Abstract
The objective of this exploratory, multicenter, randomized, double-blind study, was to evaluate the efficacy and safety/tolerability of milnacipran and venlafaxine administered at flexible doses (100, 150 or 200 mg/day, bid administration) for 24 weeks (including 4 weeks up titration period) in the outpatient treatment of adults presenting with a moderate or severe episode of major depressive disorder (MDD) without high suicidal risk (MINI-DSM IV-TR). Of the 195 patients included, 134 (68.7%) completed the study. At baseline the two groups were similar, except there was a higher proportion of patients whose episode was severe-DSM IV in the milnacipran group (63.3% versus 54.0% in the venlafaxine group). The initial MADRS score (mean 31.0) decreased progressively during the study, and this decrease was in the two treatment groups (n = 177: 90 milnacipran; 87 venlafaxine) at week 24 (observed case/OC, mean change −23.1 milnacipran; −22.4 venlafaxine). The rate of MADRS response (reduction ≥ 50%) at week 8 and week 24-last observation carried forward/LOCF was similar in the two groups (week 8: 64.4% milnacipran; 65.5% venlafaxine; week 24: 70% milnacipran; 77% venlafaxine), as was the rate of MADRS remission (score ≤ 10) (week 8: 42.2% milnacipran; 42.5% venlafaxine; week 24: 52.2% milnacipran; 62.1% venlafaxine). In both groups, the most common adverse events were: nausea, dizziness, headache, hyperhidrosis and, in males, genito-urinary problems. The overall safety/tolerability and efficacy profiles of milnacipran and venlafaxine administered at flexible dosages (up to 200 mg/day) were similar in the long term treatment of adults during episodes of MDD in an outpatient setting.
Introduction
With a prevalence of around 10% in the general population, major depressive disorder (MDD) is the most common psychiatric problem.Citation1 Strongly associated with general and psychiatric co-morbidity (anxiety problems and addictions),Citation2 it is the primary cause of physical and mental incapacity.Citation3 Suicide risk is a characteristic of this disorder, and 5% of cases of severe depression result in suicide.Citation4 Despite its prevalence and detrimental consequences for both individuals and society in general, depression is only diagnosed in one in two cases, and it is particularly poorly managed, with less than 10% of patients with depression benefiting from antidepressant treatment which is appropriate in terms of dose and duration.Citation5 Although it is now recognized that the minimum duration of treatment with antidepressants necessary in order to prevent relapse of a current depressive episode is 6 months, only a few randomized, double-blind clinical studies investigating the treatment of major depression over this period have been carried out.
The antidepressant activity of a molecule is linked to its capacity to increase the synaptic concentration of serotonin (5-HT) and/or norepinephrine (NE), generally by inhibiting the reuptake of one or both of these neurotransmitters. The tricyclic antidepressants (TCAs) have, for a long time, been the cornerstone of treatment for major depression, but their safety/tolerability profile is poor due to the fact that their pharmacological action is not limited to those two neurotransmitters. They have been succeeded first by the selective serotonin reuptake inhibitors (SSRIs), which are slightly less effective, but much better tolerated, and then by the serotonin noradrenalin reuptake inhibitors (SNRIs), which combine the efficacy of the TCAs with the tolerability of the SSRIs.Citation6 Milnacipran (Ixel®) and venlafaxine (Effexor®) are both SNRIs, which differ in the ratio of potential reuptake inhibition of NE:5-HT (estimated to be 1:1 and 1:30 respectively).Citation7
The recommended dose of milnacipran in major depression is 100 mg/day. Different studies carried out in this indication with doses from 150 to 300 mg/day have suggested that some patients may benefit from higher doses.Citation8–Citation10 These studies and others carried out in fibromyalgia with a dose of 200 mg/day,Citation11,Citation12 have shown that milnacipran administered at these higher dosages both as loading dose and over the long term has a good safety/tolerability profile. Venlafaxine is prescribed in this indication at recommended doses in outpatients from 75 to 225 mg/day with uptitration at the start of treatment and down titration at the end.
The objective of this study was to explore the efficacy and long-term safety/tolerability of milnacipran and venlafaxine administered in an outpatient setting at doses of 100, 150 and 200 mg/day for episodes of MDD in adults.
Methods
Study design
This was a multi-center, randomized (1:1), double-blind study with two parallel treatment arms (milnacipran and venlafaxine), conducted over a treatment period of 24 weeks in 195 adult outpatients presenting with an episode of recurrent, unipolar, moderate-to-severe MDD.
Pre-inclusion procedures
The study protocol was approved by the Comité de Protection des Personnes Ile de France III and authorized by the Agence Française de Sécurité Sanitaire des Produits de Santé. All patients gave written informed consent before participating in the study, having first been informed of the methods and constraints of the study and their rights about access and corrections to the data concerning them.
The patients were selected and followed as outpatients through the duration of the study by 50 psychiatrists (hospital, private or a combination) in 21 centers in France. To ensure the data collected were homogeneous, all investigators were trained at study set-up in the standardized use of evaluation scales in the study. After a washout period of 4 to 21 days (depending on ongoing treatments), eligible patients were randomized to either milnacipran or venlafaxine according to a pre-established randomization list. Baseline evaluations were carried out prior to any study treatment being taken.
Study population
Included patients were male or female outpatients aged from 18 to 70 years who met the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) and using Mini International Neuropsychiatric Interview (MINI) French version 5.0.0Citation13 criteria for recurrent, unipolar, moderate-to-severe MDD, with neither psychotic features nor high suicidal risk. A Montgomery-Åsberg Depression Rating Scale (MADRS)Citation14 score ≥ 23 was required at inclusion to confirm that the intensity of the episode was moderate or severe. Patients with anxiety linked to the depression could participate in the study.
Treatment
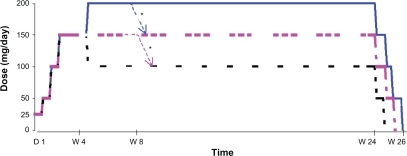
The maximum duration of exposure for each patient was 26 weeks (). During the first 4 weeks, the dose was administered according to an up titration schedule: 25 mg/day (D1–D3), 50 mg/day (D4–D6), 100 mg/day (D7–D13) and 150 mg/day (D14–D28). At D28, the investigators could decide to maintain the dose at 150 mg/day, reduce the dose to 100 mg/day or increase the dose to 200 mg/day. At any time during the following 20 weeks, the doses of 150 or 200 mg/day could be reduced for reasons relating to safety down to a minimal dose of 100 mg/day. After 24 weeks of treatment, the dose was reduced by in stages, by 50 mg/day every 5 days. The daily doses were administered in two identical doses morning and evening, except the initial dose of 25 mg/day (1 dose AM only) and the dose of 150 mg/day (50 mg AM and 100 mg PM).
Data collected: methods and timing
Data were collected by the investigators during the following study visits:
Pre-treatment: selection visit and randomization visit (D1),
During treatment: at weeks 2, 4, 6, 8, 12, 18 and 24,
Post treatment: end-of-study visit after 5 to 15 days of down titration and 10 days without treatment.
The evaluation scales used included the MADRS and the Clinical Global Impression of Severity (CGI-S) scales.Citation15
All the scales were assessed at the pre-treatment and end-of-study visits. During the study, the MADRS was assessed at all the visits, and the CGI-S was assessed at weeks 8 and 24.
The rate of MADRS response (reduction in initial score of ≥50%) and remission (score ≤ 10) were calculated at weeks 8 and 24. The rate of MADRS response and remission was also calculated at these time points in the subgroups of patients whose episode of depression was severe in intensity (DSM-IV-TR) at inclusion and in those patients who had a suicidal risk (low or moderate according to the MINI as required for inclusion).
Safety/tolerability was evaluated at each visit by the collection of adverse events and a complete physical examination including measurement of cardiovascular parameters (arterial blood pressure and heart rate). In addition, ECGs were carried out at weeks 4, 6, 8 and 24 and blood tests for standard hematology and biochemistry parameters at weeks 8 and 24. The procedure for collecting and following up adverse events was strictly in compliance with the current regulations.
Statistical analysis
The statistical analysis was carried out using the program SAS (version 9.1.3).
Standard distribution statistics: mean, standard deviation (SD), minimum (min) and maximum (max) scores and their changes from baseline, were calculated using the observed-case (OC) approach at all assessment times for the MADRS score and the last-observation-carried-forward (LOCF) at 8 and 24 weeks for both MADRS and CGI-S scores.
The rate of MADRS response and remission at weeks 8 and 24 (LOCF) was calculated by normal approximation of the binomial distribution.
Bilateral exploratory statistical tests at the 5% significance level were performed on results at 8 and 24 weeks of:
changes from baseline in MADRS score (OC) at each visit: mixed model for repeated measures (MMRM),
global MADRS response and remission rates (LOCF): chi squared.
Results
Study population
Of 195 patients randomized (97 milnacipran; 98 venlafaxine), 134 (68.7%) completed 24 weeks of treatment (60 milnacipran; 74 venlafaxine). In the two groups the main reason for premature withdrawal from the study was adverse events (19/37 on milnacipran, 10/24 on venlafaxine). No patients were lost to follow-up.
The analysis of safety/tolerability was carried out on the data for 181 patients (90 milnacipran; 91 venlafaxine); 14 patients from a center which did not respect good clinical practice were excluded. The efficacy analysis was carried out on data from 177 patients (90 milnacipran; 87 venlafaxine); 4 patients from the venlafaxine group did not complete the MADRS evaluation during the first 8 weeks of treatment.
The demographic characteristics of the patients at inclusion () were similar, except that the proportion of patients whose episode of depression was severe (DSM-IV) was greater in the milnacipran group (63.3% versus 54% in the venlafaxine group). The majority of the study population was female (60.8%); patient ages ranged from 18 to 69 years, with a mean age of 43.7 years. The mean duration of the disease was 12.6 years, and the mean duration of the current episode was 3.4 months. A previous episode had been treated with antidepressants in 87% of patients.
Table 1 Patient characteristics at inclusion
The initial mean MADRS and CGI-S scores in the overall population were 31.0 (SD: 4.5), and 5.2 (SD: 0.7) respectively, and were not significantly different between the treatment groups. This demonstrates the moderate to severe intensity of the disease and its symptoms.
For the majority of patients, the dose achieved at the end of the up titration period was either 150 mg/day (30.4% with milnacipran and 43.8% with venlafaxine) or 200 mg/day (58.2% and 46.3% respectively). At the end of treatment, the distribution of doses had not significantly differed from D28 in the two groups. Treatment compliance in the two groups was good (≥80% of the planned treatment) in 99% of patients at the 8-week visit and 93% of patients at the end of treatment visit.
Efficacy
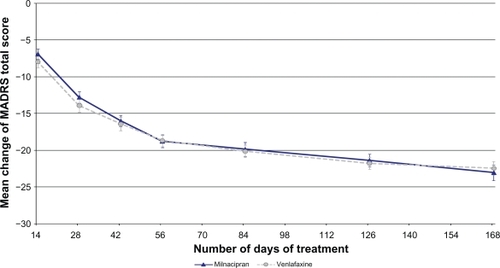
The MADRS score decreased progressively throughout the study and was comparable in the two treatment groups (). The mean [SD] decrease (OC) in the milnacipran and venlafaxine groups respectively was: 6.9 [6.7] and 7.9 [7.4] after 2 weeks of treatment, 18.8 [7.7] and 18.6 [7.3] (pMMRM = 0.95) after 8 weeks of treatment, and 23.1 [7.8] and 22.4 [7.3] (pMMRM = 0.37) after 24 weeks of treatment.
Figure 2 Changes over time from baseline MADRS total score (OC).
The rate of MADRS response and remission (LOCF) was similar, or not clinically different (or statistically different where applicable) in the two treatment groups at week 8 and, after a further increase, at week 24:
In the overall population (n = 177) ():
○ Response: 64.4% milnacipran; 65.5% venlafaxine at week 8 (pchi2 = 0.88), 70.0% milnacipran; 77.0% venlafaxine at week 24 (pchi2 = 0.29),
○ Remission: 42.2% milnacipran; 42.5% venlafaxine at week 8 (pchi2 = 0.97), 52.2% milnacipran; 62.1% venlafaxine at week 24 (pchi2 = 0.19);
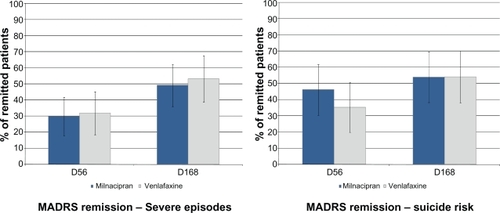
In patients whose episode of depression was severe in intensity at inclusion (n = 104) ():
○ Response: 61.4% milnacipran; 61.7% venlafaxine at week 8, 71.9% milnacipran; 74.5% venlafaxine at week 24,
○ Remission: 29.8% milnacipran; 31.9% venlafaxine at week 8, 49.1% milnacipran and 53.2% venlafaxine at week 24;
Or, in patients with a suicide risk according to the MINI (“low” or “moderate” as required for inclusion; n = 75 patients) ():
○ Response: 65.8% milnacipran; 59.5% venlafaxine at week 8, 71.1% milnacipran; 64.9% venlafaxine at week 24,
○ Remission (): 44.7% milnacipran; 35.1% venlafaxine at week 8, 52.6% milnacipran; 54.1% venlafaxine at week 24.
Figure 3 Rate of MADRS response and remission at weeks 8 and 24.
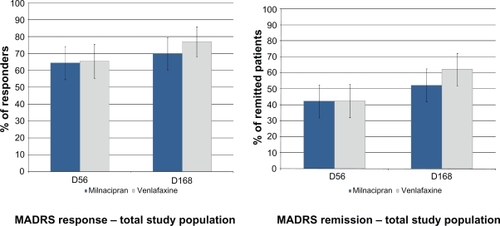
Figure 4 Rate of MADRS remission at weeks 8 and 24 in patients presenting at inclusion with i) a severe episode or ii) a suicide risk.
In the vast majority of cases, the therapeutic benefit achieved at week 8 was maintained over the long term: Overall, 93% of the patients who were MADRS responders at week 8, were also responders at week 24 (95% milnacipran, 87% venlafaxine), while 89.3% of the patients who had MADRS remission at week 8 also had remission at week 24 (87% milnacipran; 92% venlafaxine). Twelve (7%) patients (6 in each group) had response or remission at week 8 which was not maintained at week 24. However, for 7 of these patients (3 milnacipran; 4 venlafaxine) the increase in MADRS score between week 8 and week 24 was not clinically significant (<25% between weeks 8 and 24).
The global CGI-S score of the severity of the disease had significantly decreased at week 8, and this decrease was comparable in the two groups: mean [SD] decrease of −2.0 [1.4] milnacipran; −2.3 [1.2] venlafaxine: This improvement was maintained at week 24, with a mean [SD] decrease in CGI-S of −2.3 [1.2] milnacipran and of −2.7 [1.4] venlafaxine.
Safety/tolerability
During the treatment period, at least one adverse event (AE) was reported in 65 patients (72%) in the milnacipran group and 67 patients (74%) in the venlafaxine group. The majority of AEs (85% in both groups) were mild or moderate in severity.
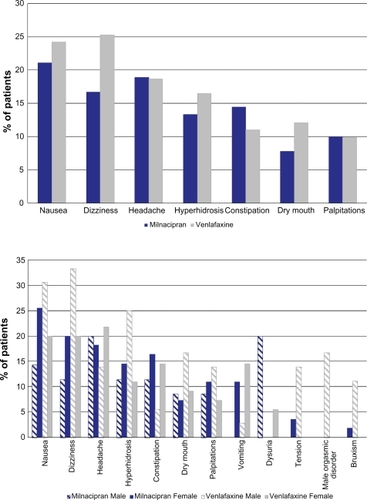
In the two groups, the most common treatment emergent AEs (occurring in ≥ 10% of patients in either group) () were gastrointestinal (nausea, constipation and dry mouth), neurological (dizziness and headache), cutaneous (hyperhidrosis) and cardiac (palpitations). In males, genitourinary disorders were also common: testicular pain and dysuria were experienced only in the milnacipran group (9% and 20% respectively), while orgasmic disorders were only reported in the venlafaxine group (17%). In males, there was a higher incidence in the venlafaxine group of the following AEs: dizziness (33% versus 11%), nausea (31% versus 14%), hyperhidrosis (25% versus 11%) and tension/agitation and bruxism (only in the venlafaxine group, in 14% and 11% of patients respectively).
Onset of the majority of the most frequent AEs occurred within the first 3 weeks of treatment (except for dizziness in the venlafaxine group which occurred over the first 3 months). Nausea was the event that had the earliest onset (within the first 2 days in the majority of cases).
After the end of treatment, the incidence of AEs (particularly dizziness and headache) was greater in the venlafaxine group (14% versus 7% in the milnacipran group).
Five patients in the milnacipran group and 7 patients in the venlafaxine group experience at least one serious AE (SAE) (). One death occurred during the study in a 46-year-old patient who was receiving milnacipran 150 mg/day, following the rupture of a cerebral arterial aneurysm (D88). This event was judged by the investigator to be unrelated to the study treatment. Three SAEs (all in the venlafaxine group) were judged to be possibly related to the study treatment (1 event of worsening depression, 1 of anxiety related to an overdose of venlafaxine and 1 of biliary colic). There were 4 cases of worsening depression (3 with venlafaxine and 1 with milnacipran), all of which led to hospitalization and had favorable outcome. The three treatment-emergent cases (2 with venlafaxine and 1 with milnacipran) all occurred in males and were severe. They all occurred either during or at the end of the uptitration period and they were the main reason for premature withdrawal from the study in 2 of the cases (1 from each treatment group).
Table 2 Serious adverse events
AEs which were potentially related to the study treatment and which led to definitive treatment withdrawal were reported in 17 patients (19%) in the milnacipran group (8 during uptitration, 1 at 100 mg, 4 at 150 mg and 4 at 200 mg) and 12 patients (13%) in the venlafaxine group (6 during uptitration, 3 at 150 mg, and 3 at 200 mg). They were as follows: i) divided equally between the two groups, 10 gastrointestinal events, 7 psychiatric events, 6 neurological events and 2 events of hyperhidrosis); ii) more frequent in the venlafaxine group: 5 psychiatric events (versus 2), iii) only observed in the milnacipran group: 6 events of genito-urinary problems in males (notably dysuria or testicular pain) and 4 events of increase in blood pressure or heart rate.
No clinically relevant biological abnormalities were observed, except for increased transaminases at week 8 in 1 patient in each group (ASAT 10 times the upper limit of normality [ULN] in 1 patient in the milnacipran group and ALAT and ASAT at 8.5 and 21 times the ULN respectively in 1 patient in the venlafaxine group). In both cases the values had returned to normal at week 24.
The two products showed a tendency to increase heart rate (mean increase of +4 bpm and +6 bpm at week 24 with venlafaxine and milnacipran respectively). There was also a tendency for increased systolic and diastolic blood pressure (SBP and DBP), which was more marked with milnacipran (on average at week 24, for both SBP and DBP: +1 mmHg with venlafaxine and +4 mmHg with milnacipran).
There was no clinically significant lengthening of corrected QT ECG interval in either group.
Discussion
The objective of this multicenter, controlled, randomized, double-blind study was to evaluate the efficacy and safety/tolerability of long term administration of milnacipran at flexible doses of up to 200 mg/day in adults presenting with a moderate or severe episode of MDD. Venlafaxine was chosen as the active control in this study and has recently been described as the treatment of reference for MDD.Citation16,Citation17
In the present study, which was carried out in 195 adults suffering from MDD, the efficacy profile of the two SNRIs at identical doses was found to be similar. The rates of responders and remissions showed no relevant differences between groups at all evaluation time-points, from 8 weeks to 6 months. In clinical practice, achieving a long-lasting remission remains the major objective; after 6 months of treatment, the rate of remission in the two treatments groups (>50%) was greater than that normally reported in the literature.Citation18 The similarity of the efficacy profiles of these two dual-aminergic antidepressants in this long term study indicates the strength of action of milnacipran in MDD. In addition, milnacipran has been shown to have comparable efficacy to clomipramine, another reference treatment for major depression, when given at equivalent doses during a period of 6 months.Citation19
Regarding safety/tolerability, the similar incidence in both groups of symptoms related to the serotonergic and noradrenergic modes of action of the two molecules suggests that milnacipran and venlafaxine, at the doses administered (mainly 150 or 200 mg/day) have similar selectivity at NA and 5-HT receptors. It was noted that in men, orgasmic disorders were more frequent with venlafaxine, while urinary symptoms were more frequent with milnacipran.
The statistical power required to carry out a non-inferiority analysis was not achieved. However, when considering this limitation, it should be noted that the level of recruitment required to reach this statistical power proved to be unfeasible for a double-blind trial over this study duration (6 months). The statistical comparisons of efficacy presented here must therefore be considered to be exploratory. This does not affect the interest and the originality of this study, the sample size of which is still considerable clinically.
One of the strengths of this study was the total length of the protocol, which had the objective to evaluate the two treatments over a long period whilst maintaining the double-blind control for 6 months of follow-up. For the outpatient follow-up period, the rate of study withdrawal was reasonable (more than two-thirds of patients finished the study), taking into account the fact that similar withdrawal rates have been reported in the literature for follow-up periods which were often considerably shorter.Citation20,Citation21 In addition, the conditions in which this study was carried out are similar to real-life practice; outpatient follow-up of patients, use of flexible doses and moderate and severe forms of the disorder.
Overall, the results of this study show that milnacipran and venlafaxine have similar efficacy and safety profiles when administered at flexible doses of up to 200 mg/day. As suggested by a recent meta-analysis,Citation16 this study confirms milnacipran’s place among the most effective antidepressant molecules.
Acknowledgements
The authors would like to thank the investigating psychiatrists:
M Abbar (30006 Nîmes), I Amado (75074 Paris), J Audet* (16000 Angoulême), GP Badet (21033 Dijon), B Baranovski* (35000 Rennes), R Benadhira (93200 Saint-Denis), B Bonin (21033 Dijon), F Boulet (30006 Nîmes), C Bourbon* (31400 Toulouse), P Bourgoin* (16000 Angoulême), Y Caer (30006 Nîmes), M Cazenave (33076 Bordeaux), N Chapel (13274 Marseille), F Chapelle* (31300 Toulouse), JC Chauvet-Gelinier (21033 Dijon), G Collin (34295 Montpellier), P Courtet (34295 Montpellier), R Didi (21033 Dijon), W De Carvalho (75116 Paris), D Drapier (35703 Rennes), J Farisse (13274 Marseille), M Faure* (37000 Tours), G Fischman (75005 Paris), C Gaussares* (33120 Arcachon), C Géraud* (53200 Château-Gontier), GC Girod (21033 Dijon), R Gourevitch (75074 Paris), JM Guibaud* (31200 Toulouse), B Kastler (67091 Strasbourg), D Januel (93200 Saint-Denis), Pr F Juan (44035 Nantes), Pr C Lancon (13274 Marseille), F Lanvin* (59139 Wattignies), P Leclercq* (68100 Mulhouse), N Le Garzic (35703 Rennes), P Le Goubey* (50100 Cherbourg), T Loiseau* (78500 Sartrouville), Pr B Millet (35703 Rennes), D Modavi (31200 Toulouse), R Mhiri* (78230 Le Pecq), E Neuman* (78230 Le Pecq), N Parant-Lucena* (31000 Toulouse), M Patris (67091 Strasbourg), J Pon* (31200 Toulouse), S Torres (34295 Montpellier), B Trojak (21033 Dijon), JM Vanelle (44035 Nantes), A Viala (75674 Paris).
*Members of the group GICIPI (Groupe d’investigateurs cliniciens indépendants pour des études pivotales de qualité [Group of independent clinical investigators for quality pivotal studies]).
A report of this study has been published in French.22
The study was sponsored by Pierre Fabre Médicament, manufacturers of milnacipran.
Disclosures
AM and MR are employees of Pierre Fabre Médicament.
References
- WeissmanMMBlandRCCaninoGJCross national epidemiology of major depression and bipolar disorderJAMA1996272932998656541
- LépineJPGastparMMendlewiczJTyleeADepression in the community: the first pan-European study DEPRES (Depression Research in European Society)Int Clin Psychopharmacol199711929
- MurrayCJLLopezAThe Global Burden of Disease (volume I in The Global Burden of Disease and Injury Series) Harvard University Press Boston, MA, USA1996
- GuzeSBRobinsESuicide and primary affective disordersBr J Psychiatry19701174374385481206
- Le PapeALecomteTPrévalence et prise en charge médicale de la dépressionFrance 1996–1997. CREDES Paris1999
- MachadoMIskedjianMRuizIEinarsonTRRemission, dropouts, and adverse drug reaction rates in major depressive disorder: a meta-analysis of head-to-head trialsCurr Med Res Opin2006221825183716968586
- StahlSMGradyMMMoretCBrileyMSNRIs: their pharmacology, clinical efficacy, and tolerability in comparison with other classes of antidepressantsCNS Spectr20051073274716142213
- AnsseauMVon FrenckellRGérardMAInterest of a loading dose of milnacipran in endogenous depressive inpatients. Comparison with the standard regimen and with fluvoxamineEur Neuropsychopharmacol199111131211821700
- HayashiMMimuraMOtsuboTKamijimaKEffect of high-dose milnacipran in patients with depressionNeuropsychiatr Dis Treat2007369970219300602
- OkumuraKFurukawaTARemission rates with milnacipran 100 mg/day and 150 mg/day in the long term treatment of major depressionClin Drug Invest200626135142
- ClauwDJMeasePPalmerRHGendreauRMWangYMilnacipran treatment for fibromyalgia syndrome: one-year durability of responseNeurology200870suppl 1A162 (abstract)
- MeasePJClauwDJGendreauRMThe efficacy and safety of milnacipran for treatment of fibromyalgia: a randomized, double-blind, placebo-controlled trialJ Rheumatology200936112
- LecrubierYWeillerEHerguetaTMINI Mini International Neuropsychiatric Interview. French version 5.0.0Hôpital de la Pitié Salpétrière, INSERM, Paris;1998
- MontgomerySAAsbergMA new depression scale designed to be sensitive to changeBr J Psychiatry197913382389444788
- GuyWClinical Global ImpressionsECDEU Assessment Manual for PsychopharmacologyPublication ADM No. 76–338National Institute of Mental Health Rockville, MD, USA1976
- MontgomerySABaldwinDSBlierPWhich antidepressants have demonstrated superior efficacy? A review of the evidenceInt Clin Psychopharmacol20072232332917917550
- BauerMTharmanathanPVolzHPMoellerHJFreemantleNThe effect of venlafaxine compared with other antidepressants and placebo in the treatment of major depression: a meta-analysisEur Arch Psychiatry Clin Neurosci200925917218519165525
- KasperSEveryday problems in treating depression: focus on SNRIsCNS Spectr2008137 Suppl 114518622367
- SteenADen BoerJAA double-blind six months comparative study of milnacipran and clomipramine in major depressive disorderInt Clin Psychopharmacol19971252692819466161
- DeakinJFWDoes selectivity matter?Int Clin Psychopharmacol199611Suppl 113178732439
- MöllerHJVolzHPDrug treatment of depression in the 1990s: an overview of achievements and future possibilitiesDrugs1996526256389118813
- OliéJPGourionDMontagneARostinMPoirierMFMilnacipran et venlafaxine à posologie flexible (jusqu’à 200 mg/j) dans le traitement ambulatoire des épisodes dépressifs majeurs modérés à sévères de l’adulte: étude exploratoire de 24 semaines, randomisée en double insuEncephale20093559560420004291