Abstract
Steroidal neuromuscular blocking agents (NMBAs), such as rocuronium, are widely used in clinical anesthesia and emergency medicine to facilitate endotracheal intubation and artificial ventilation and to allow surgical access to body cavities. Reversal of neuromuscular blockade is important for the acceleration of patient recovery and prevention of postoperative residual neuromuscular blockade and reduces the incidence of severe morbidity and mortality associated with anesthesia management. Sugammadex is the first selective relaxant binding agent (SRBA) and has been designed to reverse the steroidal neuromuscular blocking drug rocuronium. Encapsulation of the rocuronium molecule by sugammadex results in a rapid decrease in free rocuronium in the plasma and subsequently at the nicotinic receptor at the motor endplate. After encapsulation, rocuronium is not available to bind to the nicotinic receptor in the neuromuscular junction. This promotes the liberation of acetylcholine receptors, and muscle activity reappears. This new concept of reversal of neuromuscular block induced by rocuronium (or vecuronium) led to impressive results in animal and phase 1 and 2 studies. Sugammadex is currently in phase 3 clinical studies and may be commercially available by 2008.
Introduction
Steroidal neuromuscular blocking agents (NMBAs), such as rocuronium, are widely used in clinical anesthesia and emergency medicine to facilitate endotracheal intubation and artificial ventilation and to allow surgical access to body cavities (CitationHunter 1995). Although the use of NMBAs has significantly reduced the incidence of laryngopharyngeal lesions due to endotracheal intubation, their use is still associated with higher morbidity and mortality compared with anesthetic techniques that do not use NMBAs (CitationPedersen et al 1992; CitationShorten 1993; CitationMencke et al 2003). This is mainly attributable to the development of postoperative residual neuromuscular blockade, resulting in hypoventilation, airway obstruction, and hypoxia (CitationPedersen et al 1992; CitationShorten 1993). Reversal of neuromuscular blockade is important for the acceleration of patient recovery and prevention of postoperative residual neuromuscular blockade, and reduces the incidence of severe morbidity and mortality associated with anesthesia management (CitationArbous et al 2005).
At present, the reversal of neuromuscular blockade is achieved by the administration of cholinesterase inhibitors (neostigmine, edrophonium, or pyridostigmine) (CitationOsmer et al 1996). Importantly, cholinesterase inhibitors have a number of undesirable side-effects (bradycardia, bronchoconstriction, hypersalivation, abdominal cramps and nausea and vomiting) (CitationVan Vlymen and Parlow 1997) which can be counteracted by co-administration of muscarinic antagonists (atropine or glycopyrrolate) (CitationOsmer et al 1996; Citationvan Vlymen and Parlow 1997). However, muscarinic antagonists also have side-effects (blurred vision, dry mouth, and tachycardia) (Citationvan Vlymen and Parlow 1997; CitationZhang 2003). Furthermore, due to their mechanism of action, cholinesterase inhibitors are not capable of reversing deeper levels of neuromuscular blockade (CitationBooij et al 2002; CitationZhang 2003). Return of muscle paralysis after apparent reversal of neuromuscular block by cholinesterase inhibitors, recurarization, is another limitation of the currently used reversal strategy. Thus, there is clearly a clinical need for a new reversal agent, with minimal side-effects and the capability to reverse neuromuscular blockade effectively, independently of its depth.
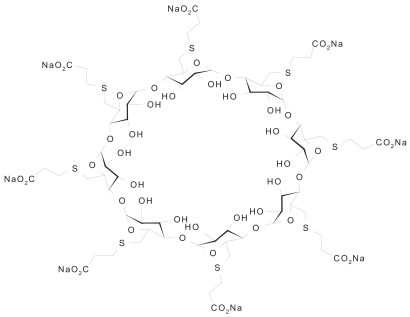
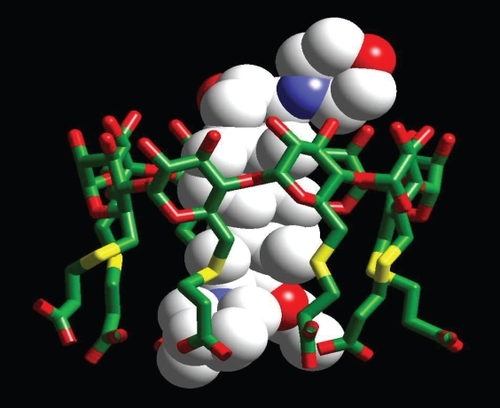
Sugammadex is the first selective relaxant binding agent (SRBA) and has been designed to reverse the steroidal neuromuscular blocking drug rocuronium (CitationBooij et al 2002; CitationBom et al 2002; Citationde Boer et al 2006a). Sugammadex, per-6-(2-carboxyethylthio)-per-6-deoxy-γ-cyclodextrin sodium salt (), is a synthetic γ-cyclodextrin (CD) derivative designed to bind selectively to the steroidal NMBA molecule (CitationBooij et al 2002; CitationBom et al 2002; Citationde Boer et al 2006a). Cyclodextrins, a group of cyclic oligosaccharides, are ring-shaped molecules with lipophilic inner cavities and hydrophilic outer surfaces that form complexes by inclusion of specific guest molecules, such as steroids (CitationBom et al 2002; CitationChalla et al 2005). Structurally, they consist of either 6 (α-CDs), 7 (β-CDs), or 8 (γ-CDs) glucose units and each type has its own characteristics (CitationChalla et al 2005). CDs are highly water soluble, particularly γ-CDs compared with α- and β-CDs and are also biologically well tolerated (CitationBom 2002). Chemically modified CDs have been used in the clinic to increase the stability, solubility, and bioavailability of an encapsulated drug, thereby delivering the required dose of the drug to the appropriate target sites (CitationBom et al 2002). The use of a modified CD to reverse a rocuronium-induced neuromuscular blockade, by removing rocuronium from the effector site, thus represents a paradigm shift from current methodology. Encapsulation of the rocuronium molecule by sugammadex results in a rapid decrease in free rocuronium in the plasma and subsequently at the nicotinic receptor at the motor endplate (). After encapsulation, rocuronium is not available to bind to the nicotinic receptor in the neuromuscular junction. This promotes the liberation of acetylcholine receptors, and muscle activity reappears (CitationBom et al 2002; CitationEpemolu et al 2003). This new concept of reversal of neuromuscular block induced by rocuronium (or vecuronium) led to impressive results in animal and phase 1 and 2 studies. Sugammadex is currently in phase 3 clinical studies and may be commercially available by 2008.
Animal studies
The efficacy of sugammadex as a reversal agent was evaluated using different animal models (mouse, guinea pig, cat, and Rhesus monkeys). In vitro studies by CitationMiller and Bom (2001), in the isolated mouse hemidiaphragm, showed that sugammadex effectively reversed a 90% neuromuscular block induced by the steroidal NMBAs agents rocuronium, rapacuronium (no longer commercially available), vecuronium, and pancuronium. Most efficient reversal was seen in the rocuronium-treated group followed by a neuromuscular block induced by rapacuronium, vecuronium, and pancuronium. However, sugammadex was not effective against the non-steroidal NMBAs mivacurium, atracurium and succinylcholine (CitationMiller and Bom 2001). These findings were confirmed by CitationMason and Bom (2001) who performed in vivo experiments in guinea pig. In this study guinea pigs were treated with steroidal and non-steroidal NMBAs to induce a 90% neuromuscular block. The time of spontaneous recovery from this neuromuscular block was compared with the recovery time of the same neuromuscular block after the administration of 1.0 mg/kg sugammadex. The results showed that sugammadex caused a rapid reversal of neuromuscular block induced by steroidal NMBAs. No signs of residual blockade or recurarization were observed. The injection of sugammadex did not cause significant changes in heart rate or blood pressure. In this study, they confirmed and extended earlier findings that sugammadex was not effective in reversing neuromuscular block induced by non-steroidal NMBAs. This confirms that the size of the cyclodextrin cavity is too small to accommodate the bulky molecules of mivacurium and atracurium. Another study by Hope and colleagues evaluated the effects of sugammadex on rocuronium-induced steady-state neuromuscular block of the tibialis muscle of the anesthetized cat (Hope and Bom 2003). A neuromuscular block of 90% was rapidly reversed by 1.0 mg/kg sugammadex, without significant changes in heart rate and blood pressure and again no signs of residual blockade or recurarization were reported. CitationEpemolu et al (2002) found in experiments in guinea pigs that the high affinity of sugammadex for rocuronium results in a concentration gradient between the free rocuronium molecules in the tissue compartment, including the effect compartment (neuromuscular junction) and the central compartment (plasma). As result of this concentration gradient free rocuronium molecules will return to the central compartment and are encapsulated by unsaturated sugammadex. Due to this process rocuronium is less available at the effect site, the neuromuscular junction and in turn muscle activity will return. Citationde Boer et al (2006a) evaluated the capability of nine CD derivatives (one β-CD and eight γ-CDs of increasing molecular weight) to reverse a constant neuromuscular block of 90% induced by rocuronium. The ability of these CD derivatives to reverse neuromuscular block was compared with the reversal of the same neuromuscular block by the commonly used combination of neostigmine and atropine. Furthermore the cardiovascular stability was evaluated by monitoring heart rate and blood pressure. The results of these experiments showed that two of the γ-CD derivatives (Org 26148 and Org 25969, sugammadex) caused a rapid and effective reversal of rocuronium-induced neuromuscular block versus spontaneous recovery without signs of residual blockade or recurarization. Both these CD derivatives were also significantly faster and more efficient compared with reversal by the currently used combination of neostigmine and atropine. There were no observable side-effects or significant effects on blood pressure or heart rate caused by any of the nine synthetic CD derivatives. In contrast, in the experiments with neostigmine–atropine combination, two of the four animals showed abdominal discomfort (retching) and mean arterial pressure and heart increased significantly to more than 10% of the baseline values in two of the four experiments. This study in Rhesus monkeys confirmed earlier findings in in vitro studies that the potency of natural α-, β-, and γ-CDs to reverse neuromuscular block induced by rocuronium correlates with their cavity sizes, resulting in a clear measurable effect for γ-CDs (CitationBom et al 2002; Citationde Boer et al 2006a). The best fit for cavity size can be improved by adding side chains to the molecules. The electric charge distribution in these side chains interacting with the charges in the guest molecule results in even greater affinity. A rapid and effective reversal of 90% rocuronium-induced neuromuscular block could be achieved without signs of residual blockade or recurarization. However, little data about the reversal of profound neuromuscular block induced by rocuronium are available. The only study available on profound neuromuscular block was reported by Bom et al: they showed that a 10 × ED90 dose of rocuronium can be reversed by sugammadex in guinea pigs (CitationBom et al 2001). De Boer and colleagues designed a study to determine the feasibility of reversal of rocuronium-induced profound neuromuscular block in Rhesus monkeys (Citationde Boer et al 2006b). A profound neuromuscular block was induced by injection of a 500 μg/kg dose of rocuronium (100 μg/kg resulted in a mean neuromuscular block of 93%). One minute after the administration of rocuronium, either 1.0 or 2.5 mg/kg sugammadex or saline (placebo) was injected. The results of this study showed a significant reversal of profound rocuronium-induced neuromuscular block after a dose of 2.5 mg/kg sugammadex and a partial reversal after 1.0 mg/kg sugammadex. Cardiovascular changes were not observed in this study.
Sugammadex-rocuronium complexes are highly hydrophilic, and it has been demonstrated that sugammadex is excreted rapidly and dose dependently in urine of anesthetized guinea pigs (CitationEpemolu et al 2002). CitationBom et al (2003) designed a study to determine the influence of renal impairment on the reversibility of rocuronium-induced neuromuscular block by sugammadex. This study showed that the effectiveness of sugammadex in reversal of neuromuscular block was not affected by cessation of renal blood flow. Furthermore acid-base imbalances were also studied to evaluate the effectiveness in changed circumstances (CitationMason et al 2002). The effectiveness of sugammadex was not affected by changes in pH in anesthetized guinea pigs.
Human studies
The first human exposure of sugammadex was reported by CitationGijsenbergh et al (2005). Twenty-nine healthy male volunteers received either sugammadex or placebo. In the first part of the study19 subjects received sugammadex up to 8.0 mg/kg without administration of a neuromuscular blocking agent. In the second part of this study the subjects were anesthetized and received an intubation dose of 0.6 mg/kg rocuronium or placebo. This was followed by a single bolus injection of sugammadex (0.1–8.0 mg/kg) or placebo. Assessment of efficacy showed a large reduction in recovery time as compared with placebo. A dose of sugammadex of 8.0 mg/kg resulted in a recovery time to a train-of-four ratio of 0.9 (normal neuromuscular function) of 1 minute compared with 52 minutes for placebo. Residual blockade or recurarization were not reported. There were no adverse events and sugammadex was well tolerated in doses up to 8.0 mg/kg. CitationShields et al (2006) studied sugammadex for reversal of prolonged (>2 hours) rocuronium-induced neuromuscular block. Thirty patients were anesthetised and received rocuronium 0.6 mg/kg as an initial dose followed by increments to maintain a deep block. Neuromuscular monitoring was carried out using acceleromyography, in the train-of-four mode. After at least 2 hours of neuromuscular block, at recovery of the second twitch of the train-of-four, the patients received either sugammadex in a dose of 0.5–6.0 mg/kg or placebo. The results showed a dose-related decrease in recovery time to a normal neuromuscular function (train-of-four ratio 0.9) within 2 minutes. No signs of recurarization were observed and no adverse events were reported. The conclusion of this study was that the effective dose to reverse a deep and prolonged rocuromium-induced neuromuscular block appears to be 2–4 mg/kg. CitationSorgenfrei et al (2006) investigated the dose-response, safety, and pharmacokinetics of sugammadex in a dose up to 4.0 mg/kg in reversing neuromuscular block induced by 0.6 mg/kg rocuronium. Sugammadex decreased the reversal time in a dose-dependent manner from 21.0 minutes in the placebo group to 1.1 minute in the 4.0 mg/kg sugammadex dose group. No signs of recurarization were observed and no adverse events were reported. Sugammadex enhanced renal excretion of rocuronium and was excreted unchanged in urine. Two patients experienced hypotension after the administration of 2.0 and 3.0 mg/kg sugammadex. These adverse events were considered to be possibly related to sugammadex. Reversal of high dose rocuronium (1.0 mg/kg) by sugammadex at 3 and 15 minutes after the administration of rocuronium was evaluated in a study by CitationKhunl-Bradey et al (2005). The patients were treated with either placebo or sugammadex in a dose up to 16.0 mg/kg. This study showed that a profound rocuronium-induced neuromuscular block was on average reversed within 2.5 minutes for a dose of 8.0 mg/kg sugammadex or higher. None of the reported adverse events were considered related to sugammadex. Another study investigated the efficacy of sugammadex in reversing rocuronium-induced neuromuscular block with either sevoflurane or propofol maintenance anesthesia (CitationVanacker et al 2005). After 2.0 mg/kg sugammadex, recovery to a normal neuromuscular function was equivalent under propofol and sevoflurane maintenance anesthesia. A multicenter dose-finding and safety study performed by Citationde Boer et al (2005) investigated the reversal or rocuronium-induced neuromuscular block by sugammadex at 5 min after the administration of rocuronium. After a high dose rocuronium (1.2 mg/kg) for intubation, the patients received either placebo or sugammadex in a dose op to 16.0 mg/kg 5 minutes after the injection of rocuronium. A dose of sugammadex of 16.0 mg/kg resulted in a recovery time of less than 2 minutes compared with 122 minutes for placebo. Residual blockade or recurarization were not observed. Sugammadex caused a dose-dependent, fast, and efficient reversal of profound rocuronium-induced neuromuscular block. Evaluation of safety data indicates that sugammadex was well tolerated at doses up to 16.0 mg/kg. CitationSuy et al (2007) evaluated the dose-response relationship of sugammadex for the reversal of rocuronium and vecuronium-induced neuromuscular block. Thirty-nine patients received 0.6 mg/kg rocuronium and 40 received 0.1 mg/g vecuronium. Both groups were treated with either placebo or up to 8.0 mg/g sugammadex at reappearance of the second twitch of the train-of-four ratio. Again, a normal neuromuscular function was the primary end-point of this study. Sugammadex showed a fast and effective recovery after a rocuronium and vecuronium-induced neuromuscular block. A clear dose-response relationship was observed. Again, residual blockade or recurarization were not reported. No adverse events related to sugammadex were reported and sugammadex showed a good safety profile. A multicenter study further evaluated the efficacy of sugammadex in reversing profound neuromuscular block induced by 1.2 mg/kg rocuronium (CitationRex et al 2005). Randomly at 3 or 15 minutes after the injection of rocuronium, sugammadex was administered in doses up to 16.0 mg/g. A dose-dependent time to recovery to a normal neuromuscular function was found. The reversal time was significantly decreased compared with placebo. Only one adverse event was reported possibly related to sugammadex (QT-prolongation). An interesting study was conducted by CitationSacan et al (2006). Reversal of rocuronium-induced neuromuscular block by sugammadex was compared with the reversal of the currently used combination of cholinesterase inhibitors and muscarinic acetylcholine receptor antagonists, neostigmine-glycopyrrolate, and edrophonium-atropine. The reversal of rocuronium-induced neuromuscular block by sugammadex was more rapid and efficient compared with neostigmine-glycopyrrolate and edrophonium-atropine. Treatment with sugammadex was also associated with fewer side-effects frequently reported after reversal with cholinesterase inhibitors.
Conclusion
The results of recent studies demonstrate that sugammadex is effective for reversal of rocuronium- and vecuronium-induced neuromuscular block without apparent side-effects. This is in contrast to the currently available cholinesterase inhibitors used to reverse neuromuscular block, which are ineffective even against profound neuromuscular block and have a number of undesirable side-effects. Sugammadex–rocuronium complexes are highly hydrophilic and it has been demonstrated that sugammadex is excreted in a rapid and dose-dependent manner in urine, resulting in a complete elimination from the body. Although sugammadex-induced reversal is tolerated in patients with renal disease, the fate of the sugammadex-rocuronium/vecuronium complex remains unclear. Clinical studies should be conducted in order to determine this. The ability of sugammadex to reverse rocuronium- and vecuronium-induced neuromuscular block may have major implications for routine anesthetic practice. Once sugammadex becomes commercially available, anesthesiologists will be capable of maintaining the desired depth of neuromuscular block at any time, thereby assuring optimal surgical conditions. It has been speculated that sugammadex might also be used to rapidly terminate the effects of rocuronium in the dangerous and feared “cannot intubate, cannot ventilate” situation, and that it could improve the suitability of rocuronium rapid sequence induction techniques. The mechanism by which sugammadex encapsulates rocuronium and vecuronium appears to be superior to currently used neuromuscular block reversal strategies in terms of speed, efficacy, incidence of residual neuromuscular block and recurarization, and side-effects.
References
- ArbousMSMeursingAEEvan KleefJW2005Impact of anesthesia management characteristics on severe morbidity and mortalityAnesthesiology1022576815681938
- BomABradleyMCameronK2002A novel concept of reversing neuromuscular block: chemical encapsulation of rocuronium bromide by a cyclodextrin-based synthetic hostAngew Chem Int Ed Engl412667012491405
- BomAMasonRHopeFvan EgmondJMuirA2001The cyclodextrin derivative Org 25969, which forms complexes with steroidal neuromuscular blocking agents, causes selective reversal of normal and profound neuromuscular blockAnesthesiology95A1020
- BomAHvan EgmondJHopeF2003Rapid reversal of rocuronium-induced neuromuscular block by Org 25969 is independent of renal perfusionAnesthesiology99A1158
- BooijLHDJDe BoerHDVan EgmondJ2002Reversal agents for non-depolarizing neuromuscular blockade: reasons for and development of a new conceptSemin Anesth Periop Med Pain21928
- ChallaRAhujaAAliJ2005Cyclodextrins in drug delivery: an updated reviewAAPS PharmSciTech632957
- de BoerHDMarcusMSchoutenP2005Reversal of rocuronium-induced (1.2 mg/kg) neuromuscular block by Org 25969: a multi-center dose finding and safety studyAnesthesiology103A1117
- de BoerHDvan EgmondJvan de PolF2006aChemical encapsulation of rocuronium by synthetic cyclodextrin derivatives: reversal of neuromuscular block in anaesthetized Rhesus monkeysBr J Anaesth96201616377646
- de BoerHDvan EgmondJvan de PolF2006bReversal of profound rocuronium neuromuscular blockade by sugammadex in anesthetized Rhesus monkeysAnesthesiology1047182316571967
- EpemoluOBomAHopeF2003Reversal of neuromuscular blockade and simultaneous increase in plasma rocuronium concentration after the intravenous infusion of the novel reversal agent Org 25969Anesthesiology99632712960547
- EpemoluOMayerIHopeF2002Liquid chromatography/mass spectrometric bioanalysis of a modified γ cyclodextrin (Org 25969) and rocuronium bromide (Org 9426) in guinea pig plasma and urine: its application to determine the plasma pharmacokinetics of Org 25969Rapid Commun Mass Spectrom1619465212362386
- GijsenberghFRamaelSHouwingN2005First human exposure of Org 25969, a novel agent to reverse the action of rocuronium bromideAnesthesiology10369570316192761
- HopeFBomA2001Org 25969 reverses rocuronium-induced neuromuscular blockade in the cat without important hemodynamic effectsEur J Anaesthesiol18Suppl 2310011270018
- HunterJM1995New neuromuscular blocking drugsN Engl J Med332169197760871
- Khunl-BradyKRexCKjaerCC2005Reversal of high dose rocuronium with Org 25969Eur J Anaesthesiol22Suppl 34121
- MasonRBomA2001Org 25969 causes selective reversal of neuromuscular block induced by steroidal NMBs in anaesthetized guinea pigsEur J Anaesthesiol18Suppl 2310011270018
- MasonRBomAMcIndewarI2002Org 25969 causes rapid reversal of rocuronium-induced neuromuscular block, independent of acid-base statusEur J AnaesthesiolA-1009
- MenckeTEchternachMKleinschmidtS2003Laryngeal morbidity and quality of tracheal intubation: a randomized controlled trialAnesthesiology9810495612717124
- MillerSBomA2001Org 25969 causes selective reversal of neuromuscular blockade induced by steroidal NMBs in the mouse hemi-diaphragm preparationEur J Anaesthesiol18Suppl 2310011270018
- OsmerCVogeleCZickmanB1996Comparative use of muscle relaxants and their reversal in three European countries: a survey in France, Germany and Great BritainEur J Anaesthesiol13389998842663
- PedersenTViby-MogensenJRingstedC1992Anaesthetic practice and postoperative pulmonary complicationsActa Anaesthesiol Scand36812181466220
- RexCKhunl-BradyKSielenkaemperA2005Reversal of high dose rocuronium (1.2 mg/kg) with Org 25969Anesthesiology103A1129
- SacanOKleinKWhitePF2006Sugammadex reversal of rocuronium-induced neuromuscular blockade: a comparision wth neostigmine-glycopyrrolate and edrophonium-atropineEur J Anaesthesiol23Suppl 37143
- ShieldsMMirakhurRKMoppettI2006Org 25969 (sugamadex), a selective relaxant binding agent for antagonism of prolonged rocuronium-induced neuromuscular blockBr J Anaesth96364316357116
- ShortenGD1993Postoperative residual curarisation: incidence, aetiology and associated morbidityAnaesth Intensive Care2178298122734
- SorgenfreiIFNorrildKLarsenPB2006Reversal of rocuronium-induced neuromuscular block by the selective relaxant binding agent sugammadex: a dose-finding and safety studyAnesthesiology1046677416571960
- SuyKMoriasKHansP2007Fast, effective and safe reversal of rocuronium and vecuronium-induced moderate neuromuscular block by the selective relaxant binding agent Org 25969Anesthesiology106283817264722
- VanackerBVermeyenKStruysMRF2005Reversal by Org 25969 is not affected by sevoflurane when compared with propofolEur J Anaesthesiol22Suppl 34119
- Van VlymenJMParlowJL1997The effects of reversal of neuromuscular blockade on autonomic control in the perioperative periodAnesth Analg84148548989016
- ZhangMQ2003Drug-specific cyclodextrins: the future of rapid neuromuscular block reversalDrugs Future234754