Abstract
We have previously reported that prior exposure to inescapable tailshock stress increased avoidance responding 24 hours later. We argued previously that this might model the avoidance behavior characteristic of post-traumatic stress disorder (PTSD). The current experiment was conducted to determine whether a more ethologically relevant stressor would produce similar effects on avoidance responding. Therefore, rats were restrained for 2 hours and exposed to trimethylthiazoline (TMT), a component of fox feces, restrained only, or served as home cage controls. Twenty-four hours later, subjects received a 4-hour escape–avoidance session. Animals exposed to TMT made more escape responses overall, and made more avoidance responses than the other two groups by the 4th hour of the session. Differences between the TMT-exposed animals and restraint alone could not be explained by differences in corticosterone (CORT) levels. Results are discussed in terms of the possible neural changes induced by TMT exposure and the relationship to the behavioral aspects of PTSD or acute stress.
Introduction
Animal models are critical to understand the etiology and neurobiology of psychiatric conditions. The most frequently used animal model of post-traumatic stress disorder (PTSD), for example, is exposure to inescapable electric shock (eg, CitationMaier 2001). Exposure to inescapable shock in rats leads to a variety of physiological sequelae, including increases in basal plasma corticosterone (CitationOttenweller et al 1992), urinary corticosterone (CitationBrennan et al 2000), as well as persistent increases in acoustic startle responding (CitationServatius et al 1995). These symptoms collectively resemble the physiological symptoms seen in PTSD patients. However, since most of these changes are relatively transient, they may be better described as the effects of acute stress.
The inescapable shock model has also been criticized, however, for not being a good model of the behavioral aspects of PTSD (CitationYehuda and Antelman 1993). Inescapable shock typically produces a passivity manifested as decreases in responding (ie, learned helplessness; CitationMaier and Seligman 1976), while the avoidance aspect of PTSD could be an active process. We have recently reported increased avoidance responding 24 hours after exposure to inescapable tailshock (CitationBrennan et al 2005). We utilized a leverpress escape–avoidance model, as opposed to the more traditional shuttlebox procedure, and also conducted more trials than are typically performed in learned helplessness studies. We argued that the increase in avoidance performance in the previously stressed rats might model the behavioral aspect of PTSD (CitationBrennan et al 2005). These data will have to be supplemented with additional findings that the increased avoidance persists for a long period of time, to better model the persistence of PTSD symptoms.
Recently, a number of researchers have attempted to examine the physiological and behavioral effects of stress using more ethologically relevant stimuli (see CitationDielenberg and McGregor 2001 for a review). One of the manipulations used is exposure to trimethylthiazoline (TMT), a component of fox feces. Since the fox is a predator of the rat, exposure to this odor is apparently both a naturalistic as well as an innate stressor (CitationHolmes and Galea 2002). Laboratory-bred rats show a robust corticosterone (CORT) response to TMT (CitationMorrow et al 2000) and show increased dopamine metabolism in both the medial prefrontal cortex and amygdala (CitationMorrow et al 2000). These same changes in dopamine metabolism are also seen after restraint stress (CitationMorrow et al 1997) and suggest that TMT could be used as a qualitatively different, nonpainful stress manipulation to compare with traditional shock procedures.
The purpose of the current experiment was to assess the effect of restraint and TMT exposure on subsequent escape–avoidance performance in rats. We hypothesized that TMT would cause a similar facilitatory effect on avoidance performance as prior shock (CitationBrennan et al 2005). We also measured CORT levels in TMT-exposed animals and animals that were restrained with no odor presentation. We hypothesized an increase in CORT in both groups, and perhaps a differential increase if CORT is related to any observed behavioral differences.
Methods
Subjects
Subjects were 36 male, Sprague-Dawley rats obtained from Charles River, Kingston, NY, USA. They were approximately 60 days old, and 300–350 g at the time of testing. They were group housed (3 per cage), and all cage mates were in the same group. Subjects were maintained on ad lib food and water, except during the stress and escape–avoidance sessions. Subjects were maintained on a 12:12 light/dark cycle, with lights on at 0700 h.
Apparatus and general procedures
Restraint–TMT exposure
Twelve animals were randomly assigned to the TMT-exposure group. To ensure that subjects were exposed to the predator odor, the subjects were restrained in plastic tubes (Harvard Apparatus, Inc, Holliston, MA, USA) for 2 hours (the length of time of shock exposure; CitationBrennan et al 2005) and 25 μl of TMT was placed on to a cotton ball and placed approximately 5 cm in front of the restrainers.
To control for the effects of restraint alone (REST), a second group of 12 animals was restrained for 2 hours in a separate location that was not proximal to the odors. Finally, a third group of 12 subjects was left undisturbed in the housing room and served as home cage controls (HCC). The stress manipulations were conducted from approximately 1200 to 1400 h. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the East Orange VA Medical Center, NJ, USA.
Escape–avoidance conditioning
Twenty-four hours after restraint, animals received a single escape–avoidance session. Escape–avoidance sessions were conducted in 4 operant chambers (Coulbourn, Inc, Allentown, PA, USA) 24 hours after TMT or REST. The chambers were 30.5 cm wide × 24.4 cm deep × 30.5 cm high, and had a lever mounted on one wall. A houselight was mounted on the upper portion of the chamber in the wall directly across from the lever. Subjects were allowed approximately 1 minute to explore the chamber before the session began. The first trial began with the onset of the warning signal (WS) and houselight. The WS was a 1000-Hz tone, emitted from a speaker mounted in the chamber and clearly audible to the animal. If the animal had not made a leverpress after 60 seconds of the WS, they began to receive 1.0-mA footshock through the grid floor. The shock, WS, and houselight were all terminated by a leverpress. After a leverpress, the animal was given a 6-minute period of safety. There was a discrete safety signal, a flashing light located on the wall above the lever.
A leverpress after the shock had begun was classified as an “escape” (even if it occurred during the intermittent periods between shocks), while a response that occurred during the initial 60 seconds of the WS before the shock came on was classified an “avoidance”. A trial thus ended with a leverpress. A new trial began with the safety signal terminating, and the reintroduction of the houselight and WS. In the absence of a leverpress, the 30-second shocks were presented on a variable time 60 seconds schedule. Subjects were given a “free” escape by the experimenter if no response had occurred in 20 minutes. Subjects received a single 4-hour session. The maximum number of responses was thus 40, or 10 per hour. Subjects did not receive any leverpress training prior to the single session.
CORT levels
For the TMT and REST groups, 4 small (~0.25 ml) blood samples were drawn via a nick in the tail vein and deposited into microcentrifuge tubes. A baseline sample was drawn at approximately 0900 h. This is near the trough of the circadian CORT cycle. A second sample was drawn later that day (approximately 1400 h) after the restraint or restraint plus TMT exposure. A third sample was drawn the following morning at 0900 h. Our group has published extensively that prior stress can raise the circadian CORT trough on subsequent days (eg, CitationMoldow et al 2005). A final sample was drawn later that day (approximately 1400 h) after the escape–avoidance session. CORT was assayed via a double-antibody radioimmunoassay kit (ICN Biomedicals, Inc, Carson, CA, USA), as previously described (CitationOttenweller et al 1992).
Analyses
We analyzed the total number of escape and avoidance responses by hour across the session. Occasionally an animal would make one or more “pseudo-avoidances” while exploring the chamber during the initial warning period(s). These responses were not counted in any dependent measure. Only responses that occurred after a shock had been received were included in all analyses. Data were analyzed via mixed factor (group × hour) ANOVA models, with Newman-Keuls post hoc tests to detect specific differences.
Results
Escape responses
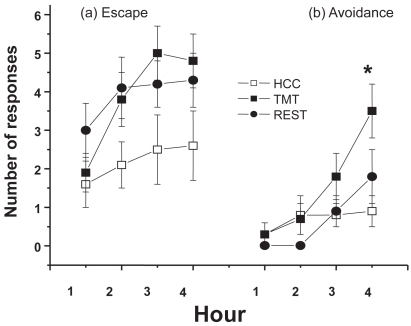
The number of escapes across the session is presented in . The number of responses for all 3 groups appeared to increase over the session. However, the TMT-exposed animals appeared to make the most escape responses. A 3 × 4 (group × time) mixed factor ANOVA was performed. The ANOVA confirmed these observations. There were significant effects of both group, (2, 144) = 3.49, p < 0.05, and time, F(3, 144) = 7.00, p < 0.0001. Newman-Keuls analyses revealed that the TMT group made more escape responses than the HCC group. The interaction was not significant, F (6, 144) = 0.99, p > 0.05.
Avoidance responses
The number of avoidance responses over the session is presented in . It appeared that the TMT group made more avoidance responses than the other groups by the end of the session. The groups generally increased the number of avoidance responses they performed across time, F(3, 144) = 13.26, p < 0.0001. The main effect of group approached significance, F(2, 144) = 2.77, p = 0.07. These effects were superseded by the significant group × time interaction, F(6, 144) = 2.79, p = 0.01. Newman-Keuls post-hoc comparisons indicated that the TMT-exposed animals made more avoidance responses than both the restraint and HCC animals during the fourth hour of the session, p < 0.05.
CORT levels
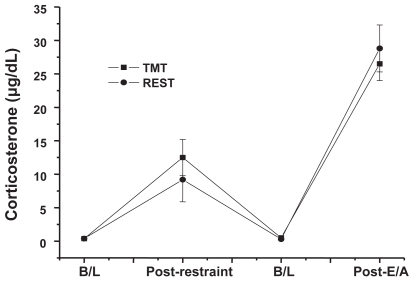
The CORT levels did not appear to differ between the two groups at any time point. These data are presented in . The ANOVA confirmed this, revealing only a main effect of time, F(3, 72) = 66.96, p < 0.0001. The two baseline samples did not differ; all other differences were significant, p < 0.05. Neither the main effect of group, F(1, 72), p < 1.0 nor the interaction, F(3, 72), p < 1.0, was significant.
Discussion
The current results demonstrate that rats restrained and exposed to the predator odor TMT performed more avoidance responses by the last hour of a 4-hour session than animals restrained alone, or HCC. Further, the differences in avoidance responding between the two restrained groups could not be attributed to plasma CORT levels, as the two groups did not differ at any time point. A study where CORT was pharmacologically depleted would further support that hypothesis. These results extend those of our previous report that inescapable shock increases avoidance responding 24 hours later (CitationBrennan et al 2005).
TMT exposure has characteristics of a stressor, producing increases in CORT (CitationMorrow et al 2000), defensive behavior (CitationHolmes and Galea 2002), freezing (CitationWallace and Rosen 2000), and activation of central dopaminergic systems (CitationMorrow et al 2000). TMT exposure appears to induce an innate fear response (CitationKing et al 2005). The CORT increase that we observed after TMT exposure replicates the results of a number of reports (CitationMorrow et al 2000; CitationDay et al 2004). Interestingly, CitationDay et al (2004) found that exposure to the noxious control odor butyric acid had no effect on the hypothalamic–pituitary–adrenal (HPA) axis. It appears that the behavioral and physiological effects of TMT are not due to its noxious odor, but rather to it being an innate stress stimulus for the rat.
A key question is what neural substrates are activated by TMT that produce the observed changes in physiology and behavior, including the increased avoidance responding. The recent study by CitationDay et al (2004) exposed rats to TMT and then measured c-fos mRNA induction in a number of brain regions. Relative to controls, the TMT-exposed animals showed significant activity in a number of areas known to be activated by other stressors, including the bed nucleus of the stria terminalis (BNST), a number of hypothalamic nuclei, and the central nucleus of the amygdala (CeA) (CitationDay et al 2004). Importantly, exposure to the control odor butyric acid did not activate any of these regions. Again, this supports the contention that the effects of TMT are due to its innately aversive properties.
We have argued that the leverpress avoidance response is dependent on dopamine systems (CitationBrennan et al 2003; CitationBrennan 2004). Stress or fear activates dopaminergic systems (CitationPezze and Feldon 2004), and performance of the leverpress avoidance response is blocked by dopamine2 (D2) receptor antagonists (CitationBrennan 2005 unpubl). CitationMorrow et al (2002) exposed rats to TMT in an open field environment and found dopamine turnover was increased in the amygdala and medial prefrontal cortex. A control odor had no effect on dopamine turnover (CitationMorrow et al 2002). Tailshock, which also facilitates learning of the avoidance response 24 hours later, has been shown to increase the density of D2 receptors selectively in the medial prefrontal cortex the next day (CitationMacLennan et al 1989). TMT exposure may thus induce a sensitization of dopamine systems that leads to increased avoidance responding when the animals are exposed to the escape–avoidance training 24 hours later. This possibility awaits further research.
In summary, restraint plus TMT exposure increased avoidance responding 24 hours later, comparable to that of shock. Restraint alone had no facilitatory effect. The fact that the two restrained groups had identical CORT levels at all time points tested appears to preclude the HPA axis as a mechanism for the behavioral difference. Future studies will assess the neural changes produced by TMT exposure, and their relationship to avoidance responding.
Acknowledgments
We acknowledge the technical assistance of Jean Willi, Sara Kelly, Tara Tuminello, and Susan Tente. This research was supported by Department of Defense DOD funds to RJS.
References
- BrennanFX2004Genetic differences in leverpress escape/avoidance conditioning in seven mouse strainsGenes Brain Behav31101415005719
- BrennanFXBeckKDRossRJ2005Stress-induced increases in avoidance responding: A model for post-traumatic stress disorder behavior?Neuropsychiatric Disease and Treatment1697218568126
- BrennanFXBeckKDServatiusRJ2003Leverpress escape/avoidance in performance in rats: safety signal length and avoidance performanceIntegr Physiol Behav Sci38364412814195
- BrennanFXOttenwellerJESeifuY2000Persistent stress-induced elevations of urinary corticosterone in ratsPhysiol Behav71441611239661
- DayHEWMasiniCVCampeauS2004The pattern of brain c-fos mRNA induced by a component of fox odor, 2,5-dihydro-2,4,5-trimethylthiazoline (TMT), in rats, suggests both systemic and processive stress characteristicsBrain Res10251395115464754
- DielenbergRAMcGregorIS2001Defensive behavior in rats towards predatory odors: a reviewNeurosci Biobehav Rev2559760911801285
- HolmesMMGaleaLAM2002Defensive behavior and hippocampal cell proliferation: Differential modulation by naltrexone during stressBehav Neurosci116160811898802
- KingJADe OliveiraWLPatelN2005Deficits in testosterone facilitate enhanced fear responsePsychoneuroendocrinology303334015694113
- MacLennanAJPelleymounterMAAtmadjaS1989D2 dopamine receptors in the rat prefrontal cortex: characterization and alteration by stressBrain Res47730072522809
- MaierSF2001Exposure to the stressor environment prevents the temporal dissipation of behavioral depression/learned helplessnessBiol Psychiatry497637311331084
- MaierSFSeligmanMEP1976Learned helplessness: theory and evidenceJ Exp Psy Gen105346
- MoldowRLBeckKDWeaverS2005Blockage of glucocorticoid, but not mineralocorticoid receptors prevents the persistent increase in circulating basal corticosterone concentrations following stress in the ratNeurosci Lett7458
- MorrowBAElsworthJDRothRH2002Fear-like biochemical and behavioral responses in rats to the predator odor, TMT, are dependent on the exposure environmentSynapse4611812211094
- MorrowBALeeEJKTaylorJR1997(S)-(-)-HA-966, a 8-hydroxybutyate-like agent, prevents enhanced mesocorticolimbic dopamine metabolism and behavioral correlates of restraint stress, conditioned fear, and cocaine sensitizationJ Pharm Exp Therap28371221
- MorrowBARedmondAJRothRH2000The predator odor, TMT, displays a unique, stress-like pattern of dopaminergic and endocrinological activation in the ratBrain Res8641465110793199
- MorrowBARothRHElsworthJD2000TMT, a predator odor, elevates mesoprefrontal dopamine metabolic activity and disrupts short-term working memory in the ratBrain Res Bull525192310974491
- OttenwellerJEServatiusRJTappWN1992A chronic stress state in rats: effects of repeated stress on basal corticosterone and behaviorPhysiol Behav51689981594666
- PezzeMAFeldonJ2004Mesolimbic dopaminergic pathways in fear conditioningProg Neurobiol743012015582224
- ServatiusRJOttenwellerJENatelsonBH1995Delayed startle sensitization distinguishes rats exposed to one or three stress sessions: further evidence toward an animal model of PTSDBiol Psychiatry38539468562666
- WallaceKJRosenJB2000Predator odor as an unconditioned fear stimulus in rats: elicitation of freezing by trimethylthiazoline, a component of fox fecesBehav Neurosci1149122211085605
- YehudaRAntelmanSM1993Criteria for rationally evaluating animal models of Posttraumatic stress disorderBiol Psychiatry33479868513032