Abstract
Modafinil, a wakefulness-promoting agent unrelated to classical sympathomimetic stimulants, has been studied in a total of 933 children and adolescents as a treatment for attention-deficit/hyperactivity disorder (ADHD). Several studies, including three double-blind, placebo-controlled studies with intent-to-treat analyses, have demonstrated the efficacy of modafinil film-coated tablets in reducing symptoms of ADHD and associated problem behaviors in children and adolescents. Modafinil is generally well tolerated, with adverse events (such as insomnia, headache, loss of appetite, weight loss, and gastrointestinal discomfort) that are generally mild to moderate, rarely leading to medication discontinuation. To minimize treatment-emergent side effects, titration to the target dose of 355–425 mg once a day should take place over 2–3 weeks. Due to reports of skin rash (including one case of possible erythema multiforme/Stevens Johnson Syndrome during pivotal studies), additional studies have been requested to better evaluate the risks of developing severe cutaneous adverse reactions.
Pharmacological management of children and adolescents with attention-deficit/hyperactivity disorder (ADHD) often results in improvements in symptoms of inattention (distractibility, forgetfulness, inability to concentrate), in symptoms of hyperactivity-impulsivity (restlessness, fidgetiness, impulsive responses), and in overall quality of life (CitationBrown et al 2005; CitationKing et al 2006). Sympathomimetic stimulants are the most common treatment for ADHD (CitationBrown et al 2005; CitationArnsten 2006), but a sizable minority of children and adolescents with ADHD do not respond adequately to these medications or have intolerable side-effects (CitationBrown et al 2005; CitationKing et al 2006). Thus, additional treatments for ADHD are sought. Modafinil has been evaluated as a potentially effective medication in reducing problematic symptoms of ADHD.
Molecular and biologic characteristics of modafinil
Modafinil (Provigil®, Cephalon, Inc., Frazer, PA, USA) is a wakefulness-promoting medication that is structurally different from the classical stimulants (methylphenidate, amphetamines, and pemoline; see ). Besides being structurally unique, the neurophysiological properties of modafinil are also unique in comparison with sympathomimetic stimulants (CitationLin et al 1992, Citation1996; CitationSimon et al 1995; CitationShelton et al 1995; CitationFerraro et al 1997). Besides a low affinity for the dopamine reuptake carrier site with only a slight increase in extracellular dopamine (CitationMignot et al 1994), modafinil does not bind to nor does it have significant agonist or antagonist activity at any of the known CNS neuronal catecholamine, cholinergic or amino acid/neuropeptide receptors or transporters (CitationCephalon 2006). In contrast to the sympathomimetic stimulants, physiological effects of modafinil are not a direct result of dopaminergic or noradrenergic activity (CitationAkaoka et al 1991; CitationDeSereville et al 1994; CitationFerraro et al 1997).
Whereas methylphenidate and amphetamine stimulants have a wide distribution of direct neuronal activity throughout the cortex, basal ganglia, and nucleus accumbens, preclinical studies of modafinil demonstrate more limited binding, primarily in the hypothalamus (CitationLin et al 1996). Increased neuronal activity (as evidenced by increased c-fos activity) was found primarily in orexin neurons of the tuberomammilary nucleus, even with administration of low doses of modafinil (CitationScammell et al 2000). This area of the brain is implicated in regulating physiologic wakefulness (CitationChemelli et al 1999; CitationScammell et al 2000). Evidence also exists for inhibition of the sleep-promoting neurons of the ventrolateral preoptic nucleus after modafinil adminstration (CitationGallopin et al 2004).
Notable in these preclinical studies is the lack of significant activity in the nucleus accumbens (CitationLin et al 1996; CitationFerraro et al 1997; CitationScammell et al 2000), the brain’s reward/reinforcement center (which mediates most of the addictive properties of drugs such as cocaine, opioids, and stimulants). Several studies have confirmed the low abuse potential of modafinil (CitationDeroche-Gamonet et al 2002; CitationMyrick et al 2004), with less cocaine-like discriminative-stimulus effects and self-reported stimulant effects than methylphenidate or amphetamines (CitationGold 1996; CitationJasinski 2000; CitationJasinski and Lovacevic-Ristanovic 2000; CitationRush et al 2002a, Citationb).
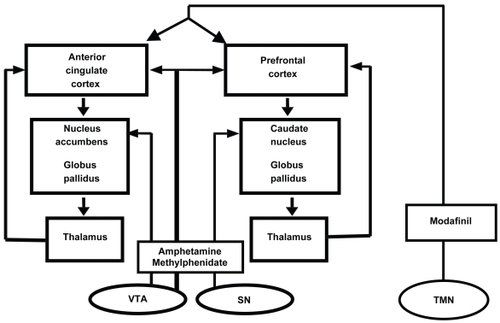
Although approved by the US Food and Drug Administration for improving wakefulness in adults with excessive sleepiness associated with narcolepsy, obstructive sleep apnea/hypopnea, and shift work sleep disorder 16 years of age and older (CitationCephalon 2004), its mechanism of action in humans is not fully understood. After administration of modafinil, functional MRI (fMRI) has shown activation of the anterior cingulate cortex during a task of working memory (CitationSpence et al 2005), and an activation of other cortical areas (particularly in individuals with low initial activation levels; CitationEllis et al 1999). This is very significant, considering that the anterior cingulate cortex was notably deficient in fMRI activity in individuals with ADHD during a working memory task (CitationBush et al 1999). A likely hypothesis regarding modafinil’s physiologic effects is that modafinil indirectly activates the cerebral cortex (including areas implicated in ADHD pathology) via ascending arousal pathways arising from the hypothalamus (eg, the tuberomammillary nucleus; see ).
Figure 2 Indirect activation of the cerebral cortex by modafinil action on the tuberomammillary nucleus. Adapted from CitationSwanson et al (1998).
Modafinil is well absorbed from the gastrointestinal tract, but bioavailability cannot he determined since low water solubility makes i.v. administration unfeasible (CitationCephalon 2004, Citation2006). Peak levels are seen at 2–4 hours after oral administration, but about 1 hour later if administered with food (CitationCephalon 2004,Citation2006). Metabolized by several cytochrome P450 (CYP450) enzymes, modafinil is a mild inducer of CYP1A2, CYP2B6, CYP3A4, and is a mild inhibitor of CYP2C19 (CitationCephalon 2004). Therefore, interactions with medicines may be seen with diazepam, propranolol, phenytoin, S-mephenytoin, and tricyclic or some SSRI antidepressants (in poor metabolizers of CYP2D6), whereas decreased efficacy of added medicines may be seen with cyclosporine and birth control hormones (CitationCephalon 2004). Strong CYP3A4 inducers (such as carbamazepine, phenobarbital, and rifampin) or inhibitors (such as ketoconazole or itraconazole) may slightly alter modafinil blood levels (CitationCephalon 2004).
Half-life in adults is approximately 15 hours, but is shorter for children and adolescents. For children 6–7 years of age, the half-life is about 7 hours (CitationCephalon 2006). The half-life gradually lengthens until 9–11 years, when a pronounced shift is seen approaching adult levels (CitationCephalon 2006).
Efficacy in children and adolescents with ADHD
After two investigator-initiated independent studies suggested possible efficacy of modafinil in children with ADHD (CitationRugino and Copley 2001; CitationRugino and Samsock 2003), a phase 2 study of 248 children and adolescents aged 6–13 years was completed to determine if divided doses were effective, tolerated, and necessary to treat children with ADHD. The subjects who received 300 mg once per day showed consistently greater improvement in home and school ADHD Rating Scale IV (ADHD-RS-IV; CitationDePaul G, et al 1998) total scores as well as in Conners Parent Rating Scales (CPRS; Connors 1997) ADHD DSM-IV total scores, whereas subjects who received 300 mg per day in divided doses showed less consistent improvements in ADHD symptoms (CitationBiederman et al 2006b). The pharmacokinetic data from this and other phase 2 studies allowed for computer modeling of the exposure – response relationship. Using a target range for the area under the plasma drug concentration versus time curve (AUC0–24) of 150 μg × hours/mL, doses of 340 mg/day were found to be the most appropriate target dose if the child’s weight was <30 kg and 425 mg/day if the child’s weight was ≥30 kg (CitationCephalon 2006).
Three phase 3 studies involving a total of 638 children and adolescents with ADHD aged 6 to 17 years confirmed the clinical efficacy of 85 mg modafinil film-coated compressed tablets when administered as once-daily doses of up to 340–425 mg over 7–9 weeks (CitationBiederman et al 2005; CitationSwanson et al 2006; CitationGreenhill et al 2006). For each of the studies, the diagnosis of ADHD was confirmed with the Diagnostic Interview Schedule for Children, Fourth Edition (DISC IV), and severity of illness was confirmed with scores ≥1.5 standard deviations above the age- and gender-based mean on the ADHD-RS-IV: School Version. Mental impairment, uncontrolled psychological comorbidity, and significant medical problems excluded subjects from these studies. As is observed in most ADHD studies involving children and adolescents, most of the subjects of these studies (CitationBiederman et al 2005; CitationSwanson et al 2006; CitationGreenhill et al 2006) had ADHD, Combined Type (59%–71%), were male (2.4–2.7:1), and were moderately to markedly ill (82%–91%).
Two flexible-dose studies (CitationBiederman et al 2005; CitationGreenhill et al 2006) and one fixed-dose study (CitationSwanson et al 2006) documented efficacy of a film-coated compressed tablet formulation of modafinil using an intent-to-treat analysis with 2:1 ratio of treatment: control and with the endpoint defined as the last obtained value carried forward. In the flexible-dose studies, the mean and modal stable doses were 361–369 mg/day and 425 mg/day (range 170–425 mg once daily every morning), whereas the final administered dose in the fixed-dose study was 340 mg for children <30 kg and 425 mg for subjects ≥30 kg. For all three studies and for pooled data (CitationCephalon 2006), ADHD-RS-IV scores demonstrated improvements consistently in favor of modafinil for the primary outcome measure (the School Version of the ADHD-RS-IV, see , , ), the Home Version of the ADHD-RS-IV, as well as for the inattentive and hyperactive-impulsive subscale scores of each version. There were no effects of race, sex, weight, ADHD subtype, or comorbidity in the improvements seen with modafinil treatment (CitationCephalon 2006). More improvement was seen in patients less than 12 years of age compared with older patients. Modafinil was efficacious in treating both stimulant-naïve patients and those who had received prior stimulant therapy. ADHD-RS-IV: School Version total scores showed a reduction of ≥30% in 64%–69% of modafinil-treated subjects (as compared with 35%–39% of controls, p < 0.0001) and a reduction of ≥50% for 44%–48% of modafinil-treated subjects as compared with 19%–20% of controls, p < 0.001) for each of the studies (CitationBiederman et al 2005; CitationSwanson et al 2006; CitationCephalon 2006; CitationGreenhill et al 2006). Using the ADHD-RS-IV: School Version total score data and Cohen’s calculations, the overall pooled effect size was 0.69; this corresponds to a medium-to-large effect (Cohen 1988). The greatest effect was seen in drug-naïve subjects, in whom the calculated effect size was 1.08 (treatment n = 221 and control n = 102).
Figure 3a ADHD Rating Scale total scores as a function of time in a flexible-dose study of efficacy of modafinil for children and adolescents with attention-deficit/hyperactivity disorder (n = 194). *p values <0.05; endpoint represents the last obtained value carried forward Reproduced from Cephalon, Inc. 2006. Modafinil (CEP-1538) tablets Supplemental NDA 20-717/S-019 ADHD indication. Briefing document for Psychopharmacologic Drugs Advisory Committee Meeting March 26, 2006. Frazer, PA: Cephalon, Inc.
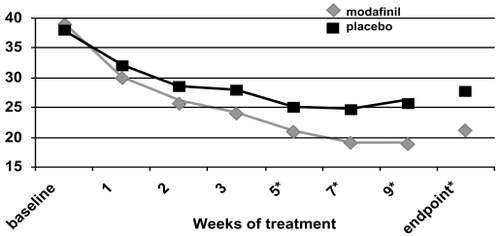
Figure 3b ADHD Rating Scale total scores as a function of time for a flexible-dose study of modafinil efficacy for children and adolescents with attention-deficit/hyperactivity disorder (n = 244). *p values <0.05; endpoint represents the last obtained value carried forward. Reproduced from: Cephalon, Inc. 2006. Modafinil (CEP-1538) tablets Supplemental NDA 20-717/S-019 ADHD indication. Briefing document for Psychopharmacologic Drugs Advisory Committee Meeting March 26, 2006. Frazer, PA: Cephalon, Inc.
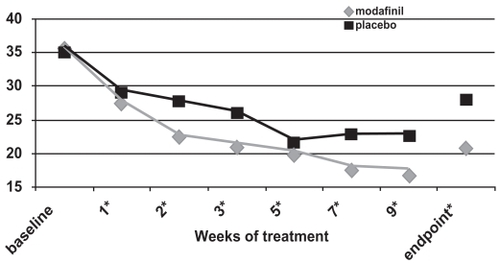
Figure 3c ADHD Rating Scale total scores as a function of time for a fixed-dose study of efficacy of modafinil for children and adolescents with attention-deficit/hyperactivity disorder (n = 183). *p values <0.05; endpoint represents the last obtained value carried forward Reproduced from Cephalon, Inc. 2006. Modafinil (CEP-1538) tablets Supplemental NDA 20-717/S-019 ADHD indication. Briefing document for Psychopharmacologic Drugs Advisory Committee Meeting March 26, 2006. Frazer, PA: Cephalon, Inc.
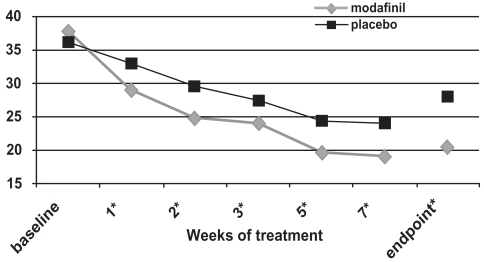
For all three of these double-blind, placebo-controlled studies (CitationCephalon 2006), the ADHD Rating Scale-IV scores continued to improve while subjects were followed week-to-week (see ). Nonetheless, improvements were often seen at the first follow-up visit (week 1 of treatment; CitationSwanson et al 2006; CitationGreenhill et al 2006).
outlines the Clinical Global Impression of Change ratings from these studies (CitationCephalon 2006), defining a clinical response as “very much improved” or “much improved.” Patients treated with modafinil showed greater improvement in overall clinical condition than those receiving placebo at all study visits and for the last observation carried forward (46% vs 18%). Based on the pooled data (CitationCephalon 2006), the number needed to treat was 3.5 (95% confidence interval = 2.8–4.7).
Figure 4a Percentage responders as a function of time for a flexible-dose study of efficacy of modafinil for children and adolescents with attention-deficit/hyperactivity disorder (n = 194). Response is defined as having a Clinical Global Impressions of Change score of 1 (“very much improved”) or 2 (“much improved”).
*p values <0.05; EP = endpoint, which represents the last obtained value carried forward. Reproduced from Cephalon, Inc. 2006. Modafinil (CEP-1538) tablets Supplemental NDA 20-717/S-019 ADHD indication. Briefing document for Psychopharmacologic Drugs Advisory Committee Meeting March 26, 2006. Frazer, PA: Cephalon, Inc.
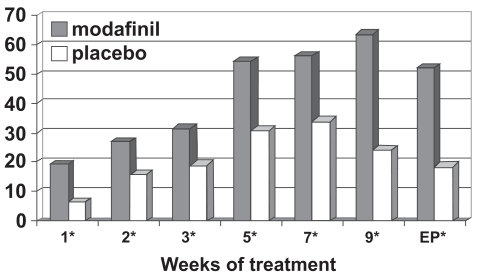
Figure 4b Percentage responders as a function of time for a flexible-dose study of efficacy of modafinil for children and adolescents with attention-deficit/hyperactivity disorder (n = 244). Response is defined as having a Clinical Global Impressions of Change score of 1 (“very much improved”) or 2 (“much improved”). *p values <0.05; EP = endpoint, which represents the last obtained value carried forward. Reproduced from Cephalon, Inc. 2006. Modafinil (CEP-1538) tablets Supplemental NDA 20-717/S-019 ADHD indication. Briefing document for Psychopharmacologic Drugs Advisory Committee Meeting March 26, 2006. Frazer, PA: Cephalon, Inc.
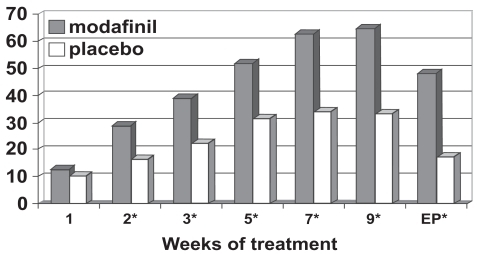
Figure 4c Percentage responders as a function of time for a fixed-dose study of efficacy of modafinil for children and adolescents with attention-deficit/hyperactivity disorder (n = 183). Response is defined as having a Clinical Global Impressions of Change score of 1 (“very much improved”) or 2 (“much improved”). *p values <0.05; EP = endpoint, which represents the last obtained value carried forward. Reproduced from Cephalon, Inc. 2006. Modafinil (CEP-1538) tablets Supplemental NDA 20-717/S-019 ADHD indication. Briefing document for Psychopharmacologic Drugs Advisory Committee Meeting March 26, 2006. Frazer, PA: Cephalon, Inc.
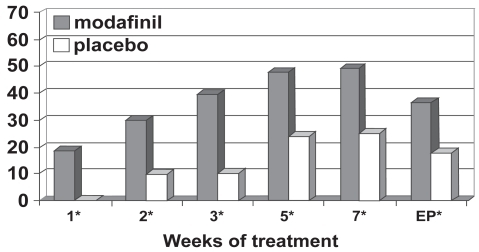
Using the Social Skills Rating Scale (SSRS; CitationGresham and Elliot 1990), statistically significant improvements favoring modafinil treatment were seen at endpoint for the externalizing subscale score, the internalizing subscale score, and the hyperactivity subscale score as well as for the problem behaviors total score (CitationBiederman et al 2005; CitationSwanson et al 2006; CitationCephalon 2006; CitationGreenhill et al 2006). Along with reductions in problem behaviors, the SSRS analysis suggested that modafinil resulted in improvements in the subscale scores for cooperation, assertion, responsibility, and self-control (CitationBiederman et al 2005; CitationSwanson et al 2006; CitationCephalon 2006; CitationGreenhill et al 2006).
Safety and tolerability
The 7-week fixed-dose study was followed by an abrupt double-blind, placebo-controlled 2-week withdrawal from treatment (CitationSwanson et al 2006). No withdrawal symptoms, rebound exacerbation of ADHD symptoms, or rebound hypersomnolence was reported from the subjects who were abruptly withdrawn from modafinil.
During the three cited double-blind, placebo-controlled studies (CitationBiederman et al 2005; CitationSwanson et al 2006; CitationGreenhill et al 2006), 420 subjects received modafinil treatment; 55% of subjects had at least one treatment-related adverse event, as compared with 29% of control patients (CitationCephalon 2006). Since the flexible-dose studies (CitationBiederman et al 2005; CitationGreenhill et al 2006) proceeded with a slower upward titration (over the course of 3 weeks) than the fixed-dose study (over the course of 7–9 days; CitationSwanson 2006), and since the flexible-dose studies halted upward titration when adequate efficacy was determined, the frequency of adverse events was lower in the flexible dose studies than in the fixed dose study. In each of the flexible dose studies (CitationBiederman et al 2005; CitationGreenhill et al 2006), modafinil-treated patients did not discontinue the study because of an adverse event at a rate higher than placebo, whereas more modafinil-treated subjects discontinued the study for an adverse event than control subjects (10% vs 0%) in the fixed dose study (CitationSwanson et al 2006).
describes the adverse events that occurred in at least 5% of the modafinil-treated patients and that occurred more frequently than in the control group (CitationCephalon 2006). When analyzing the pooled data (CitationCephalon 2006), Seventyfive percent of the adverse events developed within the first 2 weeks of treatment (during upward dose titration). Insomnia was the most frequent adverse event (CitationBiederman et al 2005; CitationSwanson et al 2006; CitationCephalon 2006; CitationGreenhill et al 2006), was described as severe in 9 cases and resulted in discontinuation of modafinil in 5 cases. Insomnia, headache, and gastrointestinal concerns (loss of appetite or abdominal pain) were the most common adverse events that resulted in discontinuation, but fever and nervousness were also rare causes for discontinuation (CitationBiederman et al 2005; CitationSwanson et al 2006; CitationCephalon 2006; CitationGreenhill et al 2006).
Table 1 Adverse events occurring in at least 5% of modafinil-treated subjects which occurred more frequently than in the control group during the three phase 3 studies (pooled data)
Anorexia/decreased appetite was reported in 16% of subjects (CitationBiederman et al 2005; CitationSwanson et al 2006; CitationCephalon 2006; CitationGreenhill et al 2006) and was associated with a 0.7 kg decrease of body weight during the 7–9 weeks of treatment of the three phase 3 studies (compared with the control group that gained 1 kg during the same time period). However, during a 12 month open-label extension study (CitationCephalon 2006) that followed the phase 3 studies, the weight z scores (compared with the norms established by the National Center for Health Statistics; Kucsmanski et al 2000) indicated that the subjects were heavier than average at baseline, and the z scores stabilized after 3 months of treatment. On the other hand, height z scores did not appear to decline over the 12-month extension period (CitationCephalon 2006).
No significant changes in resting heart rate, resting blood pressure, or electrocardiographic findings were found with administration of modafinil in doses from 85 mg to 425 mg (CitationBiederman et al 2005; CitationSwanson et al 2006; CitationCephalon 2006; CitationGreenhill et al 2006).
Among all children and adolescents who were administered modafinil as a part of clinical studies for ADHD (n = 933; CitationCephalon 2006), a total of 18 serious adverse events were described. Of these, only two were reported as “probably” or “possibly” related to modafinil: a maculopapular/morbiliform rash and a case of possible erythema multiforme/Stevens-Johnson Syndrome (CitationBiederman et al 2005). All of these skin lesions resolved without sequelae (CitationCephalon 2006). Six additional subjects (n = 933) discontinued modafinil treatment for concerns of rash, with resolution CitationCephalon 2006). Post-marketing experience was reviewed in light of these skin concerns. Five reports of severe cutaneous adverse reactions have been made since 1999, during which time approximately 673,000 adults have taken modafinil worldwide (CitationCephalon 2006). Further studies evaluating the relationship between the skin rashes and modafinil/modafinil sulfone have been requested by the United States Food and Drug Administration.
Psychiatric adverse events, such as aggression, suicidal ideation, and psychosis/mania were recently reported to be consequences of the various medications used to treat ADHD (CitationGelpirin 2006). In double-blind and open-label studies for children and adolescents with ADHD (n = 933, CitationCephalon 2006), aggression was reported for 1.4 to 1.8% of subjects taking modafinil. A total of 5 subjects reported symptoms of psychosis/mania (one of whom required hospitalization) and 5 reported transient suicidal ideation (most of whom had resolution despite continuing modafinil treatment). As observed with other ADHD treatments (Celpirin 2006), many of these subjects had no prior remarkable history of similar events.
Conclusion
Modafinil, when titrated to effect with a target dose of 340 mg (body weight <30 kg) or 425 mg (body weight ≥30 kg) over the course of 2–3 weeks, is effective in managing the symptoms of and the problem behaviors associated with attention-deficit/hyperactivity disorder in children and adolescents. It is generally well tolerated, with adverse events (such as insomnia, headache, loss of appetite, weight loss, and gastrointestinal discomfort) generally being mild to moderate (and rarely leading to medication discontinuation). Close observation (especially during the first 4 weeks of treatment) may be necessary to watch for the development of a skin rash.
Disclosures
Dr Rugino is on the Speaker’s Bureau of Novartis Pharmaceuticals, Shire Pharmaceuticals, UCB Pharma, and Cephalon, Inc. He is a research consultant for Cephalon, Inc. and Shire Pharmaceuticals. Dr Rugino’s employer has received research grant funding from Cephalon, Inc., Shire Pharmaceuticals, Novartis Pharmaceuticals, Sanofi-Aventis, and UCB Pharma.
References
- AkaokaHRousselBLinJS1991Effect of modafinil and amphetamine on the ratcatecholinergic neuron activityNeurosci Lett1232021676498
- ArnstenAF2006Stimulants:Therapeutic actions in ADHDNeuropsychopharmacology E-pub ahead of print
- BiedermanJSwansonJMWigalSB2005Efficacy and safety of modafinil film-coated tablets in children and adolescents with attention-deficit/hyperactivity disorder:results of a randomized, double-blind, placebo-controlled, flexible-dose studyPediatrics116e7778416322134
- BiedermanJSwansonJMWigalSB2006A comparison of once-daily and divided doses of modafinil in children with attention-deficit/hyperactivity disorder:a randomized, double-blind, and placebo-controlled studyJ Clin Psychiatry677273516841622
- BrownRTAmlerRWFreemanWS2005Treatment of attention-deficit/hyperactivity disorder:overview of the evidencePediatrics115e7495715930203
- BushGFrazierJARauchSL1999Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the Counting StroopBiol Psychiatry4515425210376114
- ChemelliRMWillieJTSintonCM1999Narcolepsy in orexin knockout mice:molecular genetics of sleep regulationCell984091210481905
- Cephalon, Inc.2004Prescribing Information:Provigil (Modafinil) Tablets PRO542West Chester, PACephalon, Inc.
- Cephalon, Inc.2006Modafinil (CEP- 1538) tablets Supplemental NDA 20-717/S-019 ADHD Indication:briefing document for Psychopharma-cologic Drugs Advisory Committee MeetingFrazer, PACephalon, Inc.
- CohenJ1998Statistical power analysis for the behavioral sciences2nd edHillsdale, New JerseyLawrence Eribaum Associates
- ConnersCK2000Conners Parent Rating Scales – Revised: Technical ManualNorth Tonawanda, NYMulti-Health Systems, Inc
- DePaulGPowerTJAnastopoulosAD1998ADHD Rating Scale – IV: Checklists, Norms, and Clinical InterpretationNew YorkGuilford Publications
- Deroche-GamonetVDarnauderyMBruins-SlotL2002Study of the addictive potential of modafinil in naive and cocaine-experienced ratsPsychopharmacology1613879512073166
- DeSerevilleJEBoerCRambertFA1994Lack of pre-synaptic dopaminergic involvement in modafinil activity in anesthetized mice:in vivo voltammetry studiesNeuropharmacology33755617936113
- EllisCMMonkASimmonsG1999Functional magnetic resonance imaging neuroactivation studies in normal subjects and subjects with the narcoleptic syndrome. Actions of modafinilJ Sleep Res88510389090
- FerraroLAntonelliTO’ConnorWT1997Modafinil:an antinarcoleptic drug with a different neurochemical profile to d-amphetamine and dopamine uptake blockersBiol Psychiatry42118139426889
- GallopinTLuppiPHRambertFA2004Effect of the wake-promoting agent modafinil on sleep-promoting neurons from the ventrolateral preoptic nucleus:an in vitro pharmacologic studySleep27192514998233
- GelpirinK2006Psychiatric adverse events with drug treatment of ADHD. Presentation to the Pediatric Advisory Committee to the United States Food and Drug Administration March 22, 2006 [online]Accessed August 24, 2006 URL: http://www.fda.gov/ohrms/dockets/ac/06/slides/2006-4210s-index.htm
- GoldLHBalsterRL1996Evaluation of the cocaine-like discriminative stimulus effects and reinforcing effects of modafinilPsychopharmacology126286928878344
- GreenhillLLBiedermanJBoellnerSW2006A randomized, double-blind, placebo-controlled study of modafinil film-coated tablets in children and adolescents with attention-deficit/hyperactivity disorderJ Am Acad Child Adolesc Psych4550311
- GreshamFMElliotSN1990Social Skills Rating System Manual Circle PinesMinnesotaAmerican Guidance Service
- JasinskiDR2000An evaluation of the abuse potential of modafinil using methylphenidate as a referenceJ Psychopharmacol14536010757254
- JasinskiDRLovacevic-RistanovicR2000Evaluation of the abuse liability of modafinil and other drugs for excessive daytime sleepiness associated with narcolepsyClin Neuropharmacol231495610895398
- KingSGriffinSHodgesZ2006A systematic review and economic model of the effectiveness and cost-effectiveness of methylphenidate, dexamfetamine and atomoxetine for the treatment of attention deficit hyperactivity disorder in children and adolescentsHealth Technol Assess101162
- KucsmarskiRJOgdenCLGrummer-StrawnLM2000CDC Growth Charts: United StatesAdv Data31412711183293
- LinJSHouYJouvetM1996Potential brain neuronal targets for amphetamine-, methylphenidate-, and modafinil-induced wakefulness, evidenced by c-fos immunocytochemistry in the catProc Natl Acad Sci USA9314128338943072
- LinJSRousselBAkaokaH1992Role of catecholamines in the modafinil and amphetamine induced wakefulness, a comparative pharmacological study in the catBrain Res591319261359924
- MignotENishinoSGuillemunaultC1994Modafinil binds to the dopamine uptake carrier site with low affinitySleep1743677991954
- MyrickHMalcolmRTaylorB2004Modafinil:preclinical, clinical, and post-marketing surveillance—a review of abuse liability issuesAnn Clin Psychiatry16101915328903
- RuginoTACopleyTC2001Effects of modafinil in children with attention-deficit/hyperactivity disorder:an open-label studyJ Am Acad Child Adolesc Psych40230235
- RuginoTASamsockTC2003Modafinil in children with ADHD:a double blind, placebo controlled randomized studyPediatr Neurol291364214580657
- RushCRKellyTHHaysLR2002aAcute behavioral and physiological effects of modafinil in drug abusersBehav Pharmacol1210515
- RushCRKellyTHHaysLR2002bDiscriminative-stimulus effects of modafinil in cocaine-trained humansDrug Alcohol Depend673112212127202
- ScammellTEEstabrookeIVMcCarthyMT2000Hypothalamic arousal regions are activated during modafinil-induced wakefulnessJ Neurosci208620811069971
- ShafferDFisherPLucasCP2000NIMH Diagnostic Interview Schedule for Children version IV (NIMH DISC-IV):description, differences from previous versions, and reliability of some common diagnosesJ Am Acad Child Adolesc Psych392838
- SheltonJNishinoSVaughtJ1995Comparative effects of modafinil and amphetamine on daytime sleepiness and cataplexy of narcoleptic dogsSleep18817268746387
- SimonPHemetCRamassamyC1995Non-amephtaminic mechanism of stimulant locomotor effect of modafinil in miceEur Neuropsychopharmacol5509148998404
- SpenceSAGreenRDWilkinsonID2005Modafinil modulates anterior cingulate function in chronic schizophreniaBr J Psychiatry187556115994572
- SwansonJMCastellanosFXMuriasM1998Cognitive neuroscience of attention deficit hyperactivity disorder and hyperkinetic disorderCurr Opin Neurobiol8263719635212
- SwansonJMGreenhillLLLopezFA2006Modafinil film-coated tablets in children and adolescents with attention-deficit/hyperactivity disorder:results of a randomized, double-blind, placebo-controlled, fixed-dose study followed by abrupt discontinuationJ Clin Psychiatry671374716426100