Abstract
Pharmacotherapies for schizophrenic and cocaine psychoses are complex but similar because of similarities in their brain neurochemistry and behavioral outcomes. Their neurochemical neuronal mechanisms of action, as shown in preclinical and clinical studies, involve primarily dopaminergic dysfunction and, secondarily, neuroadaptive effects that seem to involve central serotonergic function. Behavioral outcomes of both disorders include hyperactivity and antipsychotic medications can ameliorate psychotic symptoms. Patients with both disorders often arrive at emergency departments and present floridly psychotic with a predominance of positive symptoms, often prompting physicians to select a typical antipsychotic medication such as haloperidol. While this has become conventional wisdom, we believe that to use an atypical antipsychotic medication, such as risperidone, in the treatment of both psychoses is quite rational for long-term management of both positive and negative symptoms. Also, controlled clinical studies have shown that risperidone, an atypical antipsychotic medication, is successful in the treatment of cocaine dependence and withdrawal (CitationSmelson et al 1997, Citation2002; CitationGrabowski et al 2000). Furthermore, the availability and effectiveness of long-acting risperidone in injectable form opens new possibilities for the long-term management of both disorders. In this paper, we present data which show that the use of risperidone is plausible for effective pharmacotherapy of schizophrenic and cocaine psychoses.
Introduction
The treatment of schizophrenic psychosis demands complex solutions encompassing the multiplicity of variables associated with its onset, psychoneurobiology, course, and prognosis. The same can be said of cocaine psychosis. The following are clinical observations that underscore the relationship between schizophrenic and cocaine psychoses: (1) psychostimulants can produce psychotic syndromes in normal subjects; (2) psychostimulants at doses which would not be psychotogenic in normal subjects, exacerbate psychotic symptoms in a majority of schizophrenic patients; (3) stress precipitates psychotic behavior (CitationYui et al 1999); and (4) psychostimulants have been shown to produce positive psychotic symptoms in neuroleptic-naïve schizophrenic patients (CitationHietala et al 1995).
Indeed, similarities between and co-morbidity of schizophrenic and cocaine psychoses appear over and again in the clinical literature over the last two decades (CitationBrady et al 1991; CitationSerper et al 1999; CitationHarris and Batki 2000; CitationGreen et al 2004; CitationCubells et al 2005; CitationMauri et al 2006). For example, CitationCubells and colleagues (2005) have reported that cocaine induces a psychotic syndrome, which is transient, delusional and hallucinatory and covaries with the severity of cocaine-induced psychotic paranoia. CitationSerper and colleagues (1999) have shown that behavior in patients with cocaine intoxication and then acute abstinence mimics the positive and negative symptoms of schizophrenia. Clinical data show that about 50% of the patients who suffer from schizophrenia have also been substance abusers at some time during their illness and that schizophrenic patients often feel the need to alleviate psychosis by using reinforcing drugs (cf. CitationBuckley 1998). Moreover, cocaine-abusing patients are more likely to be diagnosed with schizophrenia of the paranoid subtype and to exhibit more negative symptoms in the schizophrenic psychotic syndrome than do non-substance abusing patients (CitationLysaker et al 1994). In addition, CitationGreen and colleagues (1999) have provided additional data that show that the atypical antipsychotic medication, clozapine, is beneficial for patients with comorbid substance use and schizophrenia.
Particularly relevant to the relationship between schizophrenic and cocaine-induced psychosis are biochemical data which show that both disorders are remarkably similar in their neurochemical neuronal mechanism of action. Pre-clinical and clinical studies show that both disorders involve primary dopaminergic dysfunction and that secondary neuroadaptive effects seem to involve central serotonergic function. Furthermore, concomitant behavioral outcomes for both disorders include hyperactivity (CitationBroderick and Phelix 1997; CitationAngelopoulos et al 2002; CitationCarlsson and Carlsson 2006).
Solutions for these related psychoses must address as many facets of these disorders as necessary, and at least include psychopharmacotherapy and appropriate psychotherapies. In this paper, we address psychopharmacotherapy, particularly research relating to the neuroscience and therapeutics of antipsychotic agents and their applications in schizophrenia and cocaine psychosis and abuse. Further, we highlight salient points of this research together with mechanisms and hypotheses published by other researchers, and present illustrative clinical cases.
Schizophrenia: an overview
Schizophrenia, a chronic and debilitating disorder that usually manifests in late adolescence and young adulthood, is the prototypical psychosis, a term that refers to behavior, ie, symptoms and signs, that breaks from consensual reality. Schizophrenia impairs several areas of brain functioning, but especially 1) cognition (form and content of thought, attention and concentration, insight, and judgment), 2) perception, 3) emotions, and 4) behavior (CitationAndreasen 1987). It is a major medical problem by any measure, present in all cultures, and has been described in writings going back to the 12th century BC. With lifetime prevalence in the United States ranging from 1% to 2%, schizophrenia accounts for over 2.5% of all medical expenditures, or about 50 billion dollars a year. Patients with schizophrenia occupy about 50% of all mental hospital beds and comprise about 16% of all psychiatric patients who receive treatment, two thirds of whom need hospitalization. Yet only about half of all schizophrenics receive treatment regardless of illness severity. Schizophrenia often has serious individual and social consequences and its emotional cost to patients and loved ones is immeasurable.
Although etiological factors underlying the disorder have not yet been fully elucidated, we accept the understanding that predominantly biological factors, including genetic predisposition, transact with adverse environmental, social, and psychological factors to create, precipitate, and perpetuate the disorder. Findings such as neuroimaging evidence of mesocorticolimbic decreased prefrontal cortex activity and the precipitating and perpetuating power of expressed emotions, stressful life events, and social downdrift corroborate this view (CitationVerhoeff et al 2000). Furthermore, the well-known dopamine hypothesis of schizophrenia, dopaminergic hyperfunction in mesolimbic areas of the brain (CitationStahl 2000), is now supported by direct evidence from single photon emission computed tomography (SPECT), wherein the unchallenged release of dopamine is elevated in schizophrenic patients compared with controls (Abi-Dargham et al 2000). Dopamine D2 receptor occupancy is critical to dose and to differences between typical and atypical antipsychotic medication (Naikar et al 2006). Finally, this hyperdopaminergic function in schizophrenia may be accompanied by decreased glutamate function (CitationKegeles et al 2000).
Arvid Carlsson’s group in Sweden has been conducting pioneering research in schizophrenia, developing antipsychotic compounds called “dopamine stabilizers” (stabilizers), which are capable of ameliorating schizophrenia symptoms without producing side effects (CitationRung et al 2005; Nilsson et al 2006). These dopamine stabilizers preferentially target extrasynaptic receptors while leaving synaptic transmission and basic dopamine function intact. CitationOhara (2007) has reported a further addition to the dopamine hypothesis of schizophrenia by showing that an n-3 fatty acid deficiency can lead to reduced dopamine concentration, number of dopamine vesicles and dopamine (D2) receptors at prefrontal presynaptic terminals.
Serotonin, a secondary neuroadaptive mechanism in schizophrenia, (CitationAngelopoulos et al 2002) balances dopamine in mesolimbic and mesocorticolimbic structures in the brain. This dopamine-serotonin balance provides the leading hypothesis for the mechanism of action of atypical antipsychotic medications.
Advanced paternal age has been demonstrated to be more prevalent in the history of persons with schizophrenia than in that of unaffected persons, which CitationMalaspina and colleagues (2002) suggest, owes to the possibility of de novo mutations in paternal germ cells. Age of onset is earlier for men compared to women even though both sexes end up being equally affected. Prenatal viral infections and exposure to certain drugs such as diuretics (CitationSorensen et al 2003) have also been implicated in the etiology of schizophrenia. The finding that more people with the disorder are born in the cold months (CitationSmits et al 2004) suggests that viral infection, which can affect fetal brain development, particularly during the 2nd gestational trimester, may be another causative factor. Unknown developmental factors which may cause schizophrenia in later years may be due to increased dopaminergic tone as opposed to hyperdopaminergic function (CitationCarlsson and Carlsson 2006).
Historically, CitationBleuler (1911) referred to the schizophrenic disorder in the plural, perhaps already glimpsing its heterogeneity and probable etiological multiplicity. While the uncertainties remain, today we speak of schizophrenia as a brain disorder, and we understand it better as a psychotic syndrome whose signs and symptoms we treat. Thus, its distinguishing features are grouped into two categories: positive symptoms, an exaggeration of normal functioning, and negative symptoms, a deficit in normal functioning. Thinking that is disordered both in form and content, conveyed by disordered speech, and hallucinations that are primarily auditory count among the positive symptoms. CitationAndreasen (1982) listed the following negative symptoms: Affective flattening, alogia, avolition, anhedonia, and attentional deficits. Although positive symptoms are often florid, dramatic, and demand immediate attention, while negative symptoms are insidious and low key, both categories can seriously disrupt the person’s life, markedly diminishing its quality. Negative symptoms have always been more difficult to treat, often understood as intractable proof of social downdrift.
Pharmacology of antipsychotic medications
Typical and atypical antipsychotic medications differ in side effect profile because they also differ significantly in mechanism and site of action of therapeutic effect. Regarding side effect profile, one ought to bear in mind that the medication must be effective for its intended purpose and that its benefits must outweigh its risks. Typical antipsychotics such as haloperidol that readily resolve positive symptoms, are seemingly ineffective in the treatment of negative symptoms of psychosis (CitationCarpenter et al 1988). The advent of the atypical antipsychotics brought the reversal of both symptom categories within range (CitationMeltzer 1992; CitationConley and Mahmoud 2001). This effectiveness led to important adjustments in our understanding of the mechanisms of action of both drug categories. Typicals control positive symptoms through their action as dopamine antagonists presumably through high occupancy of dopamine D2 receptors in the nigrostriatum (CitationFarde et al 1988; CitationMukherjee et al 2001), an action that can also produce anhedonia (CitationBlum et al 1989) and extrapyramidal side effects (EPS). Typicals have little effect on serotonergic mechanisms (CitationBroderick and Piercey 1998; CitationIchikawa et al 1998). Conversely, atypicals act primarily but not exclusively, on serotonin2/dopamine2 receptors in mesocorticolimbic neurons to reduce positive and negative symptoms, with little risk of EPS, likely due to serotonergic modulation of dopamine (CitationMeltzer and Nash 1991). The serotonergic function of atypical antipsychotic medications may also account for their effect of improving anhedonia and affective disorders, as CitationMeltzer (1989) reported.
Pharmacological behavioral studies in animal models also provide a means of demonstrating differences between typical and atypical antipsychotics. Typicals inhibit hyperactivity and stereotypy induced by administration of dopaminergic drugs. They also produce catalepsy in similar dose ranges. Under similar circumstances, atypicals selectively inhibit hyperactivity without inducing catalepsy (CitationWeiner et al 2000; CitationWadenberg et al 2001). In animal models, perospirone, a model serotonin2/dopamine2 receptor antagonist, contrary to typicals, showed preferential ability to induce Fos protein expression in the mesolimbic nucleus accumbens versus nigrostriatal dorsolateral terminal (CitationIshibashi et al 1999).
Schizophrenia: a clinical case
Anthony came to the psychiatrist’s office referred by his primary care physician. He came accompanied by his mother and appeared inordinately passive and dependent on her, which appeared incongruous for an 18-year-old male. The mother told the story; he often nodded in agreement.
He had gone to an Army boot camp in Virginia, just days after his 18th birthday, 8 months before the doctor visit. A month before the visit, he called the mother from the Port Authority Bus Terminal in Midtown Manhattan, New York City, and asked her to come pick him up. They lived an easy half-hour subway ride away, in Queens, another New York City borough. He had been used to traveling around the entire city and knew it “like the back of his hand,” so the mother was alarmed when he claimed he did not know how to get home or what to do. To the mother, Anthony sounded like a broken man and she was “shocked and worried sick.” She found him sitting on an overstuffed duffel bag looking lost, eyes gazing into nothingness. Expressionless, he stood up and remained halfway between limp and stiff as she hugged him and cried. He asked her not to cry, saying he was fine. When asked why he had returned, he said he could not talk about it because he was under surveillance. He claimed his thoughts and his every move were being monitored and that he was in grave danger. He asserted that he needed to refrain from speaking. He showed her, in a bizarrely secretive way, what amounted to his discharge papers from a psychiatric ward in a hospital close to the base, where he had been kept as an inpatient for most of the previous month. He whispered he was going to have a dishonorable discharge from the Army. Later he told his mother he had punched an officer who forced him to do night duty in “a clear effort” to get him killed. He “defended” himself to avoid getting killed, prompted by voices he heard running a commentary about him, saying he would be a wimp not to fight. He was urgently hospitalized. He told his mother of a conspiracy against him involving the base and hospital personnel who gave him drugs that made him sick.
Now in the psychiatrist’s office, he was calm, without suicidal, violent, or homicidal ideas, intent, or plans, but still feared for his life. Anthony had no prior acute or chronic illnesses or hospitalizations. There was no history of trauma or injury. He denied alcohol or drug abuse, but acknowledged, as did his mother, that for the first three months of the year prior to going to boot camp, he had joined the “wrong crowd” and used a “lot of weed.” He had no problems with the law. He failed the first marking period in school that year, something very uncharacteristic since he was used to being an A student. With mother’s approval he had decided to join the Army to get away.
Anthony was born to term in a Spanish-speaking South American country. His parents separated soon thereafter and his mother relocated to New York where another family member had settled. Anthony developed without trouble, as his mother struggled to establish herself, and married again a couple of years later to a man who was kind to Anthony. Anthony traveled to South America yearly to spend time with his father who died soon before his trouble in school began. His father was “an alcoholic” who had hospitalizations for medical, surgical, and psychiatric problems as he got older. The mother could recall no history of psychiatric disorders in her side of the family.
A physical exam and laboratory tests yielded no pathological findings. Anthony did not use prescribed or over-the-counter medicines. Alcohol blood level and urine toxicology for all drugs of abuse were negative.
Understanding and treating Anthony
Anthony’s history is, in most respects, a clear case of schizophrenia. Some of his behavioral difficulties (eg, lack of initiative, inability to plan) suggest diminished functioning of the prefrontal cortex. He is alert, fully oriented, and has average intelligence and intact memory in all spheres. However, his thinking, insight, judgment, emotions, perception, and behavior are seriously impaired. Anthony used a “lot of weed” for three months, about one year prior to the clear onset of symptoms. Though no clear causative relationship has been established – and none is suggested here – cannabis use has been correlated with earlier age of onset of schizophrenia, and has been suggested to play a role in its development (CitationBersani et al 2002), the illness often coursing with negative symptoms. Anthony’s treatment regimen consisted of atypical antipsychotic medication to address both his positive and significant negative symptoms and supportive psychotherapy to stabilize him emotionally, and to help him adhere to the treatment regimen, and attend to important matters such as family life, finances, and obtaining and maintaining a job.
In most cases, the administration of atypical antipsychotics in proper doses, should be the centerpiece of the standard for long term care of persons with schizophrenia, a rationale applied to the case above. In fact, in a 50 week, open-label trial, long-acting risperidone in injectable form was effective in ameliorating schizophrenic symptoms (CitationDocherty et al 2007). Also, the use of atypical antipsychotics has been demonstrated to reinforce participation in long-term psychosocial counseling, corresponding with greater efficacy of overall treatment and improved quality-of-life (CitationRosenheck et al 1998). In spite of this, one ought not to discount the potentially serious side effects of atypicals, with which one must become familiar so as to choose the most adequate among drugs of the class for each specific case. It is important to note that risperidone acts like an atypical in lower doses but more like a typical at the upper end of the prescribing range (CitationWilliams 2001).
Cocaine psychosis: an overview
Among the substances of abuse, cocaine is second only to alcohol in number of emergency room visits, hospital admissions, and generation of social problems, including family violence. The lure for its use is intense euphoria and increased sexual desire and performance, both transient. The down side is intense post-cocaine dysphoria, and compulsion for further use.
Cocaine intoxication often courses with anorexia, insomnia, anxiety, motor hyperactivity, and “speeded” thinking and speech. There is increased adrenergic tonus, manifested by diaphoresis, dilated but reactive pupils, hyperreflexia, and tachycardia. Stereotypical movements of face, mouth, and extremities and even grand mal seizures may be present. Local damage inflicted by cocaine depends on the route of administration and includes rhinitis, when snorted, and bronchitis, when inhaled as free-base. The situation may escalate to hypertensive crises, hyperpyrexia, stroke, myocardial infarction, situations that may require heroic emergency room measures aimed at preventing death. Cocaine psychosis is another consequence of cocaine abuse also commonly seen, especially in emergency rooms (CitationSatel and Edell 1991; CitationMendoza et al 1992; CitationTaylor and Staby 1992; CitationTueth 1993; CitationSchwarz et al 1998).
A person with the above presentation may not furnish a reliable history. Thus, before laboratory confirmation is at hand, it is useful to bear in mind that cocaine- and amphetamine-induced psychoses may be clinically indistinguishable and their differential diagnosis with schizophrenia, difficult. Cocaine use becomes a chronic pattern of a few days’ heavy binge followed by a “crash.” The person is often anhedonic, irritable, anxious, and has low-key mood. Since it is associated with heavy use, cocaine psychosis is considered an episodic event.
Preclinical research has shown that cocaine acts in presynaptic nigrostriatal and mesolimbic dopamine pathways, by both blocking transporter reuptake and enhancing release mechanisms, thus increasing neurotransmission (Citationde Wit and Wise 1977; CitationChurch et al 1987; CitationRitz et al 1987; CitationBradberry and Roth 1989; CitationHurd and Ungerstedt 1989; CitationKalivas and Duffy 1990; CitationBroderick 1991a, Citation1991b, Citation1992a, Citation1992b; CitationBroderick et al 1993). It is thought that increased neurotransmission in mesolimbic and mesocorticolimbic dopamine reward pathways emanates from the ventral tegmental area (CitationRoberts and Koob 1982; CitationGoeders and Smith 1983; CitationEvenden and Ryan 1988; CitationEinhorn et al 1988; CitationKalivas 1993; CitationBroderick and Phelix 1997).
In addition to its established effects on dopamine levels, cocaine has been demonstrated to stimulate increased serotonin release in the nucleus accumbens (CitationBroderick et al 1993; CitationBradberry et al 1993). In fact, serotonin has been implicated in cocaine’s electrophysiological, transporter, behavioral, and reinforcing effects (CitationCunningham and Lakoski, 1988; CitationBroderick 1991b, Citation1992a, Citation1992b, Citation2002; CitationCarroll et al 1993; CitationHall et al 2002). Mesolimbic and nigrostriatal serotonin release increased rhythmic movement during animal natural exploration whereas cocaine disrupted this rhythmic balance (CitationBroderick 2002).
We have focused on serotonin-dopamine interactions to explain cocaine’s neurochemical and behavioral properties. The literature shows that cocaine increases dopamine probably through serotonin 2C receptor action that is postsynaptically mediated. Also, adjunct mechanisms, such as feedback compensatory mechanisms from the ventral tegmental area provide additional dopamine release presynaptically by using serotonin 2A (CitationFilip and Cunningham 2002).
The evidence heavily favors the involvement of dopamine-serotonin interactions in the mechanism of action of cocaine: 1) Immunohistochemical studies (CitationSteinbusch 1981) and immunocytochemical studies (CitationBroderick and Phelix 1997). The latter study shows that ventral tegmental dopamine cell bodies contain a dense network of serotonin axonal varicosities. 2) CitationPhelix and Broderick (1995) showed extensive overlap of dopamine and serotonin axons in core and shell, through neuroanatomic localization of tyrosine hydroxylase- and serotonin-containing axons in nucleus accumbens. 3) CitationHerve and colleagues (1987) have shown that serotonin neurons innervate dopamine neurons synaptically, through light- and electron microscopy-derived ultra structural evidence. 4) CitationVan Bockstaele and Pickel (1993), CitationVan Bockstaele and colleagues (1994), CitationBroderick and Phelix (1997) have produced cellular evidence for serotonergic excitation of dopamine neurons. Thus, serotonergic dopamine modulation plays a key role in shaping the neurochemical profile of cocaine use, and is additionally an efficacious target for atypical antipsychotic therapy. Indeed, critical evidence from the Broderick research laboratory has recently shown that a biochemically deficient animal, deficient in dopamine and serotonin in nucleus accumbens, is unable to react to the administration of the psychostimulant cocaine (CitationBroderick and Hope 2006).
Cocaine psychosis: a clinical case
MA, a 29-year-old male came into the ER on a Tuesday at 3:00 am, brought by the Emergency Medical Service ambulance, after calling 911, himself. He complained of hearing voices telling him to jump in front of the subway. Afraid he might do it, he called the ambulance. He had been “binging” with friends “snorting cocaine, and smoking crack” from the previous Friday after work until late Monday evening, missing work in the process. He felt “paranoid” that evening around 10:00 pm. He was sure, then, he had been followed all day long by a Mafia gangster intent on killing him. He tried to escape from the gangster but saw him, through the corner of his eye, hide in his girlfriend’s apartment building as MA arrived hoping to be allowed to “crash” in his girlfriend’s apartment. MA rang the bell, but glancing at him from the inside, the girlfriend did not open the door. He immediately “understood” she had betrayed him with the gangster. He went to his parents’ home next but was turned away. By then he was thoroughly convinced that there was a wide plot against him. Voices in his head started commanding him to kill himself. MA roamed the streets where he got into a “couple” of shouting matches over “nonsense” with fellow street dwellers. He next went to a friend’s house and tried to sleep. Again the voices shouted at him insistently and, fearful he would end up obeying them and killing himself, he called 911. During his ER evaluation, MA had a wild appearance. He was disheveled and dirty, in a very poor state of hygiene. Although constitutionally thin, he looked like he had lost some weight and appeared dehydrated. He looked and behaved in a suspicious manner and his attitude toward the examiner was guarded, somewhat hostile, and marginally cooperative. He was also “scared” and expressed that feeling as pervasive. His affect was constricted but appropriate to the content of his thinking. He was alert, fully oriented and his memory was intact. He was distracted, his speech was rapid, and he was delusional. His insight was poor and his judgment uneven. He was treated in the ER with intra-muscular combination of haloperidol (a typical antipsychotic), lorazepam (an anti-anxiety benzodiazepine), and diphenhydramine (antihistamine with sedative effects). He slept for many hours, but upon awakening, MA was still psychotic, which prompted psychiatric hospitalization. On the ward, he was switched to an atypical antipsychotic.
MA has a long history of cocaine-related incidents including ER visits, brushes with the law, rough-handling of his girlfriend, and one instance in which he pushed and shoved his parents, causing injuries that were not life threatening, but necessitated medical attention. His girlfriend broke up with him two months later, when she saw his cocaine use was again escalating after a hiatus; he snorted cocaine occasionally during this hiatus. His parents have closed their doors to him for the same reason as did his girlfriend. He recently lost his apartment because he used rent money to feed his habit. He shuttled for shelter among cocaine-abusing friends’ apartments. Exacerbating this chaos in his life, he is on the verge of losing his job due to erratic attendance patterns.
Understanding and treating MA
As can be seen in MA’s case, cocaine-induced psychosis (CitationBrady et al 1991) is the most severe psychiatric consequence of abuse. It often courses with persecutory delusions, auditory, visual, and tactile hallucinations, the latter, of tiny insects crawling on one’s skin, called formication. CitationRosse and colleagues (1994) likened cocaine-induced “paranoia” to that of schizophrenia. In fact, SPECT studies show that cocaine-induced changes in blood flow are similar to those found in schizophrenic persons (CitationMiller et al 1992). Animal models of cocaine psychosis have long demonstrated that: 1) psychostimulant behavior depends on dopaminergic nigrostriatal neuronal pathways (CitationCools and van Rossum 1970; CitationCostall and Naylor 1973; CitationWise and Bozarth 1987; CitationBroderick 2002); 2) dopamine antagonists block psychostimulant behavior (CitationPijnenburg et al 1975).
Psychostimulant-induced neurochemistry and behavior have become an accepted animal model of certain aspects of psychoses. This is supported by these findings: 1) Typical antipsychotics, known to block mesolimbic and nigrostriatal dopamine, reduce psychotic symptoms in humans via mesolimbic pathways, but produce movement disorders through nigrostriatal circuits (CitationGawin and Kleber 1986; CitationKleber and Gawin 1986). 2) Atypical antipsychotic agents, known to act on mesolimbic/mesocorticolimbic dopaminergic neuronal pathways (CitationHuff and Adams 1980), reduce both positive and negative psychotic symptoms in humans (CitationMeltzer 1989).
We have noted earlier that atypicals are quite effective in the management of schizophrenia. In fact, the use of risperidone also has shown promise in cocaine abusing schizophrenic persons (CitationTsuang et al 2002); in controlling craving for cocaine (CitationSmelson et al 2002); on cocaine-induced euphoria (CitationNewton et al 2001), on cocaine dependence (CitationGrabowski et al 2000); and on cue-elicited craving for cocaine (CitationSmelson et al 1997). CitationBroderick and colleagues (2003) have studied the effects of risperidone on cocaine in the psychostimulant animal model of psychosis and we have proceeded further with new neuromolecular imaging (NMI) research in this area as shown in this paper in –. NMI, with nationally and internationally patented, miniature BRODERICK PROBE® sensors and real time electrochemical detection, is cutting-edge research. NMI is conducted concomitantly and simultaneously with infrared detection of locomotor (ambulatory) behavioral measurements. This research has led to the following conclusions: 1) Cocaine produced withdrawal symptoms in subacute studies, probably due to neuroadaptive mechanisms, especially in dopamine and serotonin release in nucleus accumbens. 2) Risperidone acutely blocked cocaine-enhanced neurochemistry and behavior, and subacutely improved cocaine’s withdrawal effects on accumbens neurochemistry. 3) Risperidone thus may be a viable psychopharmacological tool in the treatment of cocaine addiction, withdrawal, and psychosis.
Figure 1A Day 1 The effect of cocaine (10 mg/kg i.p.) on adult, male Sprague-Dawley laboratory rats (n = 4). Studies were done with neuromolecular imaging (NMI) based on in vivo electrochemistry. The imaging was performed with the BRODERICK PROBE®; sensors. Sensors were implanted in NAcc and verified by the blue dot perfusion method. DA and 5-HT were detected selectively in the freely moving animal (concurrent behavioral data are presented below). Cocaine increased DA release in NAcc up to 75% over baseline (unpaired t-test, p < 0.0001 compared with preadministration), and increased 5-HT by 190% over baseline (unpaired t-test p < 0.0001 compared with baseline).
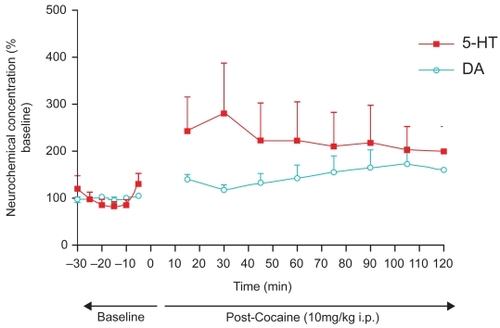
Figure 1B Day 2 Withdrawal effects after a single injection of cocaine (10 mg/kg i.p.) in adult, male Sprague-Dawley laboratory rats (n = 4) measured one day after prior administration. Studies were performed with the same paradigm as described in . Withdrawal effects were as follows: DA was significantly decreased from baseline (unpaired t-test p < 0.0001) and 5-HT was higher than at baseline only at the first point likely due to “novelty chamber effects” (unpaired t-test p < 0.05). Moreover, both DA and 5-HT were significantly lower than their Day-1 post-cocaine administration levels (unpaired t-test p < 0.0001).
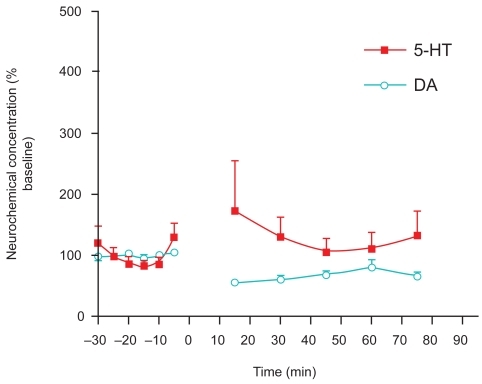
Figure 2A Day 1 The effect of co-administration of risperidone (2mg/kg s.c.) and cocaine (10 mg/kg i.p.) on adult, male Sprague-Dawley laboratory rats (n = 4). 5-HT release in NAcc after administration of risperidone and cocaine combination, did not significantly differ from baseline values (p = 0.415, unpaired t-test). As evidenced by Naiker, et al, 2mg/kg risperidone in the male laboratory rat is equivalent to a low-dose of single risperidone treatment in human psychotic patients. Furthermore, 5-HT release was found to be significantly lower when risperidone was administered with cocaine compared with cocaine alone (unpaired t-test, p < 0.0001). DA release was significantly different from its baseline upon co-administration (unpaired t-test, p < 0.05). Importantly, effects of risperidone and cocaine on DA release in NAcc was significantly decreased (p < 0.0001) from cocaine effects on DA release when cocaine was given alone.
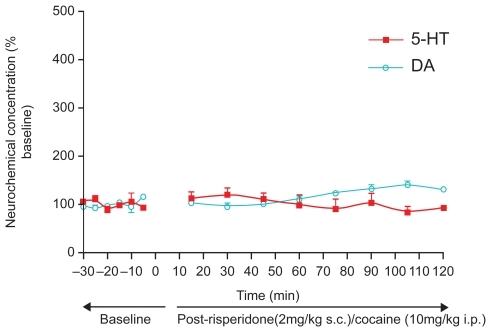
Figure 2B Day 2 Effects after a single co-administration of risperidone (2 mg/kg s.c.) and cocaine (10 mg/kg i.p.) to adult, male Sprague-Dawley laboratory rats (n = 3). Absence of typical withdrawal effects is evident relative to cocaine, as follows: risperidone-cocaine did not differ significantly from baseline on the second day (unpaired t-test, p = 0.3285 for 5-HT and p = 0.4433 for DA). Additionally, there was no significant difference between risperidone-cocaine on the first day and on the second day (p = 0.2994 for 5-HT and p = 0.0514 for DA). Thus, the data suggest that risperidone may be effective in the treatment of cocaine psychosis both for its impact on negative symptoms and its alleviation of acute withdrawal effects from cocaine.
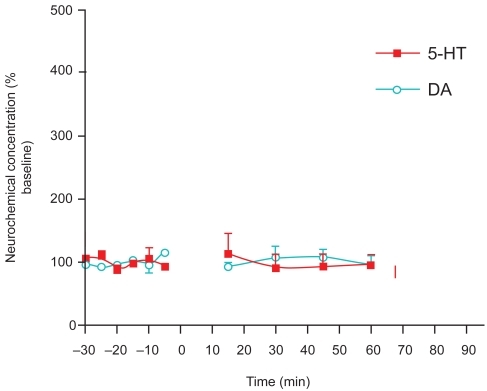
Figure 3A Day 1 The effect of cocaine (10 mg/kg i.p.) on adult male Sprague Dawley laboratory rats with respect to peripheral ambulations and the effect of co-administration of risperidone (2 mg/kg s.c.) and cocaine (10 mg/kg i.p.) are depicted. The effect of cocaine alone on ambulations post-administration was significant compared to baseline values (unpaired t-test p < 0.01). In combination with the atypical antipsychotic risperidone, ambulations are no longer significantly greater than their baseline values (unpaired t-test p = 0.1837). Therefore, risperidone has been demonstrated to block the behavioral ambulatory effects of cocaine.
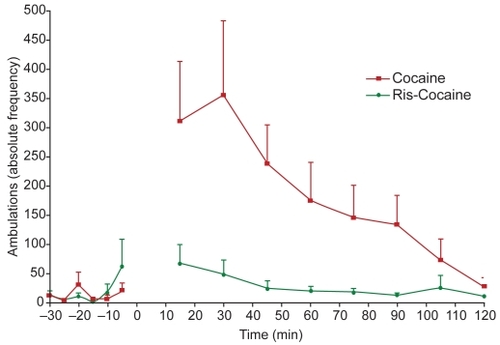
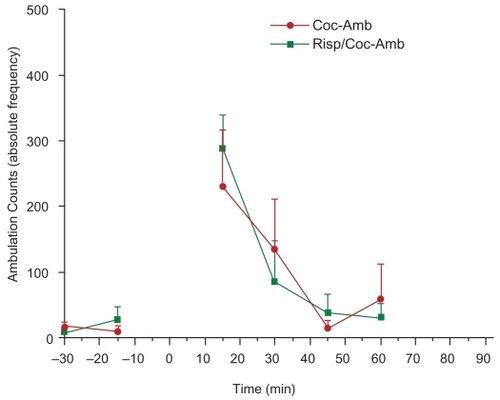
Figure 3B Day 2 Adult male Sprague-Dawley laboratory rats who received cocaine (10 mg/kg i.p.) and co-administered risperidone (2 mg/kg s.c.) and cocaine (10 mg/kg i.p.) were monitored one day after drug administration to detect possible withdrawal effects with respect to ambulations. Neither cocaine nor risperidone showed significant second-day effects over baseline (unpaired t-test, p = 0.2478 and p = 0.3605, respectively).
Closing comments on clinical management of schizophrenia and cocaine abuse
We have shown that this novel research points toward the usefulness of atypical antipsychotic agents in the treatment of schizophrenia and cocaine-related disorders. While the usefulness of atypical antipsychotic agents is well established in the treatment of schizophrenia, the potential usefulness of these agents remains very good in the treatment of cocaine-related disorders.
Again, because schizophrenia and cocaine abuse are multifaceted conditions, no simple solution exists for their clinical management. While reliance on established treatment guidelines and best practices is the optimal modus operandi, clinicians must assess patient presentation to institute the proper individually-tailored management strategy. We strongly advocate that clinicians add to their management strategies the most direct conclusions of research, such as that reported herein, whose mechanisms and hypotheses are sound. These novel translated research findings can add an important dimension to clinical protocol-building and provide up-to-the-minute potential solutions to difficult problems.
Acknowledgments
The authors gratefully acknowledge the support of the National Institutes of Health, NIH/NIGMS SCORE AWARD SO 6 GM 08168 as well as support from Professional Staff Congress/City University of New York (PSC/CUNY), RF 64282-00-33. Additional support from Pharmacia Upjohn (now part of Pfizer, Inc.), The MacKenzie Foundation and The Broderick Brain Foundation are also appreciatively acknowledged. Finally, the authors would also like to express their gratitude to (1) Vivek Murthy, (CUNY Medical School student) for excellence in preparing illustrations and (2) Bridget O’Sullivan, O.P., M.A. (Msgr. Scanlan High School) for excellent assistance with reference preparation.
References
- Abi-DarghamAGilRKrystalJ1998Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohortAm J Psychiat15576139619147
- AngelopoulosEKMarkianosMDaskalopoulouEG2002Changes in central serotonergic function as a correlate of duration of illness in paranoid schizophreniaPsychiatry Res11091712007589
- AndreasenNC1982Negative symptoms in schizophrenia. Definition and reliabilityArch Gen Psych397848
- AndreasenNC1987The diagnosis of schizophreniaSchizophr Bull139223496659
- BersaniGOrlandiVKotzalidisG2002Cannabis and Schizophrenia: impact on onset, course, psychopathology and outcomesEur Arch Psychiatry Clin Neurosci252869212111342
- BleulerE1911Dementia Praecox or Group of SchizophreniasGermany
- BlumKTrachtenbergMCKowzlowskiGP1989Cocaine therapy: the “reward. cascade” linkProf. CounselorJan–Feb32735
- BradberryCWNobilettiJBElsworthJD1993Cocaine and coca-ethylene: microdialysis comparison of brain drug levels and effects on dopamine and serotoninJ Neurochem601429358455033
- BradberryCWRothRH1989Cocaine increases extracellular dopamine in rat nucleus accumbens and ventral tegmental area as shown by in vivo microdialysisNeurosci Lett103971022779859
- BradyKTLydiardRBMalcolmR1991Cocaine-induced psychosisJ Clin Psychiatry5212509121752853
- BroderickPA1991aCocaine: on-line analysis of an accumbens amine neural basis for psychomotor behaviorPharmacol Biochem Behav40959681816582
- BroderickPA1991bIn vivo voltammetric studies on release mechanisms for cocaine with gamma-butyrolactonePharmacol Biochem Behav40969751816583
- BroderickPA1992aCocaine’s localized effects on synaptic serotonin and dopamine in ventral tegmentum in a reinforcement paradigmPharmacol Biochem Behav42889981513872
- BroderickPA1992bDistinguishing effects of cocaine I.V. and S.C. on mesoaccumbens dopamine and serotonin release with chloral hydrate anesthesiaPharmacol Biochem Behav433929371448488
- BroderickPAMassaroEJSchardienJLBroderickPA2002Microsensors detect cocaine neuroadaption: serotonin release within basal ganglia is not rhythmic with movementHandbook of NeurotoxicityTotowa, NJHumana Pr232367
- BroderickPAHopeO2006Monoamine and motor responses are codeficient in the Fawn-Hooded depressed animalProg Neuropsychopharmacol & Biol Psychiat3088798
- BroderickPAKornakEPEngF1993Real time detection of acute (IP) cocaine-enhanced dopamine and serotonin release in ventrolateral nucleus accumbens of the behaving Norway ratPharmacol Biochem Behav463715228278450
- BroderickPAPhelixCF1997I. Serotonin (5-HT) within dopamine reward circuits signals open-field behavior. II. Basis for 5-HT – dopamine interaction in cocaine dysfunctional behaviorNeurosci Behav Rev21322760
- BroderickPARahniDNZhouY2003Acute and subacute effects of risperidone and cocaine on accumbens dopamine and serotonin release using in vivo microvoltammetry on line with open-field behaviorProg Neuropsychopharmacol & Biol Psychiat276103754
- BroderickPAPierceyMF1998Clozapine, haloperidol, and the D4 antagonist PNU-101387G: in vivo effects on mesocortical, mesolimbic, and nigrostriatal dopamine and serotonin releaseJ Neural Transm1056–77497679826116
- BuckleyPF1998Substance abuse in schizophrenia: A reviewJ Clin Psychiat3SupplS2630
- CarlssonACarlssonML2006Dopaminergic deficient hypothesis of schizophrenia: the path to discoveryDialogues Clin Neurosci811374216640125
- CarpenterWTJr1998Deficit and nondeficit forms of schizophrenia: the conceptAm J Psychiatry1455578833358462
- CarrollFIGrayJLAbrahamP19933- Aryl-2-(3′-substituted-1′,2′,4′-oxadiazol-5′-yl) tropane analogues of cocaine: affinities at the cocaine binding site at the dopamine, serotonin and norepinephrine transportersJ Chem36288690
- ChurchWHJusticeJBJrByrdLD1987Extracellular dopamine in rat striatum following uptake inhibition by cocaine, nomifensine, and benztropineEur J Pharmacol139332548
- CubellsJFFeinnRPearsonD2005Rating the severity and character of transient cocaine-induced delusions and hallucinations with a new instrument, the Scale for Assessment of Positive Symptoms for Cocaine-Induced Psychosis (SAPS-CIP)Drug Alcohol Depend801233315894433
- ConleyRRMahmoudR2001A randomized double-blind study of risperidone and olanzapine in the treatment of schizophrenia or schizoaffective disorderAm J Psychiatry158576577411329400
- CoolsARvan RossumJM1970Caudal dopamine and stereotype behaviour of catsArch Int Pharmacodyn Ther1871163735480136
- CostallBNaylorRJ1973The role of the substantia nigra in the locomotor stimulant action of amphetamineBr J Pharmacol49129374787564
- CunninghamKALakoskiJM1988Electrophysiological affects of cocaine and procaine on dorsal raphe serotonin neuronsEur J Pharmacol148457623384007
- De WitHWiseRA1977Blockade of cocaine reinforcement in rats with the dopamine receptor blockade pimozide, but not with the noradrenergic blockers phentolamine or phenoxybenzamineCan J Psychol314195203608135
- DochertyJPBossieCALachauxB2007Patient-based and clinician-based support for the remission criteria in schizophreniaInt Clin Psychopharmacol2251517159460
- EinhornLCJohansenPAWhiteFJ1988Electrophysiological effects of cocaine in the mesoaccumbens dopamine system: studies in the ventral tegmental areaJ Neurosci811001123339402
- EvendenJLRyanCN1998Behavioral responses to psychomotor stimulant drugs: localization in the central nervous systemPharmacol Ther362–3151722894675
- FardeLWieselFAHallainC1988Central D2-dopamine receptor occupancy in schizophrenic patients treated with antipsychotic drugsArch Gen Psychiatry4517162892477
- FilipMCunninghamKA2002Serotonin 5-HT(2) receptors in nucleus accumbens regulate expression of the hyperlocomotive and discriminative stimulus effects of cocainePharmacol Biochem Behav7147455611888566
- GawinFKleberH1986Pharmacologic treatments of cocaine abusePsychiatr Clin North Am93573833534819
- GoedersNESmithJE1983Cortical dopaminergic involvement in cocaine reinforcementScience2214612773756879176
- GrabowskiJRhoadesHSilvermanP2000Risperidone for the treatment of cocaine dependence: randomized, double-blind trialJ Clin Psychopharmacol2033051010831016
- GreenAITohenMFHamerRM2004First episode schizophrenia-related psychosis and substance use disorders: acute response to clozapine and haloperidolSchizophr Res662–31253515061244
- GreenAIZimmetSVStrousRD1999Clozapine for comorbid substance use disorder and schizophrenia: do patients with schizophrenia have a reward-deficiency syndrome that can be ameliorated by clozapine?Harv Rev Psychiat6628796
- HallFSLiXFSoraI2002Cocaine mechanisms: enhanced cocaine, fluoxetine, and nisoxetine place preferences following monoamine transporter deletionsNeuroscience11511536112401330
- HarrisDBatkiSL2000Stimulant psychosis: symptom profile and acute clinical courseAm J Addict91283710914291
- HietalaJSyvalahtiEVuorioK1995Presynaptic dopamine function in striatum of neuroleptic-naïve schizophrenic patientsJ Lancet346113031
- HerveDPickelVMJohTH1987Serotonin axon terminals in the ventral tegmental area of the rat: fine structure and synaptic input to dopaminergic neuronsBrain Res43571832892580
- HuffRMAdamsRN1980Dopamine release in n. accumbens and striatum by clozapine: simultaneous monitoring by in vivo electrochemistryNeuropharmacology19587907402448
- HurdYLUngerstedtU1989In vivo neurochemical profile of dopamine uptake inhibitors and releasers in rat caudate-putamenEur J Pharmacol1662251602477259
- IchikawaJKurokiTDaiJ1998Effect of antipsychotic drugs on extracellular serotonin levels in rat medial prefrontal cortex and nucleus accumbensEur J Pharmacol3512163719686999
- IshibashiTTagashiraRNakamuraM1999Effects of perospirone, a novel 5-HT2 and D2 receptor antagonist, on Fos protein expression in the rat forebrainPharmacol Biochem Behav6345354110462181
- KalivasPW1993Neurotransmitter regulation of dopamine neurons in the ventral tegmental areaBrain Res Rev811751138096779
- KalivasPWDuffyP1990Effect of acute and daily neurotensin and enkephalin treatments on extracellular dopamine in the nucleus accumbensJ Neurosci109294091697899
- KegelesLSAbi-DarghamAZea-PonceY2000Modulation of amphetamine-induced striatal dopamine release by ketamine in humans: implications for schizophreniaBiol Psychiat486274011032974
- KleberHGawinF1986Psychopharmacological trials in cocaine abuse treatmentAm J Drug Alcohol Abuse12235463332559
- LysakerPBellMBeam-GouletJ1994Relationship of positive and negative symptoms to cocaine abuse in schizophreniaJ Nerv Ment Dis18221091128308528
- MauriMVolonteriLDe GaspariI2006Substance abuse in first-episode schizophrenic patients: a retrospective studyClin Prac Epidemol Ment Health24
- MalaspinaDBrownAGoetzD2002Schizophrenia risks and paternal age: a potential role for de novo mutations in schizophrenia vulnerability genesCNS Spectr7126915254446
- MeltzerH1989Serotonergic dysfunction in depressionBr J Psychiatry Suppl825312692637
- MeltzerHY1992Treatment of the neuroleptic-nonresponsive schizophrenic patientSchizophr Bull183515421357741
- MeltzerHYNashJF1991Effects of antipsychotics on serotonin receptorsPharmacol Rev4345876041685568
- MendozaRMillerBLMenaI1992Emergency room evaluation of cocaine-associated neuropsychiatric disordersRecent Dev Alcohol1073871589608
- MillerBLMenaIGiombettiR1992Neuropsychiatric effects of cocaine: SPECT measurementsJ Addict Dis11447581486093
- MukherjeeJChristianBTNarayananTK2001Evaluation of dopamine D2 receptor occupancy by clozapine, risperidone, and haloperidol in vivo in the rodent and nonhuman primate brain using 18F-fallyprideNeuropsychopharmacology2544768811557161
- NaikerDVCattsSVCattsVS2006Dose determination of haloperidol, risperidone and olanzapine using an in vivo dopamine D2-receptor occupancy method in the ratEur J Pharm5408790
- NewtonTFLingWKalechsteinAD2001Risperidone pre-treatment reduces the euphoric effects of experimentally administered cocainePsychiatry Res10232273311440773
- NilssonMCarlssonAMarkinhuhtaKR2004The dopaminergic stabiliser ACR 16 counteracts the behavioural primitivization induced by the NMDA receptor antagonist MK-801 in mice: implications for cognitionProg Neuropsychpharmacol & Biol Psychiat2867785
- OharaK2007The n-3 polyunsaturated fatty acid/dopamine hypothesis of schizophreniaProg Neuropsychopharmacol & Biol Psychiat31246974
- PhelixCFBroderickPA1995Light microscopic immunocytochemical evidence of converging serotonin and dopamine terminals in ventrolateral nucleus accumbensBrain Res Bull37137407606477
- PijnenburgAJJHonigWMMvan RossumJM1975Effects of antagonists upon locomotor stimulation induced by injection of dopamine and noradrenaline into the nucleus accumbens of nialamide-pretreated ratsPsychopharmacologia412175801153605
- RitzMCLambRJGoldbergSR1987Cocaine receptors on dopamine transporters are related to self-administration of cocaineScience23748191219232820058
- RobertsDCKoobGF1982Disruption of cocaine self-administration following 6-hydroxydopamine lesions of the ventral tegmental area in ratsPharmacol Biochem Behav17590146817350
- RosenheckRTekellJPetersJ1998Does participation in psychosocial treatment augment the benefit of clozapine?Arch Gen Psychiat557618259672052
- RosseRBCollinsJPJrFay-McCarthyM1994Phenomenologic comparison of the idiopathic psychosis of schizophrenia and drug-induced cocaine and phencyclidine psychoses: a retrospective studyClin Neuropharmacol17359699316684
- RungJPCarlssonAMarkinhuhtaKRBroderickPAGlazerWM2005The dopaminergic stabilisers (−)-OSU 6162 and ACR16 reverse (+)-MK-801-induced social withdrawal in ratsProg Neuropsychopharm & Biol Psychiat A Special Topics Issue2958339
- SatelSLEdellWS1991Cocaine-induced paranoia and psychosis pronenessAm J Psychiatry148121708111957934
- SchwarzJSchererJTrenkwalderC1998Reduced striatal dopaminergic innervation shown by IPT and SPECT in patients under neuroleptic treatment: need for levodopa therapy?Psychiatry Res8312389754702
- SerperMRChouJCAllenMH1999Symptomatic overlap of cocaine intoxication and acute schizophrenia at emergency presentationSchizophr Bull2523879410416739
- SmelsonDARoyARoyM1997Risperidone diminishes cue-elicited craving in withdrawn cocaine-dependent patientsCan J Psychiatry4299849429074
- SmelsonDALosonczyMFDavisCW2002Risperidone decreases craving and relapses in individuals with schizophrenia and cocaine dependenceJ Can Psychiatry4776715
- SmitsLPedersenCMortensonP2004Association between short birth intervals and schizophrenia in the offspringSchizophr Res701495615246463
- StahlSM2000Essential Psychopharmacology2nd EditionNew YorkCambridge Univ Pr
- SorensenHJMortensonELReinischJM2003Do hypertension and diuretic treatment in pregnancy increase the risk of schizophrenia in offspring?Am J Psychiatry1603464812611826
- SteinbuschHW1981Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminalsNeuroscience645576187017455
- TaylorWAStabyAE1992Acute treatment of alcohol and cocaine emergenciesRecent Dev Alcohol10179911589601
- TsuangJWEckmanTMarderS2002Can risperidone reduce cocaine use in substance abusing schizophrenic patients?J Clin Psychopharmacol2266293012454567
- TuethMJ1993High incidence of psychosis in cocaine intoxication and preventing violence in the EDAm J Emerg Med1166768240581
- Van BockstaeleEJCestariDMPickelVM1994Synaptic structure and connectivity of serotonin terminals in the ventral tegmental area: potential sites for modulation of mesolimbic dopamine neuronsBrain Res6472307227522922
- Van BockstaeleEJPickelVM1993Ultrastructure of serotonin-immunoreactive terminals in the core and shell of the rat nucleus accumbens: cellular substrates for interactions with catecholamine afferentsJ Comp Neurol3344603178408768
- VerhoeffNPMeyerJHKecojevicA2000A voxel-by-voxel analysis of [18F]setoperone PET data shows no substantial serotonin 5-HT2A receptor changes in schizophreniaPsychiatry Res9931233511068194
- WadenbergMLSollmanAVanderspekSC2001Dopamine D2 receptor occupancy is a common mechanism underlying animal models of antipsychotics and their clinical effectsNeuropsychopharmacology2556334111682246
- WeinerIGaislerISchillerD2000The latent inhibition model dissociates between clozapine, haloperidol, and ritanserinNeuropsychopharmacology2321516110882841
- WilliamsR2001Optimal dosing with risperidone: updated recommendationsClin Psychiatry622829
- WiseRABozarthMA1987A psychomotor stimulant theory of addictionPsychol Rev944469923317472
- YuiKGotoKIkemotoS1999Neurobiological basis of relapse prediction in stimulant-induced psychosis and schizophrenia: the role of sensitizationJ Mol Psychiat4651223