Abstract
Background
RTMS has been developed as a novel tool for treating depression but the clinical significance of this treatment has been variable, especially in the older depressed subjects.
Methods
Medication-resistant depressed patients 60 years or older were treated for two weeks (10 sessions) with high-frequency rTMS delivered to the left dorsolateral prefrontal cortex at 100% of motor threshold. Each session consisted of 20 trains at 10Hz delivered in 8-second duration. The patients continued taking their psychotropic medications throughout the study.
Results
Nineteen of the 20 subjects completed the trial. One subject dropped out after 8 sessions because of discomfort. The average age of our patients was 66.8 years (6 males and 14 females). Six patients responded and there was a 31.6% mean reduction in Hamilton Depression Rating Scale (HDRS) scores from baseline at the end of the treatment. There was statistically significant decrease from baseline in both HDRS and HARS scores at the end of treatment. rTMS was generally well tolerated.
Conclusion
These preliminary finding suggests that rTMS may be an effective treatment alternative to a subpopulation of medication resistant older depressed patients.
Introduction
Major depression is a debilitating psychiatric disorder. Of all disability and premature loss of life in developing countries, depression accounts for 3.4% in women and 1.3% in men (CitationBland et al 1988; CitationBland 1996) and depression is the most common mental health problem in the elderly. Major depression is found in 1.7% of the elderly population, while pervasive depression is found in 13.4% of the elderly population (CitationBaldwin and Simpson 1997).
Depression in the elderly has a considerable impact on their well-being and level of disability (CitationBeekman et al 2002). Late-life depression may be due to a variety of factors including a longstanding vulnerability, severe stress, and vascular risk. However, depression in the elderly may be more difficult to treat than in other populations. Antidepressants can be administered as a first line treatment; however, this can be problematic, as older patients often cannot tolerate dosages high enough to produce an antidepressant response. Predicting the antidepressant effect and side-effects of pharmacological treatment in the elderly can also be difficult due to the variation in bioavailability. Increasing the duration of antidepressant treatment to up to 12 weeks in order to get a clinical response is also suggested for treatment of depression in the elderly population. However, this approach may be difficult, especially in cases of severe depression. Antidepressant class switching and augmentation with lithium is another treatment option. However, this can also be problematic due to intolerable side-effects or potential adverse reactions with non-psychotropic medications (CitationBaldwin and Simpson 1997). Electroconvulsive therapy (ECT) is a treatment often used to treat depression in the elderly. Although ECT is quite effective and fast acting, it is associated with cognitive and other side-effects including nausea, headache and muscle aches (CitationDatto 2000). In addition, due to the risks associated with anesthetic use in the elderly ECT is not always a viable option.
Repetitive transcranial magnetic stimulation (rTMS) may be another option in the treatment of late-life depression. Recently, two reviews of the evidence for the efficacy of rTMS as a treatment of depression were completed; they focused mainly on sham-controlled studies, literature reviews, and meta-analysis. These separate reviews indicate that there is statistical evidence that rTMS is more effective than sham treatment, despite the small degree of clinical improvement (CitationLoo and Mitchell 2005; CitationRachid and Bertschy 2006). The review by CitationRachid and Bertschy (2006) also stated that rTMS treatment “is a relatively safe and well-tolerated technique with generally minor adverse effects such as localized scalp pain, headache and neck pain”.
There have only been a few studies examining the use of rTMS as an antidepressant treatment in elderly patients, for a brief review see . CitationFigiel and colleagues (1998) treated a large group of adult patients and demonstrated that overall 42% of patients responded to rTMS. However, only 23% of the elderly patients in the study responded to treatment, suggesting increased efficacy of rTMS in younger populations as compared to elderly. The authors found the rTMS treatments to be well-tolerated in all patients. However, another recent study by CitationFabre et al (2004) on the effects of rTMS in vascular depression reported that 5 out of 11 patients showed a clinically meaningful improvement in Hamilton Depression Rating Scale scores (HDRS) (CitationHamilton 1960).
Table 1 Previous findings of rTMS effect on depression in elderly populations
Another study (CitationMane et al 2001) found no significant change in HDRS (CitationHamilton 1960) scores before and after rTMS treatment in depressed elderly. They also reported 3 responders out of 10 subjects in the active treatment group and 3 responders out of 10 in the sham treatment group. These finding were attributed to insufficient stimulation parameters. Similarly, a more recent study of rTMS as add-on treatment for depression in the elderly reported no additional antide-pressant effects of real stimulation versus sham treatment. It was also reported that treatment with rTMS was safe, as adverse events were rare and the cognitive assessment demonstrated no deterioration in cognitive function (CitationMosimann et al 2004).
In contrast to ECT, the benefits of rTMS include efficiency, cost-effective administration on an outpatient basis in contrast to ECT, no need for anesthesia, no seizure induction and a lack of significant cognitive side effects. rTMS may, therefore, be another viable option for the treatment of late-life depression. However, the lack of consensus regarding the antidepressant effects of rTMS in an elderly population indicate there is a need for further research in this area.
The aim of this study is to establish the efficacy and tolerance of repetitive transcranial magnetic stimulation (rTMS) for treatment of older patients with depressive disorders not responding to psychotropic medication.
Materials and methods
Subjects
Patients were recruited from the population served by a specialized service for mood disorders (both in and out-patients were included). A local ethics board approved the study and only patients who were able to provide written informed consent were included.
Inclusion criteria were: DSM-IV (CitationAmerican Psychiatric Association, 2000) criteria for depressive disorder, unipolar or bipolar type with a HDRS score of ⩾18; 60 years of age or older and willing and capable of continuing with the same medications through out the trial. Diagnosis was confirmed by experienced psychiatrists on the basis of an unstructured clinical interview using the DSM-IV checklist (CitationAmerican Psychiatric Association 2000).
Exclusion criteria included: Personal or family history of epilepsy or seizure disorder, mass brain lesion, metal in the skull or brain, history of a head trauma, patients who were actively suicidal, with a concurrent serious medical illness, history of alcohol or drug abuse in the last 3 months.
All patients had failed to respond to treatment with at least two adequate trials of at least 6-weeks duration, with an antidepressant. All patients were required to be on stable medications for 6 weeks prior to commencing in the study. They continued taking the maximum tolerated dose of the last antidepressant throughout the two weeks of treatment with rTMS and throughout follow-up. All other psychotropic medications were to remain unchanged for the same period unless readjustments were clinically indicated.
Outcome measures
Mood assessment was performed using the HDRS-21 item, Hamilton Anxiety Rating Scale (HARS) (CitationHamilton 1969) and the Beck Depression Inventory-II (BDI) (CitationBeck et al 1961).
Visual Analogue Scale (VAS) for depression (-D), anxiety (-A) and physical discomfort (-PD).
The Clinical Global Impression (CGI) was completed as an additional scale in monitoring response to treatment.
The Mini-Mental Status Exam (MMSE) (CitationFolstein et al 1975).
The outcome measures (HDRS, HARS, BDI, MMSE, and CGI) were administered at baseline (before rTMS treatment), mid-treatment (after 5 treatments), at the end of treatment (after 10 treatments), two weeks and 1 month after the completion of rTMS. The VAS-D, VAS-A, and VAS-PD were administered after each rTMS session, two weeks and 1 month after the completion of rTMS treatment.
rTMS procedure
Patients received 10 rTMS sessions over a two-week period with all sessions employing the same stimulation parameters. A Dantec high-speed magnetic stimulator was used. A figure-8 coil was placed over the left dorsolateral prefrontal cortex (DLPFC) defined as a site 5 cm anterior to the site for optimal stimulation of the abductor pollicis brevis (APB) muscle of the contralateral side. The rTMS intensity was set at 100% of the motor threshold and 20 trains of 10 Hz stimulation with a train duration of 8 seconds were delivered at an intertrain interval of 52 seconds.
Results
Twenty patients were enrolled in the study, of these 19 patients completed all 10 rTMS treatments. One patient completed 8 of the 10 treatments but was unable to continue due to local discomfort and headache. Several of the remaining patients developed slight transient discomfort with the first treatment, but none had severe or persistent pain. Of the 20 patients, 6 (30%) were male and 14 (70%) were female, 3 (15%) suffered from bipolar depression and 17 (85%) suffered from unipolar depression. The average age was 66.8 (± 6.4) years and ranged from 60–80 years old. Fifteen patients were assessed at the 2-week follow-up and thirteen were assessed at the 1-month follow-up visit.
There was a significant decrease in the mean HDRS score for the entire population from baseline 25.3 SD 5.8 to 17.3 SD 6.5 after 10 treatments, p = 0.0003. There was a 31.6% mean reduction in HDRS from baseline. The mean HDRS score 1 month after the completion of treatment was found to be 16.6 (SD 8.7), a 34.3% mean reduction from baseline ().
Table 2 BDI, VAS-D, -A, -PD, CGI, MMSE scores at baseline, mid-treatment and 1 month follow-up
After 10 treatments 6 patients showed a reduction in HDRS scores of at least 50% and another patient showed a reduction of at least 50% at the 1-month follow-up visit. Two of the 6 patients also reached remission (defined as a HDRS score <8). The patient who showed the reduction of 50% at the 1 month follow up visit also reached remission at the 1 month follow up visit.
The mean HARS score at baseline was 19.3 (±8.2) and it reduced to 13.2 (±10.1) after 10 treatments. This is a 31.6% mean reduction from baseline. The mean HARS score 1 month after the completion of treatment was found to be 13.7 (±7.5), a 29.0% mean reduction from baseline ().
Figure 1 HDRS and HARS scores at baseline, mid-treatment, end of treatment, 2 week follow-up and 1 month follow-up. tests are needed to confirm the absence of deterioration in cognitive functioning.
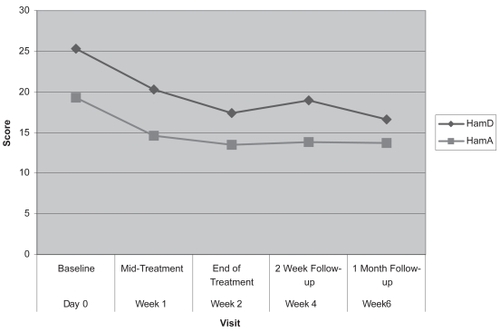
Both HDRS and HARS scores showed a significant decrease at the end of treatment (visit 10) from baseline (p= 0.0003 and p = 0.03, respectively).
As shown in , both the BDI and the CGI decreased significantly at the end of treatment from baseline (p = 0.0396 and p = 0.0088, respectively). The VAS for depression and anxiety did not show a significant change (p = 0.117 and p = 0.053, respectively) with treatment. The VAS for physical discomfort did not show a significant increase from baseline to the end of treatment (p = 0.336). Also the MMSE did not show a significant change from baseline to the end of treatment (p = 0.586).
Discussion
This study assessed the effect of rTMS applied to the left dorsal prefrontal cortex as an add-on treatment in medication-resistant depressed patients who were 60 years of age or older. After two weeks (10 rTMS treatments), 30% (6 out of 20 patients) met response criteria (>50% improvement in HDRS), with two of these patients also meeting criteria for remission (HDRS ⩽ 8).
This finding compares favorably with that of CitationNahas and colleagues (2004) who used distance adjusted TMS treatments in patients 65–75 years of age. In their study rTMS was administered for 3 weeks to the DLPFC using a stimulation intensity of 114% of the motor threshold (MT) as compared to 2 weeks at 100% of MT in our study. In Nahas et al 28% (5 of 18 subjects) met criteria for response; four of these five patients had exit HDRS scores ⩽ 8, and thus met criteria for remission. Although, our results show a higher number of responders to rTMS, Nahas’s study reported a higher number of remitters. The results of our study demonstrate a 31.6% mean reduction in HDRS scores from baseline to the end of study, increasing to 34.3% at one month follow up. Similarly, Nahas et al reported a 35.2% drop on the 28-item HDRS from baseline. Finally, the same authors reported that 10 out of 18 subjects were very much improved or much improved according to the CGI. In our study, CGI demonstrated much or very much improvement in six patients.
CitationMosimann et al (2004) treated elderly depressed patients (mean age = 62 years) with 10 daily rTMS treatments at 100% of motor threshold, similar to our stimulation parameters, and reported a 20% reduction in HDRS-21 scores in the active treatment group. Interestingly, the sham treated group showed a 17% reduction in HDRS. In the Mosimann study, 26.6% (4 of 24) of patients receiving real TMS were responders, similar to the results of our study.
Previous studies found no deterioration in cognitive function in rTMS-treated subjects (CitationMartis et al 2003; CitationSpeer et al 2001). In our study there was no evidence of cognitive deterioration, as MMSE scores remained unchanged throughout the study. However, more rigorous cognitive
CitationMosimann et al (2004) found rTMS to be a safe treatment with none of the patients withdrawing from the study or reporting serious adverse effects. One patient in our study withdrew, after 8 sessions, because of discomfort and headaches.
This is similar to the study by CitationSu et al (2005) where 3 out of 30 patients dropped out of the treatment because of pain or worsening of clinical symptoms. As well, four patients in the active and two patients in the sham group reported headaches, which is consistent with complaints reported by our study subjects.
The response rate obtained by most other studies are quite similar to our data, although in contrast to CitationSu et al (2005) who found no responder in patients older than 55 years of age. In our study we did not find any significant difference in age, sex, or degree of cognitive impairment between responders and non-responders. In addition, responders and non-responders did not differ in severity of depression, as measured by initial HDRS scores or number of previous antidepressant treatments.
It has yet to be established if rTMS’s antidepressant response rates differ between elderly and younger populations of patients with depression. Published reports indicate a lower antidepressant effect of rTMS in elderly depressed patients as compared to younger populations (CitationFigiel et al 1998; CitationPradberg et al 1999; CitationSu et al 2005). CitationFregni et al (2006) found age and treatment refractoriness to be significant negative predictors of depression improvement. They reported that TMS antidepressant therapy in younger and less treatment resistant patients resulted in better outcomes. The findings in our study of a response rate of 31.6% after only 10 daily sessions of rTMS at 100% of MT is comparable to the response rates of 30% and 35% respectively reported by CitationGarcia-TORO et al (2001) and CitationFitzgerald et al (2003) in younger adults.
Two more recent studies reported similar response rates after treating younger adults at higher intensities and for longer sessions. Response rate of 30% was reported by CitationIsenberg et al (2005) who gave 4 weeks of high frequency rTMS; and CitationAvery et al (2006) found a 32% response rate when rTMS was administered for 15 sessions at an intensity of 110% of MT. Although, we administered rTMS for 10 sessions at an intensity of 100% MT, it appears that our older patients responded well.
CitationRossini et al (2005), who reported a response rate of 61%, argued that treatment response was unrelated to the demographic and clinical characteristics of their patients. CitationFitzgerald et al (2006) have also reported no relationship between age and response to rTMS treatment. In addition, it is of interest to note that CitationJorge et al (2004) reported that reduction of depression symptoms in rTMS treated post stroke patients was not influenced by the patient’s age or type or location of ischemic strokes.
The limitations of this study require some consideration. This is non-controlled, open study with a small sample size. This is a significant limitation, which likely restricted the statistical power. Our patients all received antidepressants during the trial. We ensured that all patients stayed on a stable dose of the current medications for at least six weeks before commencement of rTMS, during the trial and follow-up period. However, changes to psychotropic medications (excluding antidepressants) were allowed, throughout the study, where clinically indicated. Since the study lacks a control group we cannot exclude the possibility of a placebo effect. The response to rTMS seen in our study may be due to a placebo effect of a cutting edge treatment, but given that all of our subjects had been taking stable doses of the same medication for at least six weeks, were not improving before the trial and had a high degree of resistance, this is unlikely. CitationKhan et al (2002) reported that more severely depressed patients responded poorly to placebo compared to antidepressant treated subjects.
Recently, it has been suggested that 10 treatments and 100% of MT may not be sufficient to produce a maximum response. But our study was planned and started a few years ago when routine rTMS treatment was a course given for two weeks. Thus, administration of a fixed number (10) of rTMS sessions may have reduced the efficacy of our treatment as a higher number of sessions optimized the clinical benefit of treatment as demonstrated in some recent studies (CitationJanicak et al 2002; CitationGrunhaus et al 2003; CitationRossini et al 2005). However the literature is lacking a study that directly compares the effects of 2 different numbers of pulses per day on mood. More rTMS sessions even at higher stimulation intensity have not consistently produced better clinical outcome as reported in newer studies (CitationIsenberg et al 2005; CitationAvery et al 2006). CitationRossini et al (2005) suggested that methodological factors might be responsible for the variable results reported in the literature.
In conclusion, rTMS is a safe and well-tolerated treatment and may be a useful adjunctive treatment to medications in elderly treatment resistant depressed patients. We believe our findings add to the growing body of literature that suggests the effectiveness of rTMS. Despite the fact that the generalizabilty of our study findings are limited, rTMS treatment in older depressed population is showing encouraging results and requires further study.
References
- American Psychiatric Association2000Diagnostic and statistical manual for mental disorders (DSM-IV-TR)4th Ed Text Revision ednWashington, DC
- AveryDHHoltzheimerPE3rdFawazW2006A controlled study of repetitive transcranial magnetic stimulation in medication-resistant major depressionBiol Psychiatry591879416139808
- BaldwinRCSimpsonS1997Treatment resistant depression in the elderly: a review of its conceptualization, management and relationship to organic brain diseaseJ Affect Disord46163739547114
- BeckATWardCHMendelsohnM1961An inventory for measuring depressionArch Gen Psychiatry45617113688369
- BeekmanATFPenninxBWJHDeegDJH2002The impact of depression on the well-being, disability and use of services in older adults: a longitudinal perspectiveActa Psychiatr Scand10520712086221
- BlandRCNewmanSCOrnH1988Epidemiology of psychiatric disorders in EdmontonActa Psych Scand77S388
- BlandRC1996International health and psychiatryCan J Psychiatry4111158919418
- DattoCJ2000Side effects of electroconvulsive therapyDepress Anxiety12130411126187
- FabreIGalinowskiAOppenheimC2004Antidepressant efficacy and cognitive effects of repetitive transcranial magnetic stimulation in vascular depression: an open label trialInt J Geriatr Psychiatry198334215352140
- FigielGSEpsteinCMacDonaldWM1998The use of rapid-rate transcranial magnetic stimulation (rTMS) in refractory depressed patientsJ Neuropsychiatr10205
- FitzgeraldPBBrownTLMarstonNA2003Transcranial magnetic stimulation in the treatment of depression: a double blind, placebo-controlled trialArch Gen Psychiatry601002814557145
- FitzgeraldPBHuntsmanS2006A randomized trial of low-frequency right-prefrontal-cortex transcranial magnetic stimulation as augmentation in treatment-resistant major depressionInt J Clin Neuropsychopharm965566
- FolsteinMFFolsteinSEMcHughPR1975Mini-mental state. A practical method for grading the state of patients for the clinicianPsychiatric Research1218998
- FregniFMarcolinMAMyczkowskiM2006Predictors of antide-pressant response in clinical trials of transcranial magnetic stimulationInt J Neuropsychopharm964154
- Garcia-ToroMMayolAAmillasH2001Modest adjunctive benefit with transcranial magnetic stimulation in medication-resistant depressionJ Affect Disord64271511313095
- GrunhausLDannonPNSchreibersS2000Repetitive transcranial magnetic stimulation is as effective as electroconvulsive therapy in the treatment of nondelusional major depressive disorder: an open studyBiol Psychiatry473142410686266
- GrunhausLSchreiberSDolbergOT2003A randomized controlled comparison of electroconvulsive therapy and repetitive transcranial magnetic stimulation in severe and resistant nonpsychotic major depressionBiol Psychiatry235662
- HamiltonM1960A rating scale for depressionJ Neurosurg Psychiatry235662
- HamiltonM1969A rating scale for anxietyBrit J Psychiatry Special Publication769
- IsenbergKDownsDPierceK2005Low frequency rTMS stimulation of the right frontal cortex is as effective as high frequency rTMS stimulation of the left frontal cortex for antidepressant free, treatment-resistant depressed patientsAnn Clin Psychiatry17153916433057
- JanicakPGDowdSMMartisB2002Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: preliminary results of a randomized trialBiol Psychiatry516596711955466
- JorgeRERobinsonRGTatenoA2004Repetitive transcranial magnetic stimulation as treatment of post stroke depression: a preliminary studyBiol Psychiatry553980514960293
- KhanALeventhalRMKhanSR2002Severity of depression and response to antidepressants and placebo: an analysis of the Food and Drug Administration databaseJ Clin Psychopharmacol2240511799341
- LooCKMitchellPB2005A review of the efficacy of transcranial magnetic stimulation (TMS) treatment for depression, and current and future strategies to optimize efficacyJ Affect Disord882556716139895
- ManesFJorgeRMorcuendeM2001A controlled study of repetitive transcranial magnetic stimulation as a treatment of depression in the elderlyInt Psychogeriatr132253111495396
- MartisBAlamDDowdSM2003Neurocognitive effects of repetitive transcranial magnetic stimulation in severe major depressionClin Neurophysiol11411253212804681
- MosimannUPSchmittWGreenbergBD2004Repetitive transcranial magnetic stimulation: a putative add-on treatment for major depression in elderly patientsPsychiatry Res1261233315123391
- NahasZXingbaoLKozelA2004Safety and benefits of distance-adjusted prefrontal transcranial magnetic stimulation in depressed patients 55–75 years of age: a pilot studyDepression Anxiety1924956
- PradbergFZwanzgerPThomasH1999Repetitive transcranial magnetic stimulation in pharmacology-refractory major depression: comparative study of fast and slow and sham rTMSPsychiatry Research881637110622338
- RachidFBertschyG2006Safety and efficacy of repetitive transcranial magnetic stimulation in the treatment of depression: a critical appraisal of the last 10 yearsNeurophysiol Clin361578317046610
- RossiniDLuccaAZanardiR2005Transcranial magnetic stimulation in treatment-resistant depressed patients: a double blind, placebo-controlled trialPsychiatry Research13711016225930
- SpeerAMRepellaJDFiguerasS2001Lack of adverse cognitive effects of 1 Hz and 20 Hz repetitive transcranial magnetic stimulation at 100% of motor threshold over the left prefrontal cortex in depressionElectroconvulsive Therapy1725963
- SuTPHuangCCWeiIH2005Add-On rTMS for Medication-Resistant Depression: a randomized, double blind, sham-controlled trial in Chinese patientsJ Clin Psychiatry66930716013911