Abstract
Delayed alternation (DA) and object alternation (OA) tasks traditionally have been used to measure defective response inhibition associated with dysfunction of frontal brain systems. However, these tasks are also sensitive to nonfrontal lesions, and cognitive processes such as the induction of rule-learning strategies also are needed in order to perform well on these tasks. Performance on DA and OA tasks was explored in 10 patients with alcohol-induced persisting amnestic disorder (Korsakoff’s syndrome), 11 abstinent long-term alcoholics, and 13 healthy non-alcoholic controls under each of two rule provision conditions: Alternation Rule and Correction Rule. Results confirmed that rule knowledge is a crucial cognitive component for solving problems such as DA and OA, and therefore, that errors on these tasks are not due to defective response inhibition alone. Further, rule-induction strategies were helpful to Korsakoff patients, despite their poorer performance on the tasks. These results stress the role of multiple cognitive abilities in successful performance on rule induction tasks. Evidence that these cognitive abilities are served by diffusely distributed neural networks should be considered when interpreting behavioral impairments on these tasks.
Introduction
The use of experimental tasks adopted from nonhuman animal models to study cognitive functions in humans has been termed “comparative neuropsychology” (CitationOscar-Berman 2004). Delayed Alternation (DA) and Object Alternation (OA), two commonly used tests of frontal lobe function in monkeys, have been used as measures of prefrontal system dysfunction in a wide variety of human clinical populations (for review, see CitationOscar-Berman and Bardenhagen 1998). Poor performances on these comparative neuropsychological tasks are traditionally interpreted to reflect impaired behavioral inhibition, or an inability to suppress prepotent responses such as a previously correct response, and are attributed to prefrontal dysfunction (CitationPribram and Mishkin 1956; CitationBardenhagen and Bowden 1998; CitationFreedman et al 1998; CitationOscar-Berman et al 2004). However, in addition to the ability to inhibit incorrect responding, both tasks require participants to deduce response rules in the absence of explicit cues (CitationVerin et al 1993).
Rule induction is a complex ability that is especially important for solving tests of prefrontal function (CitationWang 1987). Induction of task rules involves the ability to engage in conceptual change, or to test hypotheses (CitationHauser 1999). The ability to test and discard hypotheses, shown to be necessary for successful performance on OA – and presumably DA – tasks (CitationBardenhagen and Bowden 1998), is thought to involve prefrontal working memory (eg, CitationGoldman-Rakic 1987). Prefrontal working memory regulates behavior through the manipulation of sensory and mnemonic representations of experience, as well as symbolic representations such as concepts or plans, which have been elaborated in other cerebral networks (CitationGoldman-Rakic 1987; Citation1991). The integrity of working memory depends on the transmission of sensory information, conceptual knowledge, and stored schemas from other cortical areas to the prefrontal cortex (CitationFuster 1997; CitationGoldman-Rakic 1987; CitationFuster 2006). For example, there is evidence that the ability to maintain hypotheses in the face of distraction or disconfirmation may depend on limbic structures (CitationPribram et al 1969).
In a study of the cognitive components required for learning OA, neurologically intact individuals, as well as recently detoxified alcoholics, were found to make a large number of errors unrelated to abnormal inhibitory control (CitationBardenhagen and Bowden 1998). Consistent with models of prefrontal working memory (CitationGoldman-Rakic 1987; CitationFuster 2006), this finding indicated that poor performances on OA and DA tasks are due to factors more complex than impaired response inhibition, and results suggested that rule induction must be achieved before successful response selection and inhibition can occur (CitationBardenhagen and Bowden 1998).
Clarification of the abilities needed for success on DA and OA tasks is important because these tests have been widely used to assess brain function in a variety of neurological and psychiatric conditions (CitationOscar-Berman and Bardenhagen 1998). Some researchers have argued that impairments in working memory are responsible for errors on tasks of prefrontal functioning (eg, CitationGoldman-Rakic 1991; CitationKimberg et al 1997). DA is considered a measure of spatial working memory (CitationGoldman-Rakic 1987), and performance on the task is severely disrupted following bilateral lesions of the lateral surface of the frontal lobes (CitationPribram and Mishkin 1956; CitationMishkin et al 1969; CitationFreedman and Oscar-Berman 1986). Additionally, in a study of six individuals with bilateral frontal lobe lesions, DA was sensitive to medial frontal lesions, and possibly orbitofrontal lesions as well (CitationFreedman et al 1998). As a result, DA impairments have been interpreted as evidence of prefrontal dysfunction in a wide range of conditions such as closed head injury, Alzheimer’s disease, Korsakoff’s syndrome, depression, and schizophrenia (CitationOscar-Berman et al 1982; CitationFreedman 1990, Citation1994; CitationSeidman et al 1995; CitationGansler et al 1996). In contrast to DA’s role as a task of spatial working memory, OA is said to be a measure of visual working memory, and is sensitive to orbitofrontal and medial frontal damage (CitationPribram and Mishkin 1956; CitationFreedman et al 1998). Human experiments have revealed OA impairments in patients with bilateral frontal lobe damage, closed head injury, Parkinson’s disease (both with and without dementia), Alzheimer’s disease, and schizophrenia, while no impairments were found in groups of depressed or non-amnestic alcoholic individuals (CitationFreedman and Oscar-Berman 1986; CitationFreedman 1990, Citation1994; CitationSeidman et al 1995; CitationGansler et al 1996).
Although the sensitivity of DA and OA tasks to prefrontal lesions is well known, few researchers have considered the sensitivity of DA and OA to limbic system lesions. DA is sensitive to lesions of the amygdala and hippocampus in monkeys and rats (CitationOrbach et al 1960; CitationWaxler and Rosvold 1970; CitationMahut 1971; CitationWinocur 1985). DA is sensitive to lesions of the mammillary bodies and the dorsomedial nucleus of the thalamus in rats, cats, and monkeys (CitationIrle and Markowitsch 1982; CitationIsseroff et al 1982). Human studies using DA have also shown that it is not always sensitive or specific to frontal lesions. For example, one study found no difference between patients with unilateral frontal lobe lesions and non-frontal lesions (CitationChorover and Cole 1966). Another study found no group differences between controls and patients with frontal or temporal lesions or Parkinson’s disease (Canavan et al 1969). A third study reported that patients with dorsolateral prefrontal lesions performed better than controls and patients with postcentral lesions (CitationVerin et al 1993). While negative findings may be a function of procedural variations, it is clear from this research that lesions in locations other than the prefrontal cortex affects DA performance. This inference is compatible with brain models postulating widely distributed working memory networks (eg, CitationGoldman-Rakic 1991; CitationFuster 1997; CitationFox et al 2005; CitationFuster 2006). In light of the evidence of limbic involvement in DA performance, the DA impairments reported in conditions such as closed head injury, Alzheimer’s disease, depression, and schizophrenia (CitationOscar-Berman et al 1982; CitationFreedman 1990, Citation1994; CitationSeidman et al 1995; CitationGansler et al 1996) may be related to limbic involvement (CitationWright et al 2000). Similarly, DA impairments in patients with Korsakoff’s syndrome may be tied to limbic and diencephalic neuropathology (CitationBengochea and Gonzalo 1990; CitationJernigan et al 1991; CitationHarper et al 1995).
Unlike DA, OA has not been used widely to test nonhuman animals with focal lesions of regions other than the prefrontal cortex. In the one study that examined OA performance in monkeys with lesions outside the frontal lobes, the authors reported that lesions of inferotemporal cortex resulted in no impairment (CitationPribram and Mishkin 1956). However, recent positron emission tomography (PET) studies demonstrated activations in several non-frontal regions during performance of both DA and OA tasks, suggesting that areas beyond prefrontal cortex are involved in performance on these tasks (CitationZald et al 2002, Citation2005). These findings are consistent with prefrontal working memory models (CitationGoldman-Rakic 1991; CitationFuster 1997, Citation2006), in that tests of prefrontal function require activation of a number of cortical areas. The findings of nonfrontal activations may also explain the DA and OA impairments observed in conditions such as Parkinson’s disease, where lesions in the basal ganglia interfere with the transmittal of information to the prefrontal cortex.
In terms of analysis of DA and OA task performance, a main focus in the literature to date has been on interpreting total error scores and perseverative errors. As noted earlier, poor performance – commission of perseverative errors in particular – has typically been attributed to impaired response inhibition caused by prefrontal lesions (eg, CitationFreedman et al 1998). However, in general terms, abnormal perseverative behavior is not specifically attributable to frontal lesions alone (CitationSandson and Albert 1984). For example, an inability to engage in the conceptual change necessary to induce rules may result in repeated errors or “paradigmatic perseveration” (CitationHauser 1999). Hauser noted that while “perseverative actions are often the result of inhibitory problems… inhibitory problems do not always lead to perseverative actions” (CitationHauser 1999, p 214). Hauser demonstrated the importance of task-relevant knowledge in reducing paradigmatic perseveration in a series of experiments where provision of contextual information often assisted monkeys in inhibiting preferred responses. Similarly, a reduction in both perseverative and nonperseverative errors with provision of the OA response rules indicates that the ability to induce rules is a key component of the task (CitationBardenhagen and Bowden 1998).
The primary aim of the present study was to determine if rule provision improves DA and OA performance in patients with alcohol-induced persisting amnestic disorder (Korsakoff’s syndrome; KS), as previously had been shown in healthy controls and non-Korsakoff alcoholics. We reasoned that if rule provision improves performance by KS patients, it would support existing evidence of cognitive deficits in this population that extend beyond an inability to inhibit perseverative responses (CitationOscar-Berman and Evert 1997). We chose to study the role of rule learning in DA and OA tasks in this group, because KS patients are known to have difficulty in the performance of such problems (CitationOscar-Berman et al 1982; CitationDirksen et al 2006). Given the similarities between DA and OA tasks, and the classic association between abnormal inhibitory control (response perseveration) and KS (CitationOscar-Berman et al 1982), we hypothesized that if impairments in rule induction are largely responsible for poor performance on these tasks (CitationBardenhagen and Bowden 1998; CitationDirksen et al 2006), then provision of the response rules should reduce the number of trials to criterion and the number of perseverative and nonperseverative errors in amnesic and nonamnesic participants alike. However, if errors made by KS patients are due to defective response inhibition rather than to a failure to induce task rules, then rule provision should not improve their performance. In either case, results have direct relevance for describing the nature of cognitive deficits in alcoholic Korsakoff’s syndrome.
Method
Participants
A total of 34 right-handed individuals participated in the study. The first group consisted of 10 KS men who met the DSM-IV criteria for diagnosis of alcohol-induced persisting amnestic disorder (CitationAmerican Psychiatric Association 1994). All had a history of chronic alcohol dependence, and showed clinically significant memory impairments. The second group consisted of 11 abstinent previously long-term alcohol-dependent people (AL), and the third group was comprised of 13 healthy community controls without a history of alcohol dependence (NC). These last two groups were recruited as part of a larger study investigating the effects of alcohol and aging on cognitive functioning (CitationOscar-Berman et al 2004; CitationDirksen et al 2006).
Several questionnaires (eg, handedness, alcohol and drug use), tests, and interviews were administered to all participants in order to ensure that they met the inclusion criteria for the study (see below). As part of this assessment, all participants were given sections of a computerized version of the Diagnostic Interview Schedule (DIS) (CitationRobins et al 1989) that provides lifetime psychiatric diagnoses according to DSM-III-R criteria (CitationAmerican Psychiatric Association 1987). These subsections included: generalized anxiety, depression, mania, schizophrenia, alcohol abuse and dependence, drug abuse and dependence, and organic brain syndrome. A vision test was also administered at the time of testing. The demographic and medical history information was obtained from participants’ self-reports and medical records when available.
The inclusion criteria for all participants in the study included the following: normal or corrected-to-normal vision and hearing; no history of neurological dysfunction (eg, stroke, significant head trauma, loss of consciousness for longer than 15 min, or epilepsy); no history of learning disabilities, dyslexia, or attention deficit disorders; no major medical illness (eg, significant diabetes, liver disease, and heart disease); no history of polydrug abuse, extensive or recent illicit drug use; no major psychiatric disorders; not currently on antidepressants or anti-anxiety medications; and no history of electroconvulsive therapy.
Criteria for classifying someone as an alcoholic vary (CitationAbel et al 1999; CitationOscar-Berman and Marinkovic 2007). A minimum criterion of at least five years of drinking 21 or more drinks per week (one drink = 50 ml beer, 148 ml wine, or 44 ml hard liquor) is common among studies assessing alcohol-related cognitive decline (eg, CitationOscar-Berman et al 2004). It is important to note, however, that problem-based criteria, not quantity and frequency of consumption, comprise a definition for alcohol use disorders (CitationOscar-Berman and Marinkovic 2007). In the present study, the AL group met the following criteria: a minimum of five years of drinking at least 21 drinks per week; a sobriety period of at least one month so as not to confound acute alcohol effects with the long-term residual effects of alcohol dependence; and a positive diagnosis of alcohol abuse and dependence according to DSM-III-R criteria, determined by administering the Alcohol Abuse and Dependence subsection of the DIS. None of the control participants had a positive diagnosis on this measure.
Members of the AL group drank 21 or more drinks per week for an average of 19.78 years (SD = 9.94), and were sober for an average of 10.8 years (SD = 6.66) (see ). A Quantity-Frequency Index (QFI), which takes into consideration the amount, type, and frequency of use of alcoholic beverages either over the last six months (for the NC group), or over the six months preceding cessation of drinking (for the AL group), was calculated for each participant (CitationCahalan et al 1969). The average QFIfor the AL group was 8.02 (SD = 5.57; range = 0.23 to 20.35), and 0.18 for the NC group (SD = 0.23; range = 0 to 0.72), a difference that was statistically significant, t(22) = 5.09, p < 0.001. Demographic details for the three groups are presented in . The research was approved by the Institutional Review Boards of the participating institutions, and informed consent was obtained from participants or their representatives (for KS patients). The participants were compensated for their time and travel expenses.
Table 1 Participant characteristics
Apparatus
A Wisconsin General Testing Apparatus (WGTA), described previously (CitationFreedman 1990), was used for testing on DA and OA (see ). The WGTA consisted of a wooden stimulus board attached to a wooden frame from which an opaque screen was suspended. The participant and the experimenter sat facing each other across a table with the WGTA between them. The experimenter raised the screen to reveal the stimulus board to the participant during testing. Circular stimulus wells (8 cm diameter, 1 cm deep) were cut into the board, with the midpoints of each well placed half way between the front and back of the board. The centers of the wells were approximately 24 cm apart. For DA testing, identical square wooden plaques covered the wells. In OA, a three-dimensional stimulus object (eg, a small toy) was mounted on each plaque. The stimulus wells were padded with felt cloth to minimize any auditory cues during the preparation of each trial. The participant could see neither the wells nor the experimenter when the screen was down. When the screen was raised, the participant could see the stimulus board, the plaques (and objects in OA), and the experimenter’s hands, but not the experimenter’s face.
Figure 1 A modified Wisconsin General Test Apparatus (WGTA). The WGTA consisted of a wooden frame approximately 54 cm wide and 65 cm high. An opaque curtain was anchored to the top of the frame in such a way that it could be raised by the experimenter to reveal a stimulus board (53 × 28 cm) containing two circular reinforcement wells 8 cm in diameter and 1 cm deep. The reinforcement wells were covered with felt cloth to eliminate auditory cues associated with coin placement and the preparation of each trial. The reinforcement wells were 24 cm apart from center to center, and for DA tasks, the wells were covered by identical black square stimulus plaques (7.6 × 7.6 × 0.5 cm). For OA tasks, a three-dimensional stimulus object (eg, a small toy) was mounted on each plaque; each of the objects had different shapes and colors. When the curtain was in the lowered position, the participant could see neither the stimuli nor the investigator. When the curtain was raised for each trial, the participant could see the stimuli and the hands of the investigator, but not the investigator’s face.

Design and procedure
In DA, participants have to find a reward under one of two identical stimulus plaques. To do this they must determine that the reward is alternated between left and right positions on successive trials (Alternation Rule), but that if they choose the wrong (unrewarded) side on a trial, the reward remains on the same side until it is retrieved (Correction Rule). In OA, participants have to find a reward under one of two objects, the location of which randomly alternates between left and right positions. To find the reward, participants must determine that the reward alternates between the objects on successive trials (Alternation Rule), but that if they choose the wrong (unrewarded) object on a trial, the reward remains with the same object until it is retrieved (Correction Rule). In DA and OA, perseverative errors are said to occur when the participant repeatedly chooses the incorrect stimulus within one trial (CitationFreedman 1990). A nonperseverative error is defined as a single error on a trial (CitationFreedman 1990). In each task there is a learning criterion (12 consecutive correct responses) and a failure criterion for discontinuation (a maximum of 50 trials without meeting the learning criterion).
The design of the experiment was based on that described previously (CitationBardenhagen and Bowden 1998). Participants in each group were randomly assigned to one of four-rule provision conditions for both DA and OA. The four rule-provision conditions were: (1) Both Rules were explained (Both Rules); (2) the Alternation Rule only was explained (AR only); (3) the Correction Rule only was explained (CR only); and (4) neither rule was explained (No Rules; the standard form of the task). Thus, a minimum of five subjects in each group were provided with rules, and a minimum of four subjects per group were not provided with rules (see and ). This distribution allowed at least two participants from each group (KS, AL, NC) to be included in each of the specific rule provision conditions, and power analysis from our previous research (CitationBardenhagen and Bowden 1998) suggested that a minimum of two subjects per group would be needed in each condition in order to obtain significant effects of rule provision. DA and OA test order was counterbalanced, and separated by an interval of 15 to 20 minutes duration filled with other unrelated psychological testing. A three-way factorial design was used with three between-subjects factors: Group (KS, AL, NC); Alternation Rule (provided or not); and Correction Rule (provided or not). Our previous research (CitationBardenhagen and Bowden 1998) has shown that these rule manipulations may have different effects on the various scores. On the basis of our previous data, the Correction Rule was expected to reduce perseverative errors but not nonperseverative errors or trials to criterion, while the Alternation Rule was expected to reduce trials to criterion and both perseverative and nonperseverative errors on DA and OA.
Table 2 Results of ANCOVAs on DA trials to criterion, nonperseverative errors, and perseverative errors
Table 3 Results of ANCOVAs on OA trials to criterion, nonperseverative errors, and perseverative errors
DA and OA were administered in the manner described previously (CitationFreedman and Oscar-Berman 1986; CitationBardenhagen and Bowden 1998). Depending on the task, participants were told they would see two stimuli (three-dimensional objects in OA, or identical square black plaques in DA), and that there would always be a penny under one of the stimuli. The aim of the task was to try to find the penny every time the screen was raised. Participants were told they would actually earn five cents for every penny they collected. Instructions varied according to the rule provision conditions. Participants in the Both Rules and Alternation Rule Only conditions were told the Alternation Rule in addition to the standard instructions. They were told the coin would alternate between the two squares (DA) or two objects (OA) on successive trials. Participants in the Both Rules and Correction Rule Only conditions were informed that if they chose the stimulus without the coin on one trial, the coin would remain with the other object or on the other side until they retrieved it. Therefore, if they chose a stimulus without a coin under it, they should choose the other stimulus when the screen was raised again. In order to ensure that performance was not affected by participants forgetting the rules, those who were provided with either or both response rules were reminded of the rules during testing if they broke the rules with which they had been provided, but the number of reminders was not recorded.
For the first trial of each task, a coin was placed under each stimulus, and the trial began when the experimenter raised the screen. The screen was lowered after the participant removed a coin from one of the two stimulus wells. The stimuli were removed from both wells and replaced after one coin was placed in a well. After each correct response, including the first one, the position of the coin was alternated between left and right sides for DA, and between objects for OA. In OA, the objects were alternated between left and right positions on a modified random schedule (CitationGellermann 1933). When an incorrect choice was made, the coin remained with the same object in OA and in the same position for both DA and OA while the screen was down for 10 seconds. The trial was completed when the correct side was chosen. There was a 10-second intertrial interval, the learning criterion was 12 consecutive correct responses, and the failure criterion was 50 trials. The duration of each DA and OA test session depended on the number of trials completed by each participant. As each trial was separated by an intertrial interval of 10 seconds, the minimum time for completing either task in 12 trials was 120 seconds plus the response time for each of the 12 items; the maximum time for the 50 trials was 500 seconds plus the response time for each of the 50 items; additional time was needed for commission of perseverative responses. Although the testing times for each subject were not recorded, common response times were in the range of 2 to 10 seconds, and the duration of the DA or OA test session ranged from approximately 2.5 minutes for error-free performance over 12 trials, to approximately 15 minutes for participants reaching the failure criterion of 50 trials.
Results
The AL and NC groups were equivalent in age, education, and Verbal IQ (VIQ; CitationWechsler 1981; ), but the KS participants were significantly older F(2, 31) = 3.73, p < 0.05, less educated F(2, 31) = 23.52, p < 0.001, and had lower VIQs F(2, 27) = 5.77, p < 0.01. Age, education, and VIQ were, therefore, used as covariates in analyses of the data. Performances of the three groups on DA trials to criterion, nonperseverative errors, and perseverative errors were examined using three separate general linear model (GLM) univariate Analyses of Covariance (ANCOVA; SPSS version 10.0), each having three between-subjects factors (Group, Alternation Rule, Correction Rule) and three covariates (Age, Education, VIQ). There were three levels for the between-subjects factor of Group (NC, AL, KS), and two levels for each of the rule provision conditions (rule provided or not provided). These analyses were repeated for scores on OA. Although the data were positively skewed, analyses of transformed data did not change the pattern of results, nor did nonparametric analyses, so parametric analyses of untransformed data are reported. The effects of the covariates (age, education, and VIQ) were nonsignificant for all DA and OA measures (all ps > 0.05). Counterbalancing of test order in the experimental design resulted in no significant effects of order of presentation on performance (all ps > 0.05). Partial eta-squared (η2) effect sizes are reported for the 3-way factorial model used for the analyses. Effect size estimates were evaluated according to Cohen’s criteria, with a small f (0.10) corresponding to η2 = 0.0099, a medium f (0.25) corresponding to η2 = 0.0588, and a large f (0.40) corresponding to η2 = 0.1379 (CitationCohen 1988). In the KS group, one of the 10 subjects did not complete the DA test.
Delayed alternation
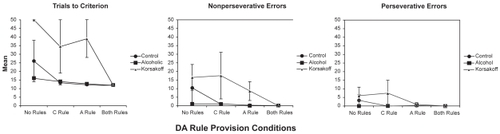
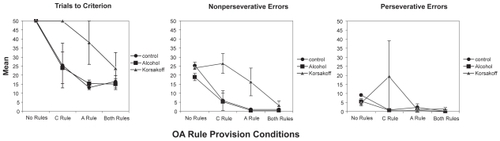
Results for the three groups in the four DA rule provision conditions are presented in . shows that, as hypothesized, provision of the Alternation Rule had significant effects on trials to criterion, nonperseverative errors, and perseverative errors. Contrary to expectations based on our previous research (CitationBardenhagen and Bowden 1998), instruction in the Correction Rule only had a significant effect on DA trials to criterion, but not on nonperseverative and perseverative errors, where effect sizes were medium and small, respectively. The three Groups differed significantly on DA trials to criterion, but not on nonperseverative or perseverative errors, where medium to large, and small to medium Group effects were found, respectively. Planned simple contrasts showed that the KS group required significantly more trials than either the AL or NC groups. There were no significant interactions involving Group, Alternation Rule, or Correction Rule on any of the DA measures.
Object alternation
Results for OA trials to criterion, nonperseverative errors, and perseverative errors are presented in . Statistical analyses, reported in , show the expected significant effects of Alternation Rule provision on trials to criterion, nonperseverative errors, and perseverative errors. Correction Rule provision resulted in significant effects on trials to criterion and, unexpectedly, on nonperseverative but not perseverative errors, where the effect size was negligible. There were no significant Group effects on any of the OA measures, but the effect sizes were medium to large for trials to criterion, large for nonperseverative errors, and medium for perseverative errors. All two-way interactions on OA performance were nonsignificant.
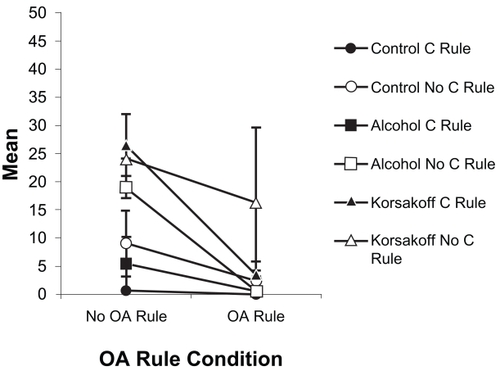
Figure 3 Mean performance (± SE) of the three groups over the four OA rule provision conditions (No Rules, Correction Rule only (CRule), Alternation Rule only (Arule), Both Rules).
There was a significant three-way interaction of Group × Alternation Rule × Correction Rule for OA nonperseverative errors, F(2, 20) = 4.85, p < 0.02, η2 = 0.327. Examination of this interaction () indicated that while provision of either the Alternation or Correction Rule alone was enough to reduce or eliminate nonperseverative errors in the AL and NC groups, the KS group did not benefit greatly from instruction in the Correction Rule alone, showed substantial variability in performance when given the Alternation Rule alone (visible in the error bars in ), and performed best when both rules were provided.
Discussion
As predicted, provision of the response rules aided DA and OA performance for all three groups, confirming that rule knowledge is a fundamental requirement for success on these tasks (CitationBardenhagen and Bowden 1998), and, importantly, that rule knowledge improves performance in KS patients, despite documented problems with impaired response inhibition in this group (eg, CitationOscar-Berman et al 1982). Provision of the Alternation Rule had the expected significant effects on all aspects of DA and OA performance for all groups. Instruction in the Correction Rule had significant effects on trials to criterion for both tasks, and on OA nonperseverative errors, while the effect on DA nonperseverative errors was medium in size. In contrast, provision of the Correction Rule did not significantly reduce OA nonperseverative errors in our previous research, but did reduce OA perseverative errors (CitationBardenhagen and Bowden 1998). In the current study, perseverative errors on DA and OA were not significantly affected by provision of the Correction Rule, with small and negligible effect sizes, respectively. Sampling differences may account for this discrepancy in results, as the procedures were identical. A larger sample size in our previous research may have helped detect an effect of Correction Rule provision on OA perseverative errors. In any case, it seems reasonable to speculate that provision of the Correction Rule may have reduced DA and OA trials to criterion and OA nonperseverative errors in this study by removing the cognitive demand of having another rule to induce in the tasks. In addition, in all three groups, provision of the Alternation Rule significantly reduced perseverative errors, presumably because it also reduced the number of nonperseverative errors, and hence the number of opportunities to make perseverative errors.
The low number of perseverative errors made by the KS group overall, even in the No Rules condition, was an interesting finding, given the classic association of abnormal perseveration with Korsakoff’s amnesia (CitationOscar-Berman et al 1982). It suggests that impaired response inhibition was not the predominant problem for the KS patients in this study. However, given the small-to-medium and medium effect sizes for Group on DA and OA perseverative errors, respectively, larger sample sizes would have resulted in the expected impairments in the KS group. Of note, a low number of perseverative errors in the KS group is not unusual, because with repetition, patients with Korsakoff’s amnesia are capable of retaining limited information, and low – but abnormal – error scores have been reported elsewhere in the literature (eg, CitationDirksen et al 2006 and CitationOscar-Berman et al 2004). Although the perseverative error data were skewed, analyses of transformed data and the use of nonparametric tests did not change the pattern of results. While perseverative errors are a feature of DA and OA performance, even in healthy controls, similar floor effects are present for perseverative errors in all the literature using these tests, and are difficult to eradicate, except by making the tasks more difficult and errors more frequent. In future research it may be interesting to test the role of rule provision after development of a pre-potent response in a reversal paradigm in a KS group.
The significant three-way interaction on OA nonperseverative errors demonstrated that KS patients benefited most from provision of both rules on this measure. While this observation is plausible, it is not clear why this result was found only on this variable, and not on others. It may be because OA is a more difficult task than DA (as suggested by the greater number of trials to criterion across all groups on OA), and that instruction in both rules was necessary to reduce the number of nonperseverative errors for the KS group, whereas instruction in either rule was helpful for the AL and NC groups. Again, this is consistent with the conclusion that the ability to induce both the Alternation and Correction Rules was the major determinant of performance in the KS group in this study.
Significant Group effects were only seen on DA trials to criterion, but the magnitude of effect sizes indicates that significant results would be predicted with larger sample sizes on all the other DA and OA measures. Indeed, before the group differences on age, education, and VIQ were considered in the analyses of covariance, GLM analyses of variance on the same data (with greater available degrees of freedom than in the ANCOVAs), indicated significant group effects for DA nonperseverative errors, F(2, 21) = 5.10, p < .05, OA trials to criterion, F(2, 22) = 5.30, p < 0.05, and OA nonperseverative errors F(2, 22) = 9.68, p < 0.005. Results for perseverative errors in these ANOVAs were not significant (all p’s > 0.05). Importantly for our understanding of KS patients, this group benefited from provision of the response rules, consistent with the hypothesis that performance on DA and OA is strongly related to rule knowledge.
The fact that the KS group benefited from rule provision, but still fared worse than the other two groups overall, suggests a number of possible interpretations. Firstly, the KS group was older, less educated, and had a lower average VIQ than the AL and NC groups, which may account for its poorer performance. Although age, education, and VIQ were not significant in the analyses of covariance, this may be related to inadequacies in analysis of covariance in such a small sample. This is an unfortunate limitation of this study, but is difficult to overcome given the infrequency of KS in the population, and the demographic characteristics of the people who volunteered to participate in the research. However, this limitation in matching the three groups does not affect the primary aim of the study, which was to examine the effects of rule provision on DA and OA performance in KS and elderly AL and NC groups. Despite the small sample size, separate analyses of the KS group indicated the same pattern of results for the effects of rule provision on performance as the analyses involving all three groups.
A second interpretation of the group differences is that they may due to cognitive impairment in the KS group, and may not be related to the demographic variables mentioned above. This latter possibility is consistent with research that found no influence of age or education on error scores on tests of set-shifting and rule induction in elderly individuals (CitationLowe and Reynolds 1999). Therefore, despite differences in demographic and VIQ variables, the KS group may have been impaired relative to the AL and NC groups in rule induction abilities, or may have had difficulty with the complexity and mnemonic demands of the tasks, even when either one of the response rules was provided. Deficits of rule detection (CitationNoël et al 2001), response inhibition/mental shifting (eg, CitationNoël et al 2001; CitationHildebrandt et al 2004), and in the manipulation of information stored in working memory (eg, CitationNoël et al 2001) are known deficits in alcoholics with and without Korsakoff’s syndrome (CitationOscar-Berman 2000).
Overall, these data extend our previous finding (CitationBardenhagen and Bowden 1998) that the ability to induce the Alternation and Correction Rules is a major component of successful performance on OA: The results apply to amnesic and nonamnesic patients alike. The significant effects of rule provision on the number of trials to criterion suggests that this measure directly reflects the ability to induce the response rules. From inspecting the trials to criterion and nonperseverative error scores for the AL and NC groups, it is clear that DA is an easier task than OA, and that the Alternation Rule is easier to induce in DA than in OA. During post-test debriefing, many of the AL and NC participants who failed OA said they had not taken notice of the objects when trying to find the reward. Several spoke of trying a number of complex hypotheses regarding the reward schedule, and they were surprised to hear it was a simple alternation between objects.
Our results suggest some hypotheses as to why OA appears to be more sensitive to abnormal perseverative responding than DA (CitationFreedman 1990, Citation1994; CitationFreedman et al 1998). The fact that neurologically intact individuals tend to perseverate on OA when unaware of the response rule in both the current study and our previous research (CitationBardenhagen and Bowden 1998) suggests that the task parameters elicit perseverative errors, which makes it more sensitive to abnormal perseverative responding (CitationFreedman 1990, Citation1994; CitationFreedman et al 1998). OA may be more sensitive to paradigmatic perseveration than DA because participants have to learn to focus on the objects and disregard the random spatial positions of the rewards. DA, in contrast, involves a simple left-right alternation of identical stimulus plaques.
In our research, instruction in the Alternation Rule appeared to have a greater effect on performance than instruction in the Correction Rule in both tasks, possibly because the Correction Rule is easier to induce. All the Correction Rule requires is choice of the other object or side after an incorrect response. In contrast, mastery of the Alternation Rule requires consistent switching between objects or sides after correct responses. It is possible that the Correction Rule may be more difficult to induce in OA than DA, as there is only the issue of side to consider in DA, while OA has the additional variable of Object to consider. For both tasks, there is no indication that the trial after an error is any different from other trials. Many participants indicated that if the reward was not on one side the last time, they assumed it must be there the next time. Perseverative errors might easily arise from this faulty assumption, and even healthy controls make perseverative errors when not provided with the task rules. While total errors and perseverative errors usually are considered a sign of defective response inhibition arising from frontal lobe damage in the literature on DA and OA, the results of this study, and our previous research (CitationBardenhagen and Bowden 1998), indicate that errors on DA and OA may reflect lack of knowledge of task rules, or the inability to induce them, consistent with CitationHauser’s (1999) concept of paradigmatic perseveration. Furthermore, there is evidence that abnormal perseveration may arise from frontal as well as nonfrontal lesions (CitationSandson and Albert 1984).
The findings of this study provide clear support for the hypothesis that poor performance on DA and OA can arise from lack of knowledge of the task rules. Still, as was the case in our earlier research, rule provision does not ensure perfect performance, suggesting that rule knowledge is not the only factor contributing to success on DA and OA (CitationBardenhagen and Bowden 1998; CitationHauser 1999). Nonetheless, rule provision resulted in improved performance for all participants, including those with KS. Similar findings may be expected with different rule induction tasks, such as the Wisconsin Card Sorting Test (WCST), and the Halstead Category Test (eg, CitationLezak 1995). Indeed, WCST improvements have been reported in patients with schizophrenia, who were given simple remediative techniques such as the instruction to verbalize the sorting category with each response, or who were provided with feedback on verbalized responses (CitationNisbet et al 1996; CitationStratta et al 1997).
From the results of the present study and research reviewed to date, we can postulate that errors on rule induction tasks may arise from a number of causes, including the following: a lack of understanding of the task; inability to deduce the rules of a task; poor memory for the task’s rules; forgetting the previous response; and failure to inhibit incorrect responses (CitationBardenhagen and Bowden 1998). Similarly, it can be argued that successful performance requires the ability to generate and evaluate competing hypotheses; to test, discard, and remember unsuccessful strategies; and to persevere until a successful strategy is found. These components of performance are thought to involve prefrontal working memory (eg, CitationGoldman-Rakic 1991; CitationFreedman et al 1998), and possibly limbic associational memory abilities (CitationJernigan et al 1991; CitationOlton et al 1992). The design of rule induction tasks ensures that they will elicit perseverative errors even in healthy control participants. As a result, we would argue that until other causes for repetitive errors are excluded, perseverative errors on rule induction tasks cannot be attributed to defective response inhibition alone.
The results of the present study support previous findings of the sensitivity of DA and OA tasks to cognitive impairment in individuals with Alcohol-Induced Persisting Amnestic Disorder (CitationOscar-Berman et al 1982; CitationDirksen et al 2006). While the poor performance of the KS group on these tasks would conventionally be attributed to prefrontal dysfunction, the DA impairments found here and reported previously (CitationOscar-Berman et al 1982) may also be a sign of their well-known diencephalic neuropathology (CitationVictor et al 1989), particularly in light of the sensitivity of DA to limbic and diencephalic lesions in nonhuman animals (CitationOrbach et al 1960; CitationWaxler and Rosvold 1970; CitationMahut 1971; CitationIrle and Markowitsch, 1982; CitationIsseroff et al 1982; CitationWinocur 1985). Given evidence of diffuse cerebral involvement, including limbic, diencephalic, and prefrontal cortical changes, in Wernicke-Korsakoff syndrome (CitationBengochea and Gonzalo 1990; CitationJernigan et al 1991; CitationHarper et al 1995), and the recent PET evidence of frontal as well as nonfrontal activation during DA and OA performance (CitationZald et al 2002, Citation2005), it may be most appropriate to conclude that the impairments on these tasks by KS patients (CitationOscar-Berman et al 1982; CitationDirksen et al 2006) is related to widespread cerebral pathology.
A preliminary version of this research was presented as a poster at the National Academy of Neuropsychology conference in San Antonio, Texas, in November 1999. The research was supported by funds from the US Department of Health and Human Services, National Institute on Alcohol Abuse and Alcoholism (R37-AA07112 and K05-AA00219) and by funds from the Medical Research Service of the US Department of Veterans Affairs to Marlene Oscar-Berman, and Australian National Health and Medical Research Council to Stephen Bowden. We gratefully acknowledge the contributions of Dorothy Weber, EdD, for data collection, and Vanessa McKay who assisted with manuscript preparation.
References
- AbelELKrugerMLFriedlJ1999How do physicians define “light,” “moderate,” and “heavy” drinking?Alcoholism:Clinical and Experimental Research2297984
- American Psychiatric Association1987Diagnostic and Statistical Manual of Mental Disorders3rd ed-revisedWashington, DCAmerican Psychiatric Association Press
- American Psychiatric Association1994Diagnostic and Statistical Manual of Mental Disorders4th edWashington, DCAmerican Psychiatric Association
- BardenhagenFJBowdenSC1998Cognitive components in perseverative and nonperseverative errors on the object alternation taskBrain and Cognition37224369665744
- BengocheaOGonzaloLM1990Effect of chronic alcoholism on the human hippocampusHistology and Histopathology5349572134390
- CahalanVCisinICrossleyHM1969American drinking practicesNew Brunswick, NJRutgers Center for Alcohol Studies
- CanavanAGMPassinghamREMarsdenCD1990Prism adaptation and other tasks involving spatial abilities in patients with Parkinson’s disease, patients with frontal lobe lesions and patients with unilateral temporal lobectomiesNeuropsychologia28969842259427
- ChoroverSLColeM1966Delayed alternation performance in patients with cerebral lesionsNeuropsychologia417
- CohenJ1988Statistical power analysis for the behavioral sciences2nd edHillsdale, New JerseyLawrence Erlbaum Associates
- DirksenCLHowardJACronin-GolombA2006Patterns of prefrontal dysfunction in alcoholics with or without Korsakoff’s syndrome, patients with Parkinson’s disease, and patients with rupture and repair of the anterior communicating arteryNeuropsychiatric Disease and Treatment232739
- FoxMDSnyderAZVincentJL2005The human brain is intrinsically organized into dynamic, anticorrelated functional networksProceedings of the National Academy of Sciences10296738
- FreedmanM1990Object alternation and orbitofrontal system dysfunction in Alzheimer’s and Parkinson’s diseaseBrain and Cognition14134432285509
- FreedmanM1994Frontal and parietal lobe dysfunction in depression: delayed alternation and tactile learning deficitsNeuropsychologia321015257969863
- FreedmanMBlackSEbertP1998Orbitofrontal function, object alternation, and perseverationCerebral Cortex818279510382
- FreedmanMOscar-BermanM1986Bilateral frontal lobe disease and selective delayed response deficits in humansBehavioral Neuroscience100337423730139
- FusterJM1997Network memoryTrends in Neuroscience204519
- FusterJM2006The cognit: a network model of cortical representationInternational Journal of Psychophysiology601253216626831
- GanslerDACovallSMcGrathN1996Measures of prefrontal dysfunction after closed head injuryBrain and Cognition301942048811997
- GellermannLW1933Chance order of alternating stimuli in visual discrimination experimentsJournal of Genetic Psychology422078
- Goldman-RakicPSCarrollBJBarrettJE1991Prefrontal cortical dysfunction in schizophrenia: The relevance of working memoryPsychopathology and the brainNew YorkRaven Press123
- Goldman-RakicPSMountcastleVBPlumF1987Circuitry of primate prefrontal cortex and regulation of behavior by representational memoryHandbook of Physiology: The Nervous System, Volume V Higher Functions of the BrainBethesda, MarylandAmerican Physiological Society373417
- Goldman-RakicPS2000Localization of function all over againNeuroImage11451710806031
- HarperCFornesPDuyckaertsC1995An international perspective on the prevalence of the Wernicke-Korsakoff syndromeMetabolic Brain Disease1017247596325
- HauserMD1999Perseveration, inhibition and the prefrontal cortex: a new lookCurrent Opinion in Neurobiology92142210322177
- HildebrandtHBrokateBElingP2004Response shifting and inhibition, but not working memory, are impaired after long-term heavy alcohol consumptionNeuropsychology182031115099142
- IrleEMarkowitschHJ1982Single and combined lesions of the cat’s thalamic mediodorsal nucleus and the mamillary bodies lead to severe deficits in the acquisition of an alternation taskBehavioral Brain Research614765
- IsseroffARosvoldHEGalkinTW1982Spatial memory impairments following damage to the mediodorsal nucleus of the thalamus in rhesus monkeysBrain Research232971137034865
- JerniganTLSchaferKButtersN1991Magnetic resonance imaging of alcoholic Korsakoff patientsNeuropsychopharmacology4175862064717
- KimbergDYD’EspositoMFarahM1997Cognitive functions in the prefrontal cortex: working memory and executive controlCurrent Directions in Psychological Science618592
- LezakMD1995Neuropsychological assessment3rd edNew YorkOxford University Press
- LowePAReynoldsCR1999Age, gender, and education may have little influence on error patterns in the assessment of set-shifting and rule induction among normal elderlyArchives of Clinical Neuropsychology143031514590598
- MahutH1971Spatial and object reversal learning in monkeys with partial temporal lobe ablationsNeuropsychologia9409245005998
- MishkinMVestBWaxlerM1969A re-examination of the effects of frontal lesions on object alternationNeuropsychologia735763
- NisbetHSeigertRHuntM1996Improving schizophrenic in-patients’ Wisconsin card-sorting performanceBritish Journal of Clinical Psychology3563138955549
- NoëlXVan Der LindenMSchmidtN2001Supervisory Attentional System in non-amnesic male alcoholic subjectsArchives of General Psychiatry581152811735844
- OltonDSMarkowskaALVoytkoMLSquireLR1992Working memory: AnimalsEncyclopedia of Learning and MemoryNew YorkMacmillan63538
- OrbachJMilnerBRasmussenT1960Learning and retention in monkeys after amygdala-hippocampus resectionArchives of Neurology32305114428979
- Oscar-BermanMNoronhaAEckardtMWarrenK2000Neuropsychological vulnerabilities in chronic alcoholismReview of NIAAA’s Neuroscience and Behavioral Research Portfolio. National Institute on Alcohol Abuse and Alcoholism Research Monograph – 34Bethesda, MDThe Institute14958
- Oscar-BermanMCraigheadWENemeroffCB2004Comparative neuropsychologyThe Concise Encyclopedia of Psychology and Behavioral Science3rd edNYWiley1979
- Oscar-BermanMBardenhagenFTrosterAI1998Nonhuman primate models of memory dysfunction in neurodegenerative disease: contributions from comparative neuropsychologyMemory in Neurodegenerative Disease: Biological, Cognitive, and Clinical PerspectivesCambridgeCambridge University Press320
- Oscar-BermanMEvertDNussbaumPD1997Alcoholic Korsakoff’s syndromeHandbook of Neuropsychology and AgingNYPlenum20115
- Oscar-BermanMKirkleySMGanslerDA2004Comparisons of Korsakoff and non-Korsakoff alcoholics on neuropsychological tests of prefrontal brain functioningAlcoholism: Clinical and Experimental Research2866775
- Oscar-BermanMMarinkovićK2007Cognitive, sensory, and motor functions and relevant brain systems affected by alcoholismNeuropsychology Review
- Oscar-BermanMZola-MorganSObergRGE1982Comparative neuropsychology and Korsakoff’s syndrome. III – delayed response, delayed alternation, and DRL performanceNeuropsychologia201872026211634
- PribramKHDouglasRJPribramBJ1969The nature of nonlimbic learningJournal of Comparative and Physiological Psychology69765724982640
- PribramKHMishkinM1956Analysis of the effects of frontal lesions in monkeys: III. Object alternationJournal of Comparative and Physiological Psychology4941513295406
- RobinsLHelzerJCottlerL1989NIMH Diagnostic Interview Schedule: Version III Revised (DIS-III-R) Computer Edition: CDIS Management Group, Inc, OttawaSt. Louis, MOWashington University
- SandsonJAlbertML1984Varieties of perseverationNeuropsychologia22715326084826
- SeidmanLJOscar-BermanMKalinowskiAG1995Experimental and clinical neuropsychological measures of prefrontal dysfunction in schizophreniaNeuropsychology948190
- StrattaPManciniFMatteiP1997Remediation of Wisconsin Card Sorting Test performance in schizophreniaPsychopathology3059669168560
- VerinMPartiotAPillonB1993Delayed response tasks and prefrontal lesions in man – evidence for self-generated patterns of behavior with poor environmental modulationNeuropsychologia311379968127434
- VictorMAdamsRDCollinsGH1989The Wernicke-Korsakoff syndrome and related neurologic disorders due to alcoholism and malnutrition2nd edPhiladelphiaDavis
- WangPLPerecmanE1987Concept formation and frontal lobe function: The search for a clinical frontal lobe testThe frontal lobes revisited189205New YorkThe IRBN Press
- WaxlerMRosvoldHE1970Delayed alternation in monkeys after removal of the hippocampusNeuropsychologia8137465001391
- WechslerD1981Wechsler Adult Intelligence Scale-RevisedNew York, NYThe Psychological Corporation
- WechslerD1987Wechsler Memory Scale-RevisedNYThe Psychological Corporation
- WinocurG1985The hippocampus and thalamus: their roles in short- and long-term memory and the effects of interferenceBehavioral Brain Research3813552
- WrightICRabe-HeskethSWoodruffP2000Meta-analysis of regional brain volumes in schizophreniaAmerican Journal of Psychiatry157162510618008
- ZaldDHCurtisCFolleyBS2002Prefrontal contributions to delayed spatial and object alternation: A positron emission tomography studyNeuropsychology16182911949710
- ZaldDHCurtisCChernitskyLA2005Frontal lobe activation during object alternation acquisitionNeuropsychology199710515656767