26%; immediate-release CBZ, 62%; p = 0.012) in patients with “classical” bipolar I disorder (without mood-incongruent features and psychiatric comorbidity and without mixed states; n = 67), while immediate-release CBZ showed a trend toward being more effective (2.5-year hospitalization rates: lithium, 44%; immediate-release CBZ, 31%; p = 0.34) in the more heterogeneous “nonclassical” subgroup of patients (n = 104).These findings suggest that patients with classical features benefit more from prophylactic therapy with lithium, while those with nonclassical features may benefit more from prophylaxis with CBZ, which appears to provide a broader spectrum of activity (CitationGreil et al 1998; CitationKleindienst et al 2000).
Efficacy of CBZ-ERC in acute mania
Recently, two large 3-week, double-blind, randomized, placebo-controlled, multicenter trials demonstrated that monotherapy with twice-daily CBZ-ERC was effective in the treatment of acute mania in patients with bipolar I disorder experiencing manic or mixed episodes (CitationWeisler et al 2004c, Citation2005). To be eligible for enrollment in these studies, patients had to be ≥18 years old and meet DSM-IV criteria for bipolar I disorder with current episode manic or mixed and with manic symptoms severe enough to necessitate hospitalization. A history of ≥1 previous manic or mixed episode and a minimum baseline total score of 20 on the YMRS were required. The initial 400-mg dose of CBZ-ERC or placebo could be titrated by the investigators between 200 mg and 1600 mg in daily increments of 200 mg. Efficacy assessments were carried out weekly and included the YMRS, the Clinical Global Impression (CGI) scale, and the HDRS.
To better elucidate the results from these two similar trials, the data were combined and the efficacy and safety of CBZ-ERC were evaluated based on the combined study population. Pooled data from these two pivotal studies (which took place across a total of 40 study sites – 34 in the United States and 6 in India) included a total of 443 patients, 223 of whom were randomized to double-blind treatment with CBZ-ERC and 220 of whom were randomized to receive placebo. A total of 240 randomized patients (54%) in the pooled analysis completed the 21-day study period, and the rate of discontinuation due to lack of efficacy was found to be higher in the placebo group (22%) than in the CBZ-ERC group (10%).
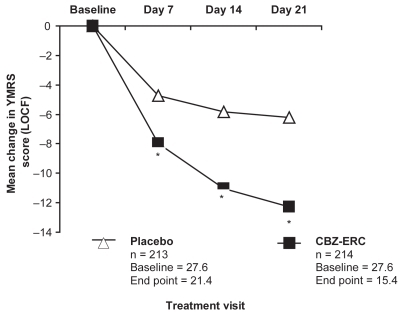
The primary efficacy endpoint was the YMRS total score at the end of the double-blind treatment period. As shown in , using the last observation carried forward analysis, CBZ-ERC-treated patients in the combined patient population had significantly greater decreases in mean YMRS scores from baseline than those in the placebo group at day 7, 14, and day 21, the primary endpoint (p < 0.0001 at all time points). By the end of the trial, 52% of CBZ-ERC-treated patients in the combined population had responded (≥50% reduction in YMRS score) vs 26% of placebo-treated patients (p < 0.0001). Patients receiving CBZ-ERC also experienced significantly greater improvements in symptoms ratings on both CGI-Improvement (CGI-I) and CGI-Severity (CGI-S) scales than those treated with placebo (p < 0.0001) (CitationWeisler et al 2004a).
Figure 1 Mean change in YMRS total scores in pooled analysis of 3-week studies of CBZ-ERC in acute mania.
*p < 0.0001 compared with placebo following analysis of covariance with baseline score as covariate.
Subgroup analyses of the pooled data from these two pivotal studies showed that CBZ-ERC reduced both manic and depressive symptoms. At endpoint, there were significant reductions in mean YMRS total scores in both manic (p < 0.0001) and mixed (p < 0.01) bipolar patients treated with CBZ-ERC. Importantly, CBZ-ERC treatment led to significant improvement in the severity of depressive symptoms in mixed bipolar I patients (p < 0.05) and in the combined patient population (p = 0.01) as evidenced by significant decreases in HDRS total scores. The reduction in depressive symptoms experienced by patients treated with CBZ-ERC therapy in this trial suggests that CBZ may have true bimodal effects in bipolar disorder (CitationWeisler et al 2004a).
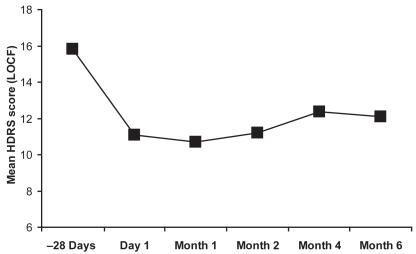
As little is known about the efficacy of CBZ-ERC in long-term treatment, a 6-month open-label extension study was conducted among patients who had completed one of two previous 3-week, double-blind, placebo-controlled evaluations of CBZ-ERC for acute mania (CitationKetter et al 2004). Ninety-two patients were enrolled in the study; 67% were mixed and 33% manic. Of the 77 patients treated with CBZ-ERC and evaluated in the intent-to-treat population, 14.3% relapsed during the 6-month study (7 had received prior treatment with CBZ-ERC and 4 with placebo). The observed mean time to relapse was approximately 2 months. Interestingly, a relapse rate of 14% compares favorably with a previously reported relapse rate of 29% for lithium therapy in a meta-analysis of 19 double-blind, randomized controlled trials of lithium prophylaxis in 865 patients (CitationDavis et al 1999). During this open-label treatment with CBZ-ERC, patients who had previously been treated with placebo had significant improvements in YMRS and HDRS in the first month of active therapy, which were maintained over the 6-month period ( and ). In addition, patients who had received prior CBZ-ERC therapy showed a trend toward continued improvement in YMRS scores (CitationKetter et al 2004).
Figure 2 Mean change in YMRS total scores with long-term CBZ-ERC treatment in bipolar disorder patients with manic or mixed episodes.
*p < 0.01; †p < 0.001; ‡p < 0.0001 vs baseline (one-sample t-test of mean change from day 1). aPatients who were previously placebo in the acute 3-week trials before entrance into the 6-month study.
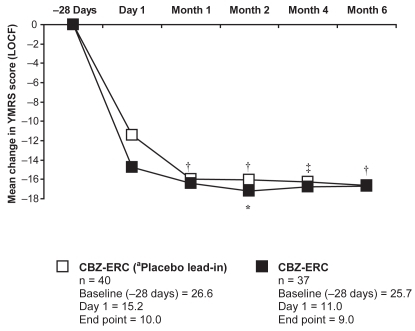
Figure 3 HDRS total scores from the 6-month CBZ-ERC study including baseline score in the pooled analysis of the 3-week studies.
*p < 0.0001, †p = 0.0004, ‡p = 0.0002 based on one sample t-test of mean change from baseline scores of pooled analysis from the 3-week studies.
Notes: When scores were based on one sample t-test of mean change from baseline values at day 1 in 6-month study, no statistical significance was detected.
Tolerability and patient acceptability
The pooled analysis of safety data from the two 3-week trials indicated that CBZ-ERC was generally well tolerated in both manic and mixed bipolar patients. The most frequently reported treatment-emergent adverse events among CBZ-ERC-treated patients (with an incidence ≥5% and at least twice the rate of placebo patients) included dizziness, somnolence, nausea, vomiting, ataxia, and pruritis (CitationWeisler et al 2004b). The most common adverse events observed with long-term CBZ-ERC therapy were typical of CBZ and included headache, dizziness, and mild rash (CitationKetter et al 2004).
Aplastic anemia and agranulocytosis have been observed rarely with CBZ, and no incidences were reported with CBZ-ERC treatment in both the acute and long-term studies. There was no clinically significant change in mean weight gain (≥7% increase from baseline) among the pooled study population in 3-week studies or in the 6-month trial, although a small percentage (5%) of patients did experience clinically significant weight gain during the trials (CitationKetter et al 2004; CitationWeisler et al 2004, Citation2005). No statistically significant alterations in electrocardiogram variables (QTc) and no meaningful change in non-fasting blood glucose were reported with long-term CBZ-ERC therapy (CitationKetter et al 2004).
Total plasma cholesterol (TC) concentrations have been shown to increase with CBZ-ERC treatment. In one of the 3-week studies, TC, low-density lipoprotein cholesterol (p < 0.0001), and high-density lipoprotein-cholesterol (HDL-C) increased significantly (p < 0.01) from baseline measurements in patients treated with CBZ-ERC (CitationWeisler et al 2005). Despite these increases in cholesterol, TC to HDL-C ratio was 4 to 1 at baseline and endpoint, which is not thought to be problematic for cardiovascular disease. Importantly, results from a 6-month CBZ-ERC extension study showed no further increase in plasma cholesterol concentrations in patients who were previously on CBZ-ERC in the 3-week studies (CitationKetter et al 2004). This finding suggests that the increase in TC concentrations is not continuous, although no definitive conclusion on the effects of CBZ-ERC on cholesterol concentrations can be made without fasting cholesterol measurements.
Studies have shown that patients with epilepsy transitioned from immediate-release CBZ formulations to CBZ-ERC have reported fewer and less severe adverse events and greater patient satisfaction (CitationFicker 2004; Miller et al 2004a). In one 3-month, open-label study, patients with epilepsy who switched from their current immediate-release CBZ product to an equal total daily dose of CBZ-ERC experienced both a decreased occurrence and a decreased severity of adverse events, demonstrating the benefits of CBZ-ERC in terms of safety and tolerability (CitationFicker 2004). To further define the improved tolerability of CBZ-ERC, a 3-month, multicenter, open-label study employed the Adverse Event Profile (AEP) questionnaire to measure the adverse event changes associated with switching adult patients with epilepsy from immediate-release CBZ to equal daily doses of CBZ-ERC. Analysis of the AEP total scores at end point showed that patients converted to CBZ-ERC exhibited statistically significant mean decreases in all common CNS side effects (sedation, vertigo, ataxia, difficulty in coordination, confusion, and diplopia) as a result of transitioning from immediate-release CBZ to CBZ-ERC (p < 0.0001 for all side effects). In addition, there was a 46% decrease from baseline in the number of patients with “toxic” AEP scores (≥ 45) (Miller et al 2004a). Overall, it appears that CBZ-ERC may be more tolerable than immediate-release CBZ, as transition to CBZ-ERC therapy significantly reduces the frequency and severity of drug-induced side effects.
Future needs
While randomized clinical trials have definitively confirmed the efficacy of CBZ-ERC in the treatment of manic symptoms in patients with manic or mixed bipolar disorder, the full spectrum of benefit provided by CBZ-ERC awaits comprehensive characterization in future studies. For example, CBZ-ERC has been linked to improvements in safety, tolerability, and patient satisfaction relative to what is seen in association with immediate-release CBZ in patients with epilepsy, and it has been hypothesized that CBZ-ERC provides similar benefits in patients with bipolar disorder. Nonetheless, because epilepsy and bipolar disorder are separate entities with distinct pathophysiological mechanisms, the potential merits of CBZ-ERC in comparison with immediate-release CBZ remain to be examined in a population specifically consisting of patients with bipolar disorder.
Aside from the relative benefits of extended-release delivery of carbamazepine, another area that warrants further study is the efficacy of CBZ-ERC in the prophylaxis of bipolar disorder. Results from a recent 6-month, open-label study were promising, with only 14% of patients experiencing recurrences during maintenance therapy with CBZ-ERC. However, because of the open-label nature of that study, and since the study involved only a relatively small number of patients, all of whom had completed a full course of treatment (CBZ-ERC or placebo) in one of two previous randomized trials, the generalised findings that were made remain to be assessed in a double-blind trial involving a more inclusive study population. Another notable finding made in these recent clinical trials is that of significantly reduced depressive symptoms in patients receiving CBZ-ERC for bipolar disorder, and studies designed specifically to evaluate the antidepressant effects of CBZ-ERC may therefore also be valuable in providing a clearer picture of the benefits associated with this agent.
Conclusion
The clinical trials discussed in this review are the only known double-blind, placebo-controlled studies of CBZ-ERC in the treatment of acute mania in bipolar I disorder. In these trials, CBZ-ERC demonstrated response rates comparable to those of lithium, valproate, and atypical antipsychotics reported in other double-blind, placebo-controlled studies in acute bipolar mania (CitationMcElroy et al 2000). In the pooled analysis of clinical data from the two 3-week studies, significant improvement in manic symptoms was noted within 1 week of initiation of CBZ-ERC therapy (the first measurement taken), with continued reduction in manic symptoms over the treatment period (CitationWeisler et al 2004a). Additionally, the extended-release formulation allowed a more rapid dosage titration (starting at 200 mg bid on day 1 and increasing by up to 200 mg per day), permitting faster responses for some patients relative to what is usually achievable with immediate-release CBZ. A subgroup analysis of the same combined patient population revealed that CBZ-ERC improved both manic and depressive symptoms in patients experiencing a mixed episode (CitationWeisler et al 2004, Citation2005). Importantly, CBZ-ERC-treated patients with mixed episodes who participated in the 6-month extension study maintained a significant decrease in HDRS score from the baseline of the double-blind studies to day 1 (p < 0.0001) of the extension study and end point (month 6) (p = 0.0003). Overall, there was no evidence of worsening of depressive symptoms among patients receiving up to 6 months of CBZ-ERC therapy (CitationKetter et al 2004).
Patients with mixed episodes have been shown to have a poor response to lithium monotherapy and often require multiple agents for successful treatment of the manic and depressive components of the mixed state (CitationMontgomery et al 2000). The significant reduction in both manic and depressive symptoms demonstrated in patients with mixed episodes during CBZ-ERC therapy suggests further investigation into CBZ-ERC as a treatment for bipolar depression. Moreover, as the extent of improvement in depressive symptoms may not have been captured in the relatively brief 3-week studies and 6-month extension study, additional controlled trials of longer duration would be warranted to assess the efficacy of CBZ-ERC in patients with bipolar depression, including those with bipolar I or bipolar II disorder.
Safety and tolerability are major factors in the selection of a therapeutic agent for bipolar patients. In the 3-week studies discussed in this review, the rapid dose-titration schedule of CBZ-ERC was aggressive at 200 mg/day and may have played a role in patient tolerability to CBZ during the first week of the study (CitationWeisler et al 2004c, Citation2005). In outpatient settings, titration may be slower, which may minimize the occurrence of common CNS and GI side effects.
Two very rare but serious adverse events linked to CBZ treatment are aplastic anemia and agranulocytosis, for which CBZ carries box warnings. However, some clinicians may have misconceptions about the frequency of aplastic anemia and agranulocytosis in patients treated with CBZ. The overall risk of developing these reactions is very low – about 5–8 times the natural occurrence (6 per million patient years for aplastic anemia and 2 per million patient years for agranulocytosis) (CitationShire 2005; CitationPellock 1987). No occurrences of aplastic anemia or agranulocytosis were reported with CBZ-ERC in either the short-term or long-term studies, although none would be expected since the patient populations were inadequately large to encounter such a reaction.
Disclosures
Dr Richard H Weisler is Adjunct Professor of Psychiatry at the University of North Carolina, Chapel Hill, and Adjunct Assistant Professor of Psychiatry at Duke University, in Durham. He is a consultant to, on the Speaker’s Bureaus of, and/or receives research support from Abbott, AstraZeneca, Biovail, Bristol-Myers Squibb, Cephalon, Corcept, Eisai, Eli Lilly, Forest, GlaxoSmithKline, Janssen, Johnson & Johnson, Lundbeck, Medicinova, Merck, Novartis, Organon, Pfizer, Saegis, Sanofi-Synthelabo, Schwabe, Shire, Solvay, Synaptic, TAP, UCB Pharma, Vela, and Wyeth in addition to the National Institute of Mental Health. He has been a stockholder in Bristol-Myers Squibb, Merck, and Pfizer.
References
- [APA] American Psychiatric Association2000Diagnostic and statistical manual of mental disorders4th ed (text revision)Washington, DCAmerican Psychiatric Association
- [APA] American Psychiatric Association Steering Committee on Practice Guidelines2002Practice guideline for the treatment of patients with bipolar disorder (revision)Am J Psychiatry159150
- AngstJ1998The emerging epidemiology of hypomania and bipolar II disorderJ Affect Disord50143519858074
- BaldessariniRJTondoLSuppesTShulmanKTohenMKutcherS1996Pharmacological treatment of bipolar disorder throughout the life-cycleBipolar disorder through the life-cycleNew YorkJohn Wiley & Sons299338
- BallengerJCPostRM1978Therapeutic effects of carbamazepine in affective illness: a preliminary reportCommun Psychopharmacol215975352607
- BelmakerRH2004Bipolar disorderN Engl J Med3514768615282355
- BowdenCLBruggerAMSwannAC1994Efficacy of divalproex vs lithium and placebo in the treatment of mania. The Depakote Mania Study GroupJAMA271918248120960
- CangerRAltamuraACBelvedereO1990Conventional vs controlled-release carbamazepine: a multicentre, double- blind, crossover studyActa Neurol Scand829132239143
- DavisJMJanicakPGHoganDM1999Mood stabilizers in the prevention of recurrent affective disorders: a meta-analysisActa Psychiatr Scand1004061710626918
- DeanBBGernerDGernerRH2004A systematic review evaluating health-related quality of life, work impairment, and healthcare costs and utilization in bipolar disorderCurr Med Res Opin2013915415006007
- DenicoffKDSmith-JacksonEEDisneyER1997Comparative prophylactic efficacy of lithium, carbamazepine, and the combination in bipolar disorderJ Clin Psychiatry5847089413412
- DilsaverSCSwannSCChenYW1996Treatment of bipolar depression with carbamazepine: results of an open studyBiol Psychiatry4093578896785
- EmilienGMaloteauxJMSeghersA1996Lithium compared to valproic acid and carbamazepine in the treatment of mania: a statistical meta-analysisEur Neuropsychopharmacol6245528880085
- FickerD2004Switching from immediate-release to extended-release carbamazepine capsules: safety and tolerabilityAnnual Meeting of the American Epilepsy Society2004 December 5–7New Orleans, LA, USA
- GarnettWRLevyBMcLeanAM1998Pharmacokinetic evaluation of twice-daily extended-release carbamazepine (CBZ) and four-times-daily immediate-release CBZ in patients with epilepsyEpilepsia3927499578044
- GinsbergLD2004aEfficacy and safety of ERC-CBZ in the treatment of bipolar II disorder56th Institute on Psychiatric Services2004 October 6–10Atlanta, GA, USA
- GoodwinFK2003Rationale for using lithium in combination with other mood stabilizers in the management of bipolar disorderJ Clin Psychiatry64Suppl 5182412720480
- GoodwinFKJamisonKRGoodwinFKJamisonKR1990Maintenance medical treatmentManic-depressive illness New YorkOxford University Pr665724
- GoodwinFKMurphyDLBunneyWEJr1969Lithium-carbonate treatment in depression and mania. A longitudinal double-blind studyArch Gen Psychiatry21486964896983
- GouldTDManjiHK2002Signaling networks in the pathophysiology and treatment of mood disordersJ Psychosom Res536879712169343
- GouldTDQuirozJASinghJ2004Emerging experimental therapeutics for bipolar disorder: insights from the molecular and cellular actions of current mood stabilizersMol Psychiatry97345515136794
- GreenbergRN1984Overview of patient compliance with medication dosing: a literature reviewClin Ther659296383611
- GreilWKleindienstNErazoN1998Differential response to lithium and carbamazepine in the prophylaxis of bipolar disorderJ Clin Psychopharmacol18455609864077
- HaefeliWEMeyerPGLuscherTF1994Circadian carbamazepine toxicityEpilepsia3540028156964
- HoppenerRJKuyerAMeijerJW1980Correlation between daily fluctuations of carbamazepine serum levels and intermittent side effectsEpilepsia21341507398602
- JamisonKRAkiskalHS1983Medication compliance in patients with bipolar disorderPsychiatr Clin North Am6175926889171
- KeckPEJrMcElroySLStrakowskiSM1996Factors associated with pharmacologic noncompliance in patients with maniaJ Clin Psychiatry5729278666570
- KesslerRCChiuWTDemlerO2005Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey ReplicationArch Gen Psychiatry626172715939839
- KetterTAKalaliAHWeislerRH2004A 6-month, multicenter, open-label evaluation of extended-release carbamazepine capsule monotherapy in bipolar disorder patients with manic or mixed episodesJ Clin Psychiatry656687315163253
- KleindienstNGreilW2000Differential efficacy of lithium and carbamazepine in the prophylaxis of bipolar disorder: results of the MAP studyNeuropsychobiology42Suppl 121011093063
- LiXKetterTAFryeMA2002Synaptic, intracellular, and neuroprotective mechanisms of anticonvulsants: are they relevant for the treatment and course of bipolar disorders?J Affect Disord6911412103447
- McElroySLKeckPEJr2000Pharmacologic agents for the treatment of acute bipolar maniaBiol Psychiatry485395711018226
- McLeanABrowneSZhangY2001The influence of food on the bioavailability of a twice-daily controlled release carbamazepine formulationJ Clin Pharmacol41183611210399
- MillerADKraussGLHamzehFM2004bImproved CNS tolerability following conversion from immediate- to extended-release carbamazepineActa Neurol Scand109374715147458
- MitchellPBMalhiGS2002The expanding pharmacopoeia for bipolar disorderAnnu Rev Med531738811818469
- MontgomerySASchatzbergAFGuelfiJD2000Pharmacotherapy of depression and mixed states in bipolar disorderJ Affect Disord59Suppl 1S395611121826
- Muller-OerlinghausenBBerghoferABauerM2002Bipolar disorderLancet359241711812578
- OkumaTKishimotoAInoueK1973Anti-manic and prophylactic effects of carbamazepine (Tegretol) on manic depressive psychosis. A preliminary reportFolia Psychiatr Neurol Jpn27283974801623
- OkumaTYamashitaITakahashiR1990Comparison of the anti-manic efficacy of carbamazepine and lithium carbonate by double-blind controlled studyPharmacopsychiatry23143501973844
- PellockJM1987Carbamazepine side effects in children and adultsEpilepsia28Suppl 3S64702961558
- RivaRAlbaniFAmbrosettoG1984Diurnal fluctuations in free and total steady-state plasma levels of carbamazepine and correlation with intermittent side effectsEpilepsia25476816540170
- Shire US Inc. 2005. Equetro (TM) [Prescribing information].
- SmallJGKlapperMHMilsteinV1991Carbamazepine compared with lithium in the treatment of maniaArch Gen Psychiatry48915211929761
- StevensRELimsakunTEvansG1998Controlled, multidose, pharmacokinetic evaluation of two extended- release carbamazepine formulations (Carbatrol and Tegretol-XR)J Pharm Sci871531410189261
- StimmelGL2004Economic grand rounds: the economic burden of bipolar disorderPsychiatr Serv551171814762232
- SuppesTDennehyEBHirschfeldRM2005The Texas implementation of medication algorithms: update to the algorithms for treatment of bipolar I disorderJ Clin Psychiatry668708616013903
- SvarstadBLShiremanTISweeneyJK2001Using drug claims data to assess the relationship of medication adherence with hospitalization and costsPsychiatr Serv528051111376229
- UlrichMLRotzingerSAsgharSJ2003Effects of dextroamphet-amine, lithium chloride, sodium valproate and carbamazepine on intraplatelet Ca2+ levelsJ Psychiatry Neurosci281152512670128
- VasudevKGoswamiUKohliK2000Carbamazepine and valproate monotherapy: feasibility, relative safety and efficacy, and therapeutic drug monitoring in manic disorderPsychopharmacology (Berl)150152310867972
- WeislerRHHirschfeldRCutlerAJ2004aEfficacy of extended-release carbamazepine in bipolar disorder: results of two pooled clinical trialsUS Psychiatric and Mental Health Congress2004 November 18–21San Diego, CA, USA
- WeislerRHHirschfeldRCutlerAJ2004bSafety and tolerability of extended-release carbamazepine in bipolar disorder: results from 2 pooled clinical trialsUS Psychiatric and Mental Health Congress2004 November 18–21San Diego, CA, USA
- WeislerRHKalaliAHKetterTA2004cA multicenter, randomized, double-blind, placebo-controlled trial of extended-release carbamaze-pine capsules as monotherapy for bipolar disorder patients with manic or mixed episodesJ Clin Psychiatry654788415119909
- WeislerRHKeckPESwannAC2005Extended-release carbamazepine capsules as monotherapy for acute mania in bipolar disorder: a multicenter, randomized, double-blind, placebo-controlled trialJ Clin Psychiatry663233015766298
- [WHO] World Health Organization2004Gender in mental health researchGenevaWHO
- WyattRJHenterI1995An economic evaluation of manic-depressive illness – 1991Soc Psychiatry Psychiatr Epidemiol30213197482006