Abstract
Objective
To determine the effect of adjunctive quetiapine therapy on the sleep architecture of patients with bipolar or unipolar depression.
Methods
This is a prospective, single-blind, repeated measures polysomnographic study. Sleep architecture was analyzed by overnight polysomnography, and subjective sleep quality was measured using the Pittsburgh Sleep Quality Index. The Hamilton Rating Scale for Depression, Montgomery Asberg Depression Rating Scale, Young Mania Rating Scale, and Clinical Global Impression-Severity Scale were employed to quantify changes in illness severity with adjunctive quetiapine treatment. Polysomnographs and clinical measures were administered at baseline, after 2–4 days of treatment, and after 21–28 days of quetiapine treatment. The average dose of quetiapine was 155 mg, ranging from 100–200 mg.
Results
Adjunctive quetiapine therapy did not significantly alter sleep efficiency, sleep continuity, or Pittsburgh Sleep Quality Index scores. Respiratory Disturbance Index and percentage of total time in rapid eye movement (REM) sleep significantly decreased and the percentage of total time in non-REM sleep, and duration of Stage 2 and non-REM sleep significantly increased after 2–4 days of quetiapine treatment. Illness severity significantly decreased over time.
Conclusions
Adjunctive quetiapine treatment alters sleep architecture in patients with major depressive disorder or bipolar disorder, which may partially explain its early antidepressant properties. Changes in sleep architecture are more robust and significant within two to four days of starting treatment.
Introduction
Quetiapine is an atypical antipsychotic agent and is approved by the United States Food and Drug Administration to treat schizophrenia, major depressive disorder (MDD) and both manic and depressive episodes associated with bipolar disorder. The drug acts as an antagonist at serotonin (5-HT1A and 5-HT2), dopamine (D1 and D2), histamine (H1), and adrenergic α1 and α2 receptors.Citation1 Results from the BOLDER I and II studies, which were two large, multicenter, randomized, placebo-controlled trials, show that quetiapine is efficacious in the treatment of acute bipolar depression as monotherapy.Citation2,Citation3 These studies led to guidelines recommending quetiapine for use as first-line monotherapy treatment for bipolar depression.Citation4 Use of an atypical antipsychotic as an adjuvant therapy with selective serotonin reuptake inhibitors (SSRIs) has shown benefits in large trials of olanzapine use with fluoxetine.Citation5,Citation6 The use of quetiapine as an adjunctive therapy has been shown to be beneficial in smaller, open-label studies of patients with bipolar depressive episodes.Citation7,Citation8 Large, randomized placebo-controlled trials have found the use of quetiapine as monotherapy, or in combination with another antidepressant, to be efficacious in the treatment of MDD.Citation9–Citation11
Patients with MDD and bipolar disorder frequently experience sleep disturbances.Citation12 Poor sleep quality and/or quantity are observed in up to 90% of depressed patients.Citation13 Insomnia is a risk factor for development of major depressive episodes and may precede the onset of depression in those with recurrent illness.Citation14–Citation16 Sleep disturbance is also a risk factor for suicide.Citation17 Studies of sleep architecture using polysomnography (PSG) have demonstrated that sleep in depressed patients tends to be characterized by decreased sleep efficiency, a reduction of slow-wave sleep (SWS), and a disinhibition of rapid eye movement (REM) sleep, manifested by a shortening of REM sleep latency, an increase in REM length, and an increased REM density.Citation18,Citation19
Low doses of quetiapine alter the sleep architecture of healthy individuals.Citation20,Citation21 Somnolence is a side effect of quetiapine treatment, leading to prescription in an off-label fashion when this side effect is desired.Citation22 The somnolence effect of quetiapine is thought to arise from its 5-HT2 and H1 receptor blockade capabilities, similar to those of medications used as sedatives, including the antidepressants mirtazapine, trazodone, and trimipramine. Qualitative sleep studies using the Pittsburgh Sleep Quality Index (PSQI) have shown improvements in the sleep quality of depressed patients after initiation of quetiapine therapy.Citation23,Citation24
The effect of quetiapine on sleep architecture, measured by PSG, has not been studied in patients experiencing a major depressive episode. Clinical practice indicates that quetiapine has sedating properties, and its sedative effects may play an important role in restoring quality of sleep in patients with MDD or bipolar disorder who frequently experience sleep disturbances as part of their illness. We hypothesize that quetiapine alters the subjective sleep quality and PSG sleep architecture of patients with MDD or bipolar disorder. We expect that quetiapine will improve sleep efficiency, decrease sleep latency and duration of REM sleep, and increase the duration of SWS. If such changes occur and can be related to the improvement of depressive symptomatology, it is suggested that part of quetiapine’s antidepressant effect may be achieved through its restoration of sleep architecture.
Methods
Study design
This was a four-week, single-blind, open-label, repeated measures PSG study of patients with MDD or bipolar disorder currently experiencing a major depressive episode, who received quetiapine fumarate treatment in addition to their current medication regime. Patients were recruited from a single-center, tertiary care mood disorders clinic. The study was approved by the Queen’s University Health Sciences and Affiliated Teaching Hospitals Research Ethics Boards as well as by the Health Canada Therapeutics Products Directorate.
Study population
Patients were recruited from inpatient and outpatient services for the evaluation and treatment of depression. All participants were 18 years of age or older and gave written informed consent. All patients had a Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) diagnosis of bipolar disorder (Type 1, Type 2, or not otherwise specified) or MDD, and were currently experiencing a major depressive episode (Hamilton Depression Rating Scale-17 item [HDRS-17] ≥ 15, Young Mania Rating Scale [YMRS] ≤ 8). Participants with a previous diagnosis of schizophrenia and/or current substance abuse (except caffeine and nicotine) were excluded. Prior antipsychotic use including quetiapine was allowed, but antipsychotics had to have been discontinued at least one week prior to study initiation. Sleep aids, including over-the-counter hypnotics, were discontinued at least one week prior to study initiation, and changes to baseline medications were not allowed in the three weeks prior to participation or during the study. Study participants were withdrawn from the study if they experienced significant side effects or withdrew consent.
Fifteen patients were enrolled in the trial. Four patients voluntarily withdrew from the study before completing a post-baseline PSG, two citing excessive sedation, one a post-menopausal bleed, and one a headache, and thus were not included in the completer analysis population. Therefore, only 11 patients were included in the analysis.
The completer population consisted of nine females and two males, with a mean age of 44.3 (± 9.1) years. Five patients had a diagnosis of MDD and six patients had a diagnosis of bipolar disorder. Ten of 11 patients were taking antidepressants (five venlafaxine, one sertraline, two bupropion, one citalopram, and one a combination of bupropion and venlafaxine). Two patients were taking lithium and topiramate as mood stabilizers. Five patients possibly took sedating medications during the trial; three were taking established regimens of regular benzodiazepines prescribed as anxiolytics (one lorazepam, one clonazepam, and one a combination of oxazepam and lorazepam) and two were taking regular trazodone prescribed as an antidepressant. One patient had occasional sleep difficulties surrounding nocturnal priapism and another patient had obstructive sleep apnea and was being treated with continuous positive airway pressure.
Quetiapine treatment
Quetiapine was given in tablet form and taken at night near bedtime. An initial dose of 50 mg was given and the dose was titrated up over the course of the study, with drug tolerance as the primary influence on titration. The average dose of quetiapine achieved was 155 mg, ranging from 100–200 mg. A fourth phase of the study, involving further titration of quetiapine to a target dose of 600 mg per day and PSG, PSQI, and mood scales at days 42–48 was optional, and is not included here due to poor participation (five of 11 patients of the completer group).
Clinical assessments
Patients were assessed at three time points, ie, baseline (before administration of study medication), and after 2–4 days and 21–28 days of quetiapine treatment. Each clinical assessment consisted of the HDRS-17, Montgomery Asberg Depression Rating Scale (MADRS), YMRS, Clinical Global Impression Severity (CGI-S) scale, and participant-reported PSQI.
Polysomnography
Objective sleep architecture measurements were derived from PSG data of study participants at defined intervals throughout the study period. A baseline PSG was taken on the day before quetiapine administration began, then once during days 2–4, and once during days 21–28. On each of the three study nights, sleep PSGs were set up by a qualified technician and recorded using the MediPalm Personal Recording Device (Braebon Corp., Ogdensburg, NY) while the patient slept at home. Patients were asked to retire and rise at their usual time. The standard overnight PSG sleep study included four electroencephalogram channels (C4-A1, C3-A2, O2-A1, O1-A2), electro-oculogram (two channels), submental electromyogram (EMG), pulse oximetry, oronasal airflow (oronasal thermistor), chest and abdominal movement (respiratory inductance plethysmography), and tibialis anterior EMG. A position sensor was used to monitor position continuously (Ultima Body Position Sensor; Braebon Corp., Ogdensburg, NY). The overnight sleep routine was applied starting at around 1900 hours each study night. Recording began at approximately 2100 hours, and ran for eight hours continuously or until the participant rose in the morning. One of two certified PSG analysts, different from the technician setting up the PSG and blinded to study design and day of study, scored the sleep record in 30-second segments according to the standardized criteria of Rechtschaffen and Kales,Citation25 using Pursuit Advanced Sleep System software (Braebon Corp., Ogdensburg, NY).
Sleep onset was defined as the beginning of the first two minutes that were not scored as awake or movement. Latencies to each sleep stage were calculated to the first two continuous minutes of the stage. Obstructive apneas and hypopneas were scored using the criteria from the American Academy of Sleep Medicine Task Force.Citation26 Events were scored when a > 50% decrease (apnea) in airflow, or clear reduction (hypopnea) in amplitude of the airflow signal (compared with stable breathing during the two minutes preceding the event), occurred associated with an arousal, a greater than 3% reduction in oxygen saturation (SaO2), or both, and the event lasted for at least 10 seconds. Arousals were scored based on American Sleep Disorders Association criteria.Citation27 Arousals had to be preceded by at least 10 seconds of sleep, have an electroencephalogram frequency shift to alpha or theta for at least three seconds and up to 15 seconds, and be associated with concurrent increased EMG tone in REM sleep. The respiratory disturbance index (RDI), which included apneas, hypopneas, and snore arousals for the number of events per hour of sleep, was calculated. Sleep efficiency (percentage) was calculated as the total sleep time divided by the total time in bed, multiplied by 100.
Statistical analysis
The completer analysis population was comprised of the patients who had initiated quetiapine during the trial and had both a baseline and at least one post-baseline PSG measurement. Calculation of the sample size was based on choosing sleep efficiency as the primary outcome measure and estimating that a 15% improvement in sleep efficiency would be a clinically meaningful finding in this population. In the power calculation, we have used baseline sleep efficiency for depressed population to be 67.4 with a standard deviation (SD) ± 18.88.Citation28 In order to detect a 15% improvement in sleep efficiency using one-sided normal distribution paired t-test analysis with a significance of 0.05 and 80% power, a total of 12 (11.277) patients is required. A sample size of 15 patients was used to allow for patient drop out.
Statistical analysis of data was done using the SPSS 17.0.1 for PC (SPSS Inc, Chicago, IL). Nonparametric analysis using the Wilcoxon Signed Rank test for two related samples, comparing pre- and post-treatment measures, was used because sample sizes were small and often not normally distributed few pairwise comparisons in which both sets of data were normally distributed, additional analysis using two-sided normal distribution paired t-test analysis with a significance of 5% was undertaken. Three of the 11 patients missed their day 21–28 PSG, one of whom had values substituted from their day 42–48 PSG administered during the optional extension period. An attempt to use multiple regression analysis to approximate missed PSG data failed to generate values consistent with prior observed values and, thus, for t-tests, the last observation was carried forward and substituted for missing values.
Results
Sleep efficiency
Quetiapine did not significantly alter sleep efficiency. Mean sleep efficiency (± SD) was 69.8% (± 20.6), 78.4% (± 9.7), and 74.5% (± 17.4) at baseline, and days 2–4, and days 21–28, respectively (P = 0.48 and P = 0.21, , ).
Figure 1 Mean ± standard error of sleep efficiency (percentage) over one month of adjunctive quetiapine treatment in 11 subjects. Sleep was assessed by polysomnography at baseline, and after 2–4 days and 21–28 days following initiation of quetiapine treatment. There was no significant difference in sleep efficiency after 2–4 days or one month of quetiapine treatment.
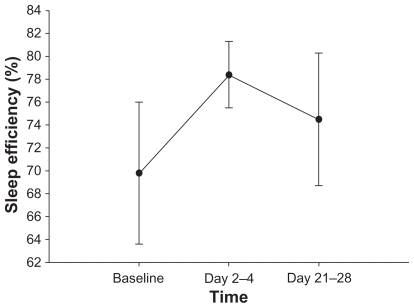
Table 1 Sleep continuity and sleep architecture measures over one month of adjunctive quetiapine treatment in 11 subjects
Sleep continuity
No significant changes in total sleep time (P = 0.18), number of awakenings (P = 0.72), time in bed (P = 0.66), and latency to sleep onset (P = 0.51) were identified after initiation of quetiapine. A significant fall in the RDI was observed after 2–4 days of quetiapine treatment compared with baseline (P = 0.041). However, RDI then increased and there was no significant difference in RDI after 21–28 days of quetiapine treatment compared with pretreatment initiation (P = 0.86, ).
Sleep architecture
Non-REM sleep
Acute quetiapine treatment significantly altered non-REM sleep stage characteristics, but longer-term treatment did not. The duration of both total Stage 2 sleep (P = 0.016) and total non-REM sleep (P = 0.05), as well as the percentage of total sleep time in non-REM sleep (P = 0.033) significantly increased 2–4 days after treatment initiation, compared with baseline, and then decreased towards baseline values. However, no significant difference in the above sleep measures was found between baseline and 21–28 days after treatment initiation ().
After 2–4 days of quetiapine treatment, total time in Stage 1 sleep (P = 0.16), percentage of total sleep time in Stage 1 sleep (P = 0.86), percentage of total sleep time in Stage 2 sleep (P = 0.06), percentage of total sleep time in SWS (P = 0.11), and total time in SWS (P = 0.20) were not significantly altered. After 3–4 weeks of treatment, percentage of total sleep time in Stage 1 sleep (P = 0.86), percentage of total sleep time in Stage 2 sleep (P = 0.95), total time in Stage 2 sleep (P = 0.86), percentage of total sleep time in non-REM sleep (P = 0.44), total time in non-REM sleep (P = 0.89), total time in SWS (P = 0.48), and percentage of total sleep time in SWS (P = 1.0) were not significantly altered compared with baseline measurements ().
REM sleep
Quetiapine initially altered REM sleep stage characteristics, but no significant changes from pretreatment measurements were observed after 21–28 days. The percentage of total sleep time in REM sleep significantly decreased 2–4 days after quetiapine treatment initiation (P = 0.033), although there was no significant difference in percentage of total sleep time in REM sleep between pretreatment and 3–4 weeks post-treatment (P = 0.49, , ). After 2–4 days of treatment, there was no significant difference in latency to REM sleep (P = 0.86) and total time in REM sleep (P = 0.18) compared with pretreatment measurements. Three to four weeks of quetiapine treatment did not significantly alter latency to REM sleep (P = 0.59), percentage of total sleep time in REM sleep (P = 0.49), and total time in REM sleep (P = 0.49, ).
Table 2 PSQI scores over one month of quetiapine adjunctive treatment in 11 subjects
Subjective sleep quality
PSQI scores did not significantly change between baseline, 2–4 days, and 21–28 days after initiation of quetiapine treatment, although PSQI scores tended to decrease over time (P > 0.05). After 21–28 days of quetiapine treatment, the subjective sleep quality subscore of the PSQI was significantly lower than before treatment (P < 0.05), indicating that quetiapine significantly improved subjective sleep quality after four weeks. However, there was no significant difference in subjective sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, and sleep dysfunction subscores after 2–4 days or 21–28 days of treatment compared with baseline, although scores declined with the exception of habitual sleep efficiency, which remained unchanged ().
Mood
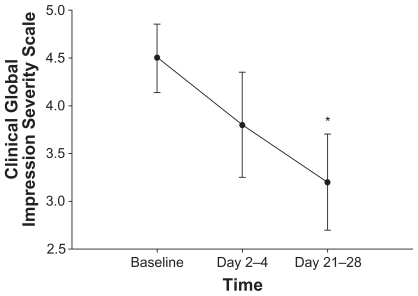
Quetiapine significantly improved mood and decreased depressive symptoms. Total MADRS scores significantly declined from baseline to days 2–4 (P = 0.01) and to days 21–28 (P = 0.008) of quetiapine treatment, to mean scores (± SD) of 28.1 (± 7.0), 22.4 (± 7.9), and 14.1 (± 8.4), respectively (). Both HDRS and CGI-S total scores significantly decreased (P = 0.01 and P = 0.02) from baseline mean scores (± SD) of 22.3 (± 4.4) and 4.5 (± 1.2), respectively, to mean scores of 12.0 (± 5.8) and 3.2 (± 1.7) after 21–28 days of quetiapine treatment ( and ). YMRS mania scores remained low and statistically unchanged over time.
Figure 2 Mean ± standard error of the MADRS score over one month of adjunctive quetiapine treatment in 11 subjects. MADRS score was assessed at baseline, after acute treatment (2–4 days), and after longer-term treatment (21–28 days) with quetiapine. After 2–4 days and 21–28 days of quetiapine treatment, MADRS scores significantly decreased from baseline measurements (P = 0.01 and P = 0.008, respectively). *P < 0.05 compared with baseline. Abbreviation: MADRS, Montgomery Asperg Depression Rating Scale.
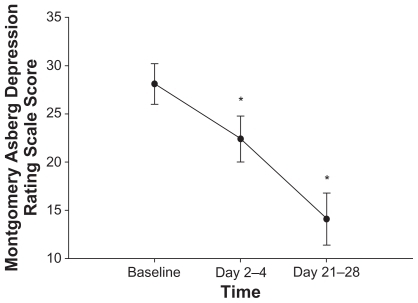
Figure 3 Mean ± standard error of the HDRS score over one month of quetiapine adjunctive treatment in 11 subjects. HDRS-17 score was assessed at baseline, after acute treatment (2–4 days), and after longer-term treatment (21–28 days) with quetiapine. After 21–28 days of quetiapine treatment, HDRS scores significantly decreased from baseline measurements (P = 0.01). *P < 0.05 compared with baseline. Abbreviation: Hamilton Rating Scale for Depression.
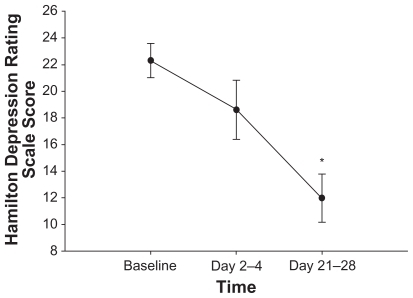
Figure 4 Mean ± standard error of the CGI-S score over one month of quetiapine adjunctive treatment in 11 subjects. CGI-S score was assessed at baseline, after acute treatment (2–4 days) and after longer-term treatment (21–28 days) with quetiapine. After 21–28 days of quetiapine treatment, CGI-S scores significantly decreased from baseline measurements (P = 0.02). *P < 0.05 compared with baseline. Abbreviation: CGI-S, Clinical Global Impression Severity Scale.
Discussion
This study demonstrates that adjunctive quetiapine treatment improves self-reported sleep quality, decreases illness severity, and initially alters sleep architecture in depressed patients. Alterations in sleep architecture are no longer seen after a longer treatment span. Specifically, quetiapine significantly increased total Stage 2 sleep time and total time spent in non-REM sleep, as well as decreased the percentage of total sleep time in REM sleep and the RDI after 2–4 days of treatment. However, these significant effects were not seen after 21–28 days of treatment. Depression significantly improved after 3–4 weeks of treatment. Therefore, it is evident that quetiapine adjunctive treatment in depressed patients induces changes in sleep architecture that are more robust and significant upon acute treatment and tend to taper off, approaching pretreatment values after 3–4 weeks of treatment. Mood continues to improve over time, with illness severity lowest after 3–4 weeks of treatment. These results emphasize that quetiapine relieves depressive symptoms effectively and rapidly and produces mild sleep benefits, with the exception of slightly increasing the number of awakenings and decreasing the latency to REM sleep.
Improvements in sleep continuity following quetiapine treatment have been observed in individuals who are not depressed.Citation21,Citation29 On the first night of treatment, Cohrs et al found that quetiapine significantly increased sleep period time, total sleep time, and sleep efficiency in healthy individuals.Citation29 Moreover, in an open-label trial of patients with insomnia, total sleep time and sleep efficiency measured by PSG increased, and this increase extended over weeks of quetiapine treatment.Citation21 To our knowledge, the current study is the first to investigate sleep architecture, as well as observe changes in sleep in depressed patients undergoing quetiapine treatment, and in particular, patients who are resistant to SSRI treatment. It is important to note that pretreatment sleep efficiency values, with a mean of 69.8 ± 20.6%, in this study are comparable with those of other PSG studies in depressed patients (67.4 ± 18.9%),Citation28 indicating that there was no selection bias regarding the baseline sleep quality of participants in this study.
The effects of atypical antipsychotics on sleep architecture in depressed patients are not well documented. However, similar improvements in sleep following quetiapine treatment are also observed in the few other antipsychotics that have been studied. Adjunctive olanzapine treatment improves sleep continuity as well as increases percentage of sleep time in both non-REM sleep and SWS sleep in treatment-resistant patients with MDD.Citation30 Risperidone decreases REM sleep and increases Stage 2 sleep in SSRI-resistant depressed patients.Citation31 This study shows that quetiapine increases non-REM sleep, as does olanzapine, in addition to increasing Stage 2 sleep and decreasing REM sleep, as does risperidone. Quetiapine appears to alter sleep architecture initially in a manner similar to other medications in its class; however, unlike treatment with other antipsychotics, these improvements in sleep do not last.
Of particular interest is the finding that quetiapine suppressed REM sleep, as identified by decreased percentage of REM sleep. Depression is associated with REM sleep abnormalities including decreased REM latency, increased REM density, and increased REM time.Citation32,Citation33 It is possible that an initial decrease in REM sleep may be related to acute improvements in mood following quetiapine treatment; however, longer-term improvements in depressive symptomatology are not related to changes in sleep.
The sleep-inducing properties of quetiapine are most likely related to the medication’s receptor-binding profile. Quetiapine is an antagonist at H1 receptors, inducing sleep upon inhibition of histamine synthesis. Histaminergic activity has more recently been implicated in the development of depression. Patients with depression show a decrease in H1 receptor binding, and this decrease is correlated with illness severity.Citation34 Therefore, the modulation of histaminergic activity by quetiapine to induce sleep may be related to the reduction of depressive symptoms in these patients.
The most substantial changes in sleep occurred after 2–4 days of treatment, after which these changes began to decline, yet patients experienced the greatest improvement in mood after 21–28 days of quetiapine treatment. This leads us to conclude that, while psychotropic medications may act to reduce depressive symptoms by improving the sleep of patients with depression or bipolar disorder, sleep improvements are only a part of the medication’s therapeutic action. Improvements in sleep contribute to a therapeutic response, but do not account on their own for the reduction in depressive symptomatology seen after quetiapine treatment.
The main limitations in this study are its open-label design, small sample size, lack of a control group, and a study population of predominantly women. Furthermore, sleep studies such as this one, acquire PSG data at distinct time points which may not be representative of the entire week. This study should be repeated as a randomized, placebo-controlled, double-blind assessment study with a larger sample size.
Conclusion
Acute quetiapine adjunctive therapy alters sleep architecture in depressed patients, after which these changes taper off towards baseline levels and are not significantly present after longer-term treatment. This pilot study suggests that further investigation of the effect of quetiapine on sleep architecture is warranted in patients experiencing a major depressive episode.
Acknowledgments
The authors would like to thank Alan Lowe, Regina du Toit, Judy Joannette, Teresa Garrah, Liane Tackaberry, Ann Shea and Dianne Groll.
Disclosure
This project was supported by an unrestricted research initiation grant from AstraZeneca.
LG, LL, DM and RJ declare no conflict of interest.
RM is on Speaker/Advisory Boards for, or has received research funds from: AstraZeneca, Biovail, BrainCells Inc., Canadian Network for Mood & Anxiety Treatments, Eli Lilly, Janssen-Ortho, Lundbeck, Pfizer, Servier, Takeda, Wyeth, Bristol-Myers Squibb and Merck.
References
- NemeroffCBKinkheadBGoldsteinJQuetiapine: Preclinical studies, pharmacokinetics, drug-interactions, and dosingJ Clin Psychiatry20026351112562141
- CalabreseJRKeckPEMacfaddenWA randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depressionAm J Psychiatry20051621351136015994719
- ThaseMEMacfaddenWWeislerRHEfficacy of quetiapine monotherapy in bipolar I and II depression: A double-blind, placebo-controlled study (the BOLDER II study)J Clin Psychopharm200626600606
- YathamLNKennedySHO’DonovanCCanadian network for mood and anxiety treatments (CANMAT) guidelines for the management of patients with bipolar disorder: Update 2007Bipolar Disord2006872173917156158
- TohenMVietaECalabreseJEfficacy of olanzapine and olanzapine/fluoxetine combination in the treatment of bipolar I depressionArch Gen Psychiatry2003601079108814609883
- CoryaSAWilliamsonDSangerTMA randomized, double-blind comparison of olanzapine/fluoxetine combination, olanzapine, fluoxetine, and venlafaxine in treatment-resistant depressionDepress Anxiety2006119
- PathakSJohnsESKowatchRAAdjunctive quetiapine for treatment-resistant adolescent major depressive disorder: A case seriesJ Child Adolesc Psychopharmacol20051569670216190801
- MilevRAbrahamGZaheerJAdd-on quetiapine for bipolar depression: A 12-month open-label trialCan J Psychiatry20065152353016933589
- CutlerAMontgomerySFeifelDLazarusAAstromMBrecherMExtended release quetiapine fumarate monotherapy in major depressive disorder: a placebo- and duloxetine-controlled studyJ Clin Psychiatry20097052653919358790
- El-KhaliliNJoyceMAtkinsonSExtended-release quetiapine fumarate (quetiapine XR) as adjunctive therapy in major depressive disorder (MDD) in patients with an inadequate response to ongoing antidepressant treatment: A multicentre, randomized, double-blind, placebo-controlled studyInt J Neuropsychopharmacol201023116
- BauerMPretoriusHWConstantELEarleyWRSzamosiJBrecherMExtended-release quetiapine as adjunct to an antidepressant in patients with major depressive disorder: Results of a randomized, placebo-controlled, double-blind studyJ Clin Psychiatry20097054054919358791
- Diagnostic and Statistical Manual of Mental Disorders4th Ed, Text Revision (DSM-IV-TR)Washington, DCAmerican Psychiatric Association2000
- RiemannDBergerMVoderholzerUSleep and depression – results from psychobiological studies: An overviewBiol Psychol2001576710311454435
- BreslauNRothTRosenthalLAndreskiPSleep disturbance and psychiatric disorders: A longitudinal epidemiological study of young adultsBiol Psychiatry1996394114188679786
- FordDECooper-PatrickLSleep disturbances and mood disorders: An epidemiologic perspectiveDepress Anxiety2001143611568977
- PerlisMGilesDEBuysseDJTuXKupferDJSelf-reported sleep disturbance as a prodromal symptom in recurrent depressionJ Affect Disord1997422092129105962
- LiuXBuysseDJSleep and youth suicidal behavior: A neglected fieldCurr Opin Psychiatry20051928829316612215
- TsunoNBessetARitchieKSleep and depressionJ Clin Psychiatry2005661254126916259539
- ThaseMEDepression and sleep: Pathophysiology and treatmentDialogues Clin Neurosci2006821722616889107
- CohrsSRodenbeckAGuanZSleep-promoting properties of quetiapine in healthy subjectsPsychopharmacology (Berl)200417442142915029469
- WiebandMHLandryFBrücknerTQuetiapine in primary insomnia: A pilot studyPsychopharmacology (Berl)200819633733817922110
- LindenMThielsCEpidemiology of prescriptions for neuroleptic drugs: Tranquilizers rather than antipsychoticsPharmacopsychiatry20013415015411518477
- BauneBTCaliskanSTodderDEffects of adjunctive antidepressant therapy with quetiapine on clinical outcome, quality of sleep and daytime motor activity in patients with treatment-resistant depressionHum Psychopharmacol2007221917191266
- EndicottJPaulssonBGustafssonUSchiölerHHassanMQuetiapine monotherapy in the treatment of depressive episodes of bipolar I and II disorder: Improvements in quality of life and quality of sleepJ Affect Disord200811130631918774180
- RechtschaffenAKalesAA Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human SubjectsBethesda, MDNational Institutes of Health1968
- Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task ForceSleep19992266768910450601
- EEG arousals: Scoring rules and examples: A preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders AssociationSleep19921517318411032543
- KerkhofsMLinkowskiPLucasFMendelwiczJTwenty-four hour patterns of sleep in depressionSleep1991145015061798882
- CohrsSRodenbeckAGuanZSleep-promoting properties of quetiapine in healthy subjectsPsychopharmacology (Berl)200417442142915029469
- SharpleyALAttenburrowMEHafiziSCowenPJOlanzapine increases slow wave sleep and sleep continuity in SSRI-resistant depressed patientsJ Clin Psychiatry20056645045415816787
- SharpleyALBhagwagarZHafiziSRisperidone augmentation decreases rapid eye movement sleep and decreases wake in treatment-resistant depressed patientsJ Clin Psychiatry20036419219612633128
- BencaRMObermeyerWHThistedRAGillinJCSleep and psychiatric disorders: A meta-analysisArch Gen Psychiatry1992496516681386215
- BencaROkawaMUchiyamaMSleep and mood disordersSleep Med Rev19971455615310523
- KanoMFukudoSTashiroADecreased histamine H1 receptor binding in the brain of depressed patientsEur J Neurosci20042080381015255990