Abstract
A number of studies have noted that the pattern of resting frontal brain electrical activity (EEG) is related to individual differences in affective style in healthy infants, children, and adults and some clinical populations when symptoms are reduced or in remission. We measured self-reported trait shyness and sociability, concurrent depressive mood, and frontal brain electrical activity (EEG) at rest and in anticipation of a speech task in a non-clinical sample of healthy young adults selected for high and low social anxiety. Although the patterns of resting and reactive frontal EEG asymmetry did not distinguish among individual differences in social anxiety, the pattern of resting frontal EEG asymmetry was related to trait shyness after controlling for concurrent depressive mood. Individuals who reported a higher degree of shyness were likely to exhibit greater relative right frontal EEG activity at rest. However, trait shyness was not related to frontal EEG asymmetry measured during the speech-preparation task, even after controlling for concurrent depressive mood. These findings replicate and extend prior work on resting frontal EEG asymmetry and individual differences in affective style in adults. Findings also highlight the importance of considering concurrent emotional states of participants when examining psychophysiological correlates of personality.
Recent studies have found that the pattern of resting frontal EEG alpha asymmetry is predictive of individual differences in affective style in healthy adults and children, some clinically depressed and anxious populations even when their symptoms are in remission (for reviews see CitationDavidson 1993, Citation2000; CitationDavidson et al 2003; CitationCoan and Allen 2004), and adults with schizophrenia whose negative symptoms are reduced (CitationJetha et al in press). Normal adults (CitationSutton and Davidson 1997; CitationSchmidt 1999) and children (for reviews see CitationFox 1991; CitationFox et al 2001) who exhibit right frontal EEG asymmetry at rest are easily distressed, fearful, and shy, whereas those who exhibit left frontal EEG asymmetry at rest are socially outgoing and extraverted. Because the pattern of frontal EEG asymmetry at rest is stable across time (CitationTomarken et al 1992) and context (CitationSchmidt et al 2003) and its appearance early in life is predictive of later personality (CitationFox et al 2001), some have argued that this metric represents a “trait-like” marker of dispositional affective style (CitationDavidson 1993, Citation2000). Here we extend these earlier findings of studies with normal individuals by examining whether the pattern of frontal EEG alpha asymmetry at rest and in response to an anticipated speech distinguished among individual differences in social anxiety in a non-clinical sample of young adults.
We previously found that a non-clinical sample of adults who were selected for high and low social anxiety (ie, high and low shyness) displayed greater relative right frontal EEG activity at rest (CitationSchmidt 1999). Others have found greater relative right frontal EEG activity in a clinical group of socially phobic adults during anticipation of public speaking (CitationDavidson et al 2000). We also noted an increase in right, but not left, frontal EEG activity in typically developing shy children in anticipation of a social speech (CitationSchmidt et al 1999), but failed to find this relation in a non-clinical sample of shy and socially anxious adults in response to anticipated social interaction (CitationSchmidt and Fox 1994).
One possible reason for these inconsistencies among earlier studies may be the failure to consider concurrent mood. For example, disparate studies have noted relations between depression and right frontal brain activity at rest (CitationHenriques and Davidson 1990, Citation1991) as well as between depression and shyness (CitationSchmidt and Fox 1995), but there appear to be no studies that have examined the possibility that concurrent depressive mood may influence the predicted shy-right frontal EEG asymmetry relation in non-clinical participants.
In the present study, participants included a healthy, non-clinical sample of young adults selected for high (HSA) and low (LSA) social anxiety. Regional brain electrical activity (EEG) was indexed both at rest and while participants were preparing a speech in anticipation of being videotaped for others viewing. Participants also completed self-report measures of trait shyness and sociability and concurrent depressive mood. We predicted that the HSA group would exhibit greater relative right frontal EEG asymmetry at rest and during the speech preparation task than the LSA group. We also predicted that shyness would be related to greater relative right frontal EEG activity at rest and during the speech preparation task, while sociability would be related to greater relative left frontal EEG activity at rest and in response to the speech task, regardless of group, after controlling for concurrent depressive mood.
Method
Participants
Participants were 330 (84 males, 246 females; M age = 20.32 years, SD = 4.18 years) undergraduate students enrolled in psychology courses at McMaster University who completed a series of prescreening questionnaires and received academic credit for their participation.
Participant selection
Of the 330 participants, 90 scored ±1 SD above or below the mean on the Social Phobia Inventory (SPIN). These 90 individuals were contacted to participate in the study. Of these 90 participants, 10 were excluded because they were left-handed (CitationHeller and Levy 1981). Of the remaining 80, 49 (12 males, 37 females) agreed to participate in the study: 24 high socially anxious (HSA; 5 males, 19 females) and 25 low socially anxious (LSA; 7 males, 18 females). There were no significant differences in age, sex, or ethnicity distribution between the two groups. All participants were healthy with no history of psychiatric or neurological problems. Participants were given more academic credit points, a chance to win a $75 gift certificate from the university bookstore, or $10 cash for their participation.
Self-report measures
Social Phobia Inventory (SPIN)
The Social Phobia Inventory (SPIN) (CitationConnor et al 2000) is a 17-item self-report measure of fear, avoidance, and physiological symptoms associated with social phobia and more generally social anxiety. The SPIN specifically focuses upon fears of talking and socializing with others, and fears of experiencing and exhibiting physiological signs of anxiety such as blushing or sweating (eg, “I avoid giving speeches”). The SPIN has demonstrated good test-retest reliability, good internal consistency, convergent validity, divergent validity, and construct validity in clinical and non-clinical samples (CitationConnor et al 2000; CitationAntony et al 2006; CitationRadomsky et al 2006).
Cheek and Buss Shyness and Sociability Scale
The five highest loaded items (see CitationBruch et al 1989) from the 13-item revised Cheek and Buss Shyness Scale (CitationCheek 1983) were used to assess shyness (eg, “I find it hard to talk to strangers”). Sociability was assessed using the 5-item Cheek and Buss Sociability Scale (1981) (eg, “I find people more stimulating than anything else”). Reliability and validity data for the two scales are provided elsewhere (CitationCheek and Buss 1981; CitationBruch et al 1989).
Depression Anxiety Stress Scales: 21-item version (DASS-21)
The DASS-21 (CitationLovibond and Lovibond 1995) is a 21-item self-report measure designed to distinguish among depression, anxiety, and symptoms of general tension. The depression scale includes items that evaluate feelings of sadness and worthlessness (eg, “I couldn’t seem to experience any positive feeling at all”). The anxiety scale includes items that evaluate symptoms of physical arousal and fear (eg, “I was worried about situations in which I might panic and make a fool of myself”). The stress scale includes items that evaluate symptoms of general tension and irritability (eg, “I felt that I was using a lot of nervous energy”). The DASS-21 has good reliability and good concurrent validity in both clinical and community samples (CitationAntony et al 1998).
Procedure
All participants were tested at the Child Emotion Laboratory at McMaster University, Hamilton, Ontario. All procedures were approved by the McMaster University Research Ethics Board. Participants were asked to refrain from consuming excessive amounts of alcohol 24 hours prior to the study and to refrain from smoking or consuming any caffeine two hours prior to the study. Upon the participants’ arrival to the laboratory, informed consent was obtained, and participants were asked if they consumed any nicotine or caffeine within the previous two hours. No participant reported having either nicotine or caffeine two hours prior to arrival or alcohol in the previous 24 hours. All participants were medication free and reported that they were not experiencing any extraordinarily stressful life events (eg, recent death in family or prolonged illness). All participants were tested between 1600 and 2000 hours. Regional EEG was continuously recorded for two minutes (1 minute eyes-open and 1 minute eyes-closed) after completion of the consent form and after the participants had a chance to acclimatize to the laboratory setting (ie, Time 1: Baseline).
Participants were then told that they were to prepare for a self-presentation task. Specifically, they were told the following:
“We are interested in your opinions about classroom presentations. For example, do you think that classroom presentations reflect the true ability of students? Are they useful learning experiences for students? We would like you to give a brief 3 minute discussion that will be videotaped and shown to some participants at a later date. We will give you 2 minutes to prepare your discussion; please make your discussion as close to 3 minutes as possible.”
Participants were then given 2 minutes to prepare their presentation, during which time regional EEG was continuously recorded (ie, Time 2: Speech Preparation Task). Following the EEG recording, the cap and electrodes were removed, and the participants were asked to give the 3 minute speech. Following the speech, participants were debriefed and thanked.
EEG data collection and data reduction
EEG recording
EEG was recorded using a lycra stretch cap (Electro-Cap, Inc.) which has electrodes sewn into it and positioned according to the International 10/20 Electrode System (CitationJasper 1958). An abrasive electrolyte gel (Omni-prep) was applied to each electrode site with a cotton swab to increase conductivity. Electrode impedances were all below 10 kΩ and within 500 Ω between homologous sites. Regional EEG was recorded from four locations: left and right mid-frontal (F3, F4) and parietal (P3, P4) regions, representing the left and right hemispheres and the anterior and posterior regions of the brain. EEG data were recorded from the parietal region to ensure that the effects were specific to the frontal sites. Electrodes were referenced to the central vertex (Cz) during recording. EEG signals were amplified using SA Instrumentation Bioamplifiers, with bandpass filters set at 1 Hz (high pass) and 100 Hz (low pass). Data from each channel were digitized on-line at a sampling rate of 512 Hz.
EEG data reduction and analysis
EEG data were visually scored for artifact (±50 μV) resulting from eye blinks, eye movements, or other motor movements using the EEG Analysis Program (James Long Company, Caroga Lake, NY, USA), which removes artifact data from all channels if artifacts are detected on any one channel. Artifact-edited data were further examined to ensure that they did not vary systematically between participants. Artifact-free epochs were analyzed using a discrete Fourier transform (DFT), with a Hanning window of one second width and 50% overlap. Power (microvolts squared) was derived from the DFT output in the alpha (8 to 13 Hz) frequency band. A natural log (ln) of EEG power was then performed for power at each of the four sites in order to reduce skewness. Separate mid-frontal and parietal EEG asymmetry scores were calculated as follows: frontal asymmetry = [ln(F4) minus ln(F3)]; and parietal asymmetry = [ln(P4) minus ln(P3)]. Because EEG power is inversely related to activity, resulting negative values reflect greater relative right hemisphere activity (CitationDavidson and Tomarken 1989). Usable baseline (resting) EEG data were available on 41 participants, and usable task (reactive) EEG data were available on 39 participants, owing to equipment problems, excessive artifacts, or refusal to wear the cap. The HSA and LSA groups did not differ in EEG data loss.
Results
A repeated measures analysis of variance (ANOVA) with Group (HSA, LSA) as a between-subjects factor and Condition (Baseline, Speech Preparation Task) as a within-subjects factor was performed on the separate frontal and parietal EEG alpha asymmetry scores. Contrary to our prediction, the HSA and LSA groups did not differ on resting (ie, Time 1: Baseline) or reactive (ie, Time 2: Speech Preparation Task) frontal EEG asymmetry. We next examined the relation between resting and reactive frontal and parietal EEG asymmetry scores separately in relation to shyness and sociability, given previous results that these traits are related to the pattern of resting (CitationSchmidt 1999) and reactive (CitationSchmidt et al 1999) frontal EEG asymmetry. A series of zero-order Pearson correlations were performed among the resting and reactive frontal and parietal EEG asymmetry scores and shyness and sociability measures. Contrary to our prediction, these relations were also not significant.
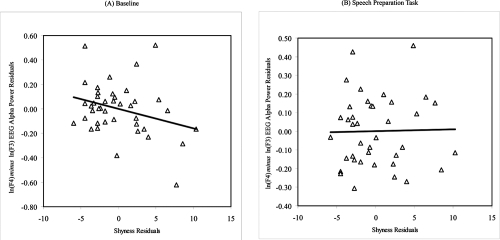
We then performed post-hoc analyses to examine whether concurrent depressive mood may have influenced the relations between resting and reactivity frontal EEG asymmetry and shyness and sociability, given that depressed individuals are known to exhibit distinct patterns of resting frontal brain activity (CitationHenriques and Davidson 1990, Citation1991). Partial correlation coefficients were calculated between the shyness and sociability measures and frontal and parietal EEG asymmetry scores at baseline and during the speech preparation task after controlling for scores on the DASS depressive mood scale. As predicted, higher levels of self-reported shyness predicted greater relative right frontal EEG asymmetry at baseline only after controlling for concurrent depressive mood (r = −0.30, p = 0.03) (see ). However, sociability did not correlate with frontal EEG asymmetry at baseline even after controlling for concurrent depressive mood (r = 0.08, ns). Contrary to our prediction, neither shyness nor sociability was related to frontal EEG asymmetry during the speech preparation task even after controlling for depressive mood (ps > 0.05). In addition, there were no significant relations between the resting and reactive parietal EEG asymmetry measures and shyness and sociability scores.
Figure 1 Residuals scatterplots of the relation between trait shyness (as measured by the Cheek and Buss Shyness Scale) and (A) baseline mid-frontal EEG alpha power asymmetry and (B) during the speech preparation task in a non-clinical sample of healthy young adults after controlling for concurrent depressive mood (Note: Negative EEG asymmetry scores reflect greater relative right frontal EEG activity.)
Discussion
Are there distinct patterns of resting and reactive frontal brain activity among socially anxious adults? Apparently, the answer to this question depends on the level of concurrent depressive mood. We found the predicted relation between shyness and right frontal EEG asymmetry at rest only after controlling for concurrent depressive mood. Individuals who self-reported a high degree of shyness exhibited greater relative right frontal EEG activity at rest after controlling for concurrent depressive mood. This finding is consistent with other recent studies that have shown concurrent symptoms may obscure the predicted relation between personality/affective style and resting frontal brain activity (CitationJetha et al in press).
There are, however, a number of limitations that need to be discussed. First, the results of the present study were based on a relatively small sample size of non-clinical undergraduates. These data need to be replicated with larger non-clinical university and community samples and a clinical sample in order to ensure the reliability and generalizability of these results. Second, there is typically a high degree of co-morbidty between anxiety and depression. Given this co-morbidity, the results may have been influenced not solely by depressed mood. Although the anxiety and stress subscales of the DASS were examined, only the depression subscale influenced the relation between trait shyness and the pattern of frontal EEG asymmetry at rest. Nevertheless, future work should examine different subtypes of co-morbid anxiety and depression with a larger sample size in order to partial out separate and joint influences of anxiety and depressive mood in relation to personality traits and the pattern of frontal EEG activity at rest. Third, we failed to find relations between the selected groups of high and low socially anxious adults. It may be that the limited number of participants in the present study contributed to the lack of statistical power needed to detect between group differences. Alternatively, the extreme group design may not have provided the variability needed to detect differences while the continuous measure of shyness did. Fourth, we failed to find differences on the task data. Again, the lack of findings may have been due to the fact that the study was statistically underpowered to detect differences, the task itself was not robust enough to elicit anxiety, or reactive frontal EEG asymmetry measures play different roles in trait shyness and anxious states. EEG asymmetries may play different roles in pathological and non-pathological disorders and states.
The results of the present study have theoretical and clinical implications. Our results suggest that concurrent depressive mood influences the relation between resting frontal brain activity and individual differences in affective style. It is possible that the predicted relations between personality traits and resting frontal EEG asymmetry may be obscured due to concurrent mood states. Accordingly, it is important for future studies to consider concurrent mood states when examining relations between personality traits and frontal EEG asymmetry measures. The results also have clinical relevance to studies that may use the frontal EEG asymmetry measures as a potential efficacy measure of pre- and post-treatment effects. That is, changes in frontal EEG asymmetry may be coincident with certain therapies used to treat mood and anxiety disorders, and the extent of change may reflect the effectiveness of the therapy. Future studies should extend the current findings to a larger sample of adults clinically diagnosed with social phobia and examine the influence of other co-morbid concurrent anxiety problems on the pattern of resting and reactive frontal brain activity.
Acknowledgements
This research was supported in part by grants from the Social Sciences and Humanities Research Council of Canada (SSHRC) awarded to Louis A. Schmidt and Martin M. Antony. Portions of this paper were based on the third author’s undergraduate honours thesis submitted in partial fulfillment of the Bachelor of Art degree conducted under the direction of LAS, MMA, and REM and presented at the 32nd Annual Ontario Undergraduate Psychology Thesis Conference. The authors would like to acknowledge Sylvia Nowakowski, Austina Reed, and Kate Spere for their help with data collection.
References
- AntonyMMBielingPCoxBJPsychometric properties of the 42-time and 21-item versions of depression anxiety stress scales in clinical groups and a community samplePsychological Assessment19981017681
- AntonyMMCoonsMJMcCabeREPsychometric properties of the Social Phobia Inventory: Further evaluationBehaviour Research and Therapy20064411778516257387
- BruchMAGorskyJMCollinsTMShyness and sociability reexamined: A multicomponent analysisJournal of Personality and Social Psychology19895790415
- CheekJMThe revised Cheek and Buss Shyness Scale1983Wellesley College
- CheekJMBussAHShyness and sociabilityJournal of Personality and Social Psychology1981413309
- CoanJAAllenJJBFrontal EEG asymmetry as a moderator and mediator of emotionBiological Psychology20046774915130524
- ConnorKMDavidsonJRTChurchillLEPsychometric properties of the social phobia inventory (SPIN)British Journal of Psychiatry20001763798610827888
- DavidsonRJLewisMHavilandJMThe neuropsychology of emotion and affective styleHandbook of emotion1993New YorkGuilford14354
- DavidsonRJAffective style, psychopathology, and resilience: Brain mechanisms and plasticityAmerican Psychologist200055119621411280935
- DavidsonRJMarshallJRTomarkenAJWhile a phobic waits: Regional brain electrical and autonomic activity in social phobics during anticipation of public speakingBiological Psychiatry200047859510664824
- DavidsonRJSchererKRGoldsmithHHHandbook of affective science2003New YorkOxford University Press
- DavidsonRJTomarkenAJBollerFGrafmanJLaterality and emotion: An electrophysiological approachHandbook of neuropsychology1989New YorkElsevier41941
- FoxNAIf it’s not left, it’s right: Electroencephalogram asymmetry and the development of emotionAmerican Psychologist199146863721928939
- FoxNAHendersonHARubinKHContinuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of lifeChild Development20017212111280472
- HellerWLevyJPerception and expression of emotion in right-handers and left-handersNeuropsychologia198119263727254505
- HenriquesJBDavidsonRJRegional brain electrical asymmetries discriminate between previously depressed and healthy control subjectsJournal of Abnormal Psychology19909922312307762
- HenriquesJBDavidsonRJLeft frontal hypoactivation in depressionJournal of Abnormal Psychology1991100535451757667
- JasperHHThe ten-twenty electrode system of the International FederationElectroencephalography and Clinical Neurophysiology19581037175
- JethaMKSchmidtLAGoldbergJOResting frontal EEG asymmetry and shyness and sociability in schizophrenia: A pilot study of community-based outpatientsInternational Journal of NeuroscienceIn press
- LovibondPFLovibondSHThe structure of negative emotional states: Comparisons of the Depression Anxiety Stress Scales (DASS) and the Beck Depression InventoriesBehavior Research and Therapy19953333543
- RadomskyASAshbaughARSaxeMLPsychometric properties of the French and English versions of the Social Phobia InventoryCanadian Journal of Behavioural Science20063835460
- SchmidtLAFrontal brain electrical activity in shyness and sociabilityPsychological Science19991031620
- SchmidtLACoteKASantessoDLFrontal electroencephalogram alpha asymmetry during sleep: Stability and its relation to affective styleEmotion20033401714674832
- SchmidtLAFoxNAPatterns of cortical electrophysiology and autonomic activity in adults’ shyness and sociabilityBiological Psychology199438183987873702
- SchmidtLAFoxNAIndividual differences in young adults’ shyness and sociability: Personality and health correlatesPersonality and Individual Differences19951945562
- SchmidtLAFoxNASchulkinJBehavioral and psychophysiological correlates of self-presentation in temperamentally shy childrenDevelopmental Psychobiology1999351193510461126
- SuttonSKDavidsonRJPrefrontal brain asymmetry: A biological substrate of the behavioral approach and inhibition systemsPsychological Science1997820410
- TomarkenAJDavidsonRJWheelerREPsychometric properties of resting anterior EEG asymmetry: Temporal stability and internal consistencyPsychophysiology199229576921410187