Abstract
The treatment of dysphoric mania is challenging given the need to treat symptoms of both depression and mania simultaneously without provoking any clinical exacerbation. The newer antiepileptic drugs such as gabapentin, lamotrogine, and carbamazepine are often used as adjuncts to either lithium or valproic acid in the treatment of bipolar disorder. We decided to undertake a monotherapy trial because previous evidence suggested mixed states may be more responsive to anticonvulsants than more traditional antimanic agents. 51 patients with a DSM IV diagnosis of dysphoric mania were randomized to three groups comprising gapbapentin, lamotrogine or carbamazepine and followed for 8 weeks. Psychiatric diagnosis was verified by the structural clinical interview for the DSM-IV (SCID). The MMPI-2 in full was used to assess symptoms at baseline and 8 weeks. All three groups showed significant changes in MMPI-2 scores for depression and mania subscales. Gabapentin showed the greatest change in depression symptom improvement relative to lamotrogine and carbamazepine, respectively. Although manic symptoms improved overall, here were no differences between groups in the degree of manic symptom improvement.
Introduction
The simultaneous presence of both manic and depressive symptoms referred to as mixed manic state or dysphoric mania has long been recognized (CitationKraepelin 1921; CitationMcElroy et al 1992; CitationSwann et al 1993; CitationAkiskal et al 1998; CitationCassidy et al 1998; CitationDilsaver et al 1999). According to the DSM-IV, for the diagnosis of mixed manic state, two of four depressive symptoms in the setting of a manic syndrome appear to suffice and occurs in 50 percent of patients with bipolar disorder at some time (CitationAkiskal 2005). Although dysphoric mania is a common disorder and the outcomes are generally poorer than pure manic and depressive episodes, little is known about how to treat such a condition and new evidence based strategies are needed.
Gabapentin, lamotrigine, and carbamazepine may be used either as monotherapies or as adjuncts to lithium and valproate, as there is some evidence that mixed states may be more responsive to anticonvulsants than more traditional anti-manic agents (CitationClothier et al 1992; CitationMcElroy et al 1992; CitationDilsaver et al 1993; CitationBowden 1995; CitationPost et al 1996; CitationKruger et al 2005). The aim of this study is to determine the clinical effectiveness of gabapentin, lamotrogine, and carbamazepine in dysphoric mania.
The newer antiepileptic drugs such as lamotrigine and gabapentin show therapeutic benefits in bipolar disorder (CitationSoutullo et al 1998; CitationBerk 1999; CitationBowden et al 1999; CitationCalabrese et al 1999; CitationSethi et al 2003) and generally have more favorable side effect profiles because of less weight gain, less drug interactions and less need for therapeutic monitoring compared with older agents (CitationMarken and Pies 2006). Whilst the anticonvulsants show growing evidence for the treatment of depressive symptoms in patients with bipolar disorder, as is seen in lamotragine (CitationBowden 1998; CitationBowden et al 1999, Citation2003; CitationCalabrese et al 1998, Citation1999; CitationMacdonald and Young 2002; CitationYatham et al 2002; CitationGhaemi and Gaughan 2005; CitationMuzina et al 2005) and gabapentin (CitationGhaemi et al 1998; CitationAltshuler et al 1999; CitationPerugi et al 1999; CitationSokolski et al 1999; CitationYoung et al 1999; CitationVieta et al 2000; CitationMacdonald and Young 2002; CitationWang et al 2002), it should be noted that more recent research does not support the use of these agents as antimanic agents (CitationPande et al 2000; CitationMaidment 2001; CitationBowden 2002; CitationCalabrese et al 2002).
An important treatment challenge in dysphoric mania is to bring about improvement in depressive symptoms without provoking the onset of manic symptoms. The studies referred to above however, were not designed to specifically treat mixed states but instead all manic episodes. In most studies, patients with mixed states accounted for a small subset of a total number of patients and thus extrapolation of these results to dysphoric mania patients as a whole should be considered with caution given the paucity of trials in the literature regarding the treatment of depressive features in mania (CitationVieta et al 2000). Despite limited evidence, concomitant anticonvulsants are used frequently in mixed manic patients. Therefore, we designed this clinical trial to compare the efficacy of monotherapy with lamotrigine, carbamazepine, or gabapentin in patients with dysphoric mania.
The MMPI-2 was selected to measure clinical symptoms of mania and depression. The MMPI-2 has been validated in Iran and multiple groups have received training at numerous academic centers resulting in excellent inter-rater reliability (CitationDuckworth and Anderson 1995; CitationMootabi Pers Comm 1995). Unfortunately the YMRS was not validated at the time of this study and the HAM-D (Hamilton Rating Scale for Depression) was not used as a single measure incorporating both mania and depression scores was required. Furthermore, the MMPI-2 is known for cross-cultural sensitivity. This has provided an instrument with religious and cultural contexts, which may be more appropriate for an Iranian study (CitationButcher 2004). For this reason the MMPI-2 in combination with the SCID were chosen as structured instruments.
Materials and methods
Subjects
A total of 59 patients entered the double blind, fixed dose, randomized study. Eight subjects were discontinued from the study due to adverse events. A total of 51 subjects completed the study, including 28 women and 23 men. The subjects ranged in age from 18–60 years (). All patients were recruited from two academic psychiatric outpatient clinics, Ghaem and Avecina hospitals, in Mashad, Iran. All patients met DSM-IV criteria for dysphoric mania. Patients had a history of bipolar I disorder with at least one prior manic episode, and a recent mild to modest but not severe mixed manic attack. No patients had current psychotic features.
Table 1 Patient baseline characteristics in an 8-week, randomized, single-blind trial of carbamazepine, lamotrigine, and gabapentin for treatment of dysphoric mania
Subjects with any other DSM-IV diagnosis requiring psychopharmacological treatment were excluded from the study. Alcohol and substance dependence or use within 1 month prior to study start date, and use of psychotropic medication for 3 months prior to study start date were also excluded from the study. Women of childbearing potential who were without adequate contraception were also excluded from the study. Subjects did not differ significantly with regard to demographic variables including age, sex, or educational level.
All subjects required a minimum reading level of grade 8 comprehension to be included in the study and have enough compliance to answer the questions necessary to participate in the MMPI-2. Therefore patients in a stage of disorganized mania were excluded from the study. All subjects underwent a medical and neurological examination and a series of laboratory tests for safety. Informed written consent was obtained from all subjects. The protocol was approved by the ethics committee of Mashhad University of Medical Sciences, Mashad, Iran. The trial was performed in accordance with the declaration of Helsinki.
Drug therapy
Subjects were randomized to three groups, group 1 (gabapentin, 900 mg/day, N = 18), group 2 (lamotrigine, 100 mg/day, N = 20), and group 3 (carbamazepine, 600 mg/day N = 13). Carbamazepin was in regular formation form. All three medications were initiated at low doses, Patients received carbamazepine 400 mg/day (twice-daily dosing), lamotrigine 25 mg/day at night and gabapentin 300 mg/day divided in three doses. The doses of carbamazepine, lamotrigine and gabapentin were increased to a target dose of 600, 100 and 900 mg/day, respectively within 2 weeks at which remained fixed until the end of study. Clinical assessments were carried out at the first visit (week 0), followed weekly until visit 3 (week 2) and then at 2-week intervals to visit 6 (8 weeks).
Clinical assessment
A detailed psychiatric interview by a psychiatrist was obtained at baseline and at the final visit. In addition, a structured clinical interview (SCID) was performed by a psychiatrist, with the patient and their family at the baseline visit. A single psychiatrist performed all ratings to reduce inter-rater error. In addition, patients with the assistance of their families filled out a questionnaire regarding gender, past medical and drug history, and other demographic information. Depression and mania symptoms were evaluated using subscales of the Minnesota multiphasic personality inventory 2 (MMPI-2). A single psychologist at baseline and at the final visit administered the 104-question test.
Blinding
Both the psychiatrist completing the interviews and the psychologist administering the MMPI-2 were blind to the treatment groups throughout the study period. Serum drug levels were not obtained in the study.
Statistical analysis
Only patients who had completed the study were included in demographic and efficacy analyses. Response to treatment regarding changes in depressive and manic scores was determined using analysis of variance (ANOVA). A t-test was used for comparisons of depression and mania scores between groups. For all analyses, p < 0.05 was defined as statistically significant. All statistical analyses were performed using SPSS version 11.
Results
The primary outcome measure was baseline versus endpoint change in the MMPI-2 scores.
Total changes in depression and mania scores following 8 weeks treatment
A significant change in mean total score on both the depression and mania subscales of MMPI-2 was observed following treatment for each of the three groups ( and ). The percent changes in depression scores for each of the three groups were as follows: gabapentin, 50% decrease (p < 0.000), lamotrigine 33% decrease (p < 0.000), and carbamazepine 13% decrease (p < 0.005). For carbamazepine, lamotrigine and gabapentin respectively, the percent change in mania score for each of the three groups were as follows: gabapentin 75% decrease (p < 0.000), lamotrigine 64% decrease (p < 0.000), and carbamazepine 59% decrease (p < 0.000) ( and ).
Figure 1 Mean change of depression subscale of MMPI-2 test from the baseline to the endpoint in an 8-week, Randomized, Single-Blind Trial of Carbamazepine, Lamotrigine and Gabapentin for treatment of Dysphoric mania.
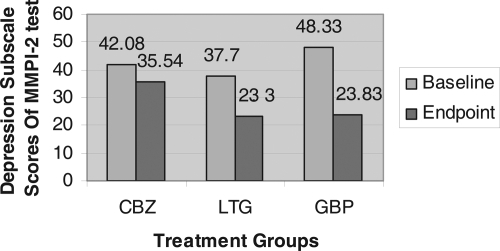
Figure 2 Mean change of mania subdimensions of hypomania subscale of MMPI-2 test from the baseline to the endpoint in an 8-week, Randomized, Single-Blind Trial of Carbamazepine, Lamotrigine and Gabapentin for treatment of Dysphoric mania.
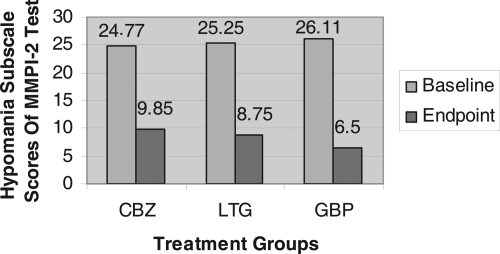
Table 2 Mean percentage change of depression and hypomania subscales of MMPI-2 test from the baseline to the endpoint in an 8-week, randomized, single-blind trial of carbamazepine, lamotrigine, and gabapentin for treatment of dysphoric mania
Table 3 Between group differences in mean percentage change of depression and hypomania subscales of MMPI-2 test scores analyzed with ANOVA test
Between group differences: depression and mania symptoms
There was a significant (p < 0.05) effect on total mania score improvement in all three groups. The mean change of total mania scores on hypomania subscale in MMPI-2 test was greater in gabapentin than carbamazepine (p = 0.046). The results were also analyzed in subdimensions of mania in MMPI-2 test, that showed a greater improvement in psychomotor acceleration (M2) in gabapentin than carbamazepine (p = 0.003). The improvement in other subdimensions of mania did not show statistically significant difference among groups ().
Table 4 Between group differences in mean change of depression and hypomania subscale of MMPI-2 test scores analyzed with ANOVA test
Significantly greater improvement in the mean total score on depression subscale of MMPI-2 test was observed in the gabapentin group when compared to lamotrigine (p = 0.000) and in gabapentin and lamotrigine as compared to carbamazepine (p = 0.000, p = 0.040, respectively) ().
Depressive symptom subscale assessment: gabapentin vs lamotrigine vs carbamazepine
An additional analysis on the depression symptoms of each patient was completed to compare the three treatments (). There are 5 subdimensions of the depression subscale. These subdimensions rate the following depressive symptoms: D1 subjective depression, D2 psychomotor retardation, D3 body dysfunction, D4 mental dullness, D5 brooding.
Figure 3 Mean Percentage Improvement in the depression subdimensions of depression subscale in MMPI-2 test in an 8-week, Randomized, Single-Blind Trial of Carbamazepine, Lamotrigine and Gabapentin for treatment of Dysphoric mania.
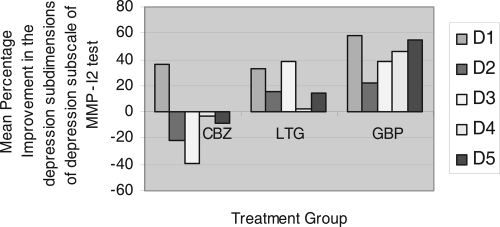
Treatment effects in 5 subdimensions of depression subscale in MMPI 2 test showed that improvement in the mean scores of psychomotor retardation and body dysfunction (D2 and D3) were significantly greater in gabapentin and lamotrigine than in carbamazepine (p = 0.023 and p = 0.0031 in D2, and p = 0.000 and p = 0.000 in D3, respectively). In the carbamazepine group, the mean scores on these subdimensions of depression increased by 0.85 in psychomotor retardation and 1.15 in body dysfunction, indicating the condition was worsened. Improvements in the mean scores of mental dullness and brooding (D4 and D5) were significantly greater (p < 0.01) in gabapentin than lamotrigine and carbamazepine.
Depression subscale: improvement in subjective depression scores
As it is important to evaluate that the improvement in depression score is related to the change in depressive associated symptoms or to the depressive mood itself, we analyzed the efficacy of drugs in reducing depressed mood (MMPI-2 subscale D1: subjective depression) scores and compared it with other subdimensions of depression subscale in MMPI-2. Mean percentage improvement in the depression subdimensions of depression subscale in MMPI-2 test showed greater improvement in depressed mood (D1-subjective depression) than other subdimenions of depression ().
Baseline differences
There were no demographic differences found between groups in age, gender or educational level. There were no differences in baseline mania scores between groups however there were differences in baseline depression scores between groups (). The total depression subscale of the MMPI-2 was significantly higher in the gabapentin than lamotrigine group (48.33 vs 37.80 p = 0.002). This effect was also seen across all subdimension scores on the depression subscale of the MMPI-2. Carbamazepine depression scores at baseline were also higher than lamotrigine scores, though not significantly higher.
Table 5 Between group differences in baseline depression and hypomania subscales of MMPI-2 test scores analyzed with ANOVA test
Rescue medication
Lorazepam up to 4 mg/day was permitted for the management of agitation or insomnia only during the first two weeks of the study. No other adjunctive psychotropic medications were permitted during the study.
Safety
Safety ratings were carried out at the first visit, followed weekly until visit three and then at 2 week intervals to visit 6 (8 weeks). Safety ratings included assessment of vital signs and adverse events detected by clinical evaluation and spontaneous reports of patients and their families at each visit. Laboratory tests were conducted at baseline of the study and included: blood count, fasting blood sugar, liver function tests, electrolytes, blood urea nitrogen, serum creatinine, thyroid function test, and urine analysis. Eight subjects were withdrawn from the study due to adverse events. Six subjects (31%) withdrew from the carbamazepine group due to gastrointestinal upset and/or vertigo and 2 subjects (11%) withdrew from the gabapentin group due to severe drowsiness and/or skin itching.
Discussion
Anticonvulsant therapy using gabapentin, lamotragine, or carbamazepine is effective in the treatment of mild to moderate dysphoric mania. All treatments showed significant improvement in depression and mania symptoms relative to baseline. Gabapentin showed the greatest change in depression symptom improvement relative to lamotrigine and carbamazepine, respectively. There were no significant differences between groups in manic symptom improvement.
To our knowledge, this is the first study to systematically evaluate monotherapy with carbamazepine, lamotrigine, and gabapentin in patients with dysphoric mania. Indeed this is one of the first randomized fixed dose trials observing the three anticonvulsants in an 8 week trial using validated clinical instruments in Iran. The study concluded that monotherapy with gabapentin, lamotrigine and carbamazepine was effective treating mixed states as shown by significant changes to the MMPI following treatment and the effects of gabapentin were superior to lamotrigine and lamotrigine was superior to carbamazepine. However in the absence or a parallel placebo control group we cannot conclude that this effectiveness is equivalent to efficacy.
In our study gabapentin provided significantly greater symptom improvement in depression scores than lamotrigine. This finding is in accordance with CitationSokolski and colleagues (1999) who showed a rapid action of gabapentin against the depressive symptoms of the mixed state. Numerous studies have reported the benefits of lamotrigine on depressive symptoms (CitationBowden 1998; CitationBowden et al 1999, Citation2003; CitationCalabrese et al 1998, Citation1999; CitationMacdonald and Young 2002; CitationYatham et al 2002; CitationGhaemi and Gaughan 2005; CitationMuzina et al 2005), and indeed lamotrigine was effective in the treatment of depressive symptoms in our study. The reason why gabapentin may have superceded lamotrigine in our study might be related to a lower baseline depression score in lamotrigine group, thus leaving little room for symptom improvement.
Gabapentin was superior to carbamazepine for reducing depression and mania symptoms. Although some trials fail to show clear antimanic efficacy of gabapentin (CitationCarta et al 2003), CitationErfruth and colleagues (1998) and CitationGrunze and colleagues (1999) suggest that monotherapy with gabapentin might be useful in selected patients to treat modest but not severe manic states. Our study suggests that gabapentin may be helpful for the treatment of depression in some bipolar patients.
Of particular concern in our study were earlier reports have suggested an association between lamotrigine and gabapentin use and subsequent manic or hypomanic episodes (CitationShort and Cooke 1995; CitationLeweke et al 1999; CitationMargolese et al 2003). However, most of these reports were isolated events influenced by a variety of confounding factors. In our study, lamotrigine and gabapentin were well tolerated and there was no evidence of exacerbation of manic symptoms. Moreover, all of these agents were more effective on manic symptoms than depressive symptoms ( and ) as shown by the mean percentage decrease in total depression and mania scores.
In our study, carbamazepine, lamotrigine, and gabapentin had a clear effect on depressed mood (D1 – subjective depression). Therefore the improvement in depression score was due to a real improvement in depressive mood, not merely in depressive associated symptoms. This finding is support by CitationBaker and colleagues (2003) on a reanalysis of the two studies performed by CitationTohen and colleagues (1999, Citation2000) who showed that the use of olanzapine in reducing total HAM-D scores was more directed to reducing somatic and paranoid symptoms than to depressive mood itself.
Our study has several limitations and include: lack of a control group, no comparison to valproate or lithium, use of the MMPI as a clinical instrument instead of the YMRS or the HAM-D, randomization imbalances due to dropouts, variance in severity of depression scores at baseline between groups, relatively small sample size, low fixed dose of medication, and no serum drug levels were obtained.
In regard to the dose, we used a relatively low fixed dose of medication, in view of the subject selection criteria, which excluded patients in severe manic episode (that commonly are treated by adjunct therapy). However, all medications were within the therapeutic window of treatment for dosage. In addition, subjects were obtained from academic psychiatric outpatient clinics and may not be representative of community patients. Finally, we could not monitor the blood levels of the drugs due to local hospital limitations. Yet, despite all of these limitations we observed highly significant improvement from baseline following treatment in all three medication groups after 8 weeks, these results may therefore indicate effectiveness of these anticonvulsants in the monotherapy treatment of mild to moderate dysphoric mania.
In comparison with other studies in the field, our study had both strengths and weaknesses. For example, our study did not allow patients to titrate to maximal efficacy nor were serum blood levels obtained to compare with therapeutic efficacy. Unlike most other studies on the anticonvulsants we did not make comparisons to either lithium or valproic acid. Our study did have excellent inter-rater reliability and advanced training on the clinical instruments, a factor often missing in other studies. In addition there were no serious adverse events reported in the study. Finally the use of the YMRS (Young mania rating scale) as a measurement for end point analysis could have been a better choice had this option been available in Iran, as this instrument has been more commonly used to assess manic symptoms in the literature.
Implications for further research
Dysphoric mania is common, severe, and resistant to treatment, therefore further studies are warranted with larger sample sizes. Future studies should examine the effects of these anticonvulsants in monotherapy and in adjunct therapy with lithium and valproate. Further research is also needed to fully clarify the role of new agents with putative antidepressant properties. In addition psychiatric structured clinical interviews like the SCID when combined with psychological test batteries such as the MMPI provide validity for studies using clinical endpoint analysis in areas of cultural diversity.
Acknowledgements
This study was undertaken as the thesis of Dr Elham Salari for graduating in psychiatrics. Her study was supported by research committee of Mashad University of Medical Sciences. The authors thank Dr Kamran Javidi Dashtbayaz and Dr Mohamad Taghi Shakeri for statistical analysis and participating in this study. Minnesota Multiphasic Personality Inventory-2 test evaluation and analysis was performed by Ms Shanz Sabouri.
References
- AltshulerLLKeckPEMcElroySLGabapentin in the acute treatment of refractory bipolar disorderBipolar Disord1999161511256659
- AkiskalHSSadockBJSadockVAMood Disorders: Clinical FeaturesComprehensive Text Book of Psychiatry20058PhiladelphiaLippincott Williams and Wilkins1636
- AkiskalHSHantoucheEGBourgeoisMLGender, temperament, and the clinical picture in dysphoric mixed mania: findings from a French national study (EPIMAN)J Affect Disord1998502–3175869858077
- BakerRWTohenMFawcettJAcute dysphoric mania: treatment response to olanzapine versus placeboJ Clin Psychopharmacol200323132712640214
- BerkMLamotrigine and the treatment of mania in bipolar disorderEur Neuropsychopharmacol19999Suppl 4S1192310524838
- BowdenCLCalabreseJRSachsGA placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently manic or hypomanic patients with bipolar I disorderArch Gen Psychiatry20036039240012695317
- BowdenCLLamotrigine in the treatment of bipolar disorderExpert Opin Pharmacother2002315131912387697
- BowdenCLCalabreseJRMcElroySLThe efficacy of lamotrigine in rapid cycling and non-rapid cycling patients with bipolar disorderBiol Psychiatry199945953810386176
- BowdenCLMitchellPSuppesTLamotrigine in the treatment of bipolar depressionEur Neuropsychopharmacol19999Suppl 4S1131710524837
- BowdenCLNew concepts in mood stabilization: evidence for the effectiveness of valproate and lamotrigineNeuropsychopharmacology19981919499653707
- BowdenCLPredictors of response to divalproex and lithiumJ Clin Psychiatry199556Suppl 325307883739
- ButcherJNPersonality assessment without borders: adaptation of the MMPI-2 across culturesJ Pers Assess2004839010415456644
- CalabreseJRSheltonMDRapportDJBipolar disorders and the effectiveness of novel anticonvulsantsJ Clin Psychiatry200265Suppl 35911908919
- CalabreseJRBowdenCLMcElroySLSpectrum of activity of lamotrigine in treatment-refractory bipolar disorderAm J Psychiatry199915610192310401445
- CalabreseJRBowdenCLSachsGSA double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study GroupJ Clin Psychiatry199960798810084633
- CalabreseJRRapportDJSheltonMDClinical studies on the use of lamotrigine in bipolar disorderNeuropsychobiology199838185919778607
- CartaMGHardoyMCHardoyMJThe clinical use of gabapentin in bipolar spectrum disordersJ Affect Disord200375839112781355
- CassidyFMurryEForestKSigns and symptoms of mania in pure and mixed episodesJ Affect Disord1998502–31872019858078
- ClothierJSwannACFreemanTDysphoric maniaJ Clin Psychopharmacol199212Suppl 113S16S1541712
- DilsaverSCChenYRShoaibAMPhenomenology of mania: evidence for distinct depressed, dysphoric, and euphoric presentationsAm J Psychiatry1999564263010080559
- DilsaverSCSwannACShoaibAMThe manic syndrome: factors which may predict a patient’s response to lithium, carbamazepine and valproateJ Psychiatry Neurosci1993186168461283
- DuckworthJCAndersonWPMMPI and MMPI-2 Interpretation Manual for Counselors and Clinicians1995Bristol, PaAccelerated Development
- ErfurthAKammererCGrunzeHAn open label study of gabapentin in the treatment of acute maniaJ Psychiatr Res19983226149789203
- GhaemiSNGaughanSNovel anticonvulsants: a new generation of mood stabilizers?Harv Rev Psychiatry200581710824292
- GhaemiSNKatzowJJDesaiSPGabapentin treatment of mood disorders: a preliminary studyJ Clin Psychiatry19985942699721823
- GrunzeHErfurthAAmannBGabapentin in the treatment of maniaFortschr Neurol Psychiatr1999672566010399045
- IchimLBerkMBrookSLamotrigine compared with lithium in mania: a double-blind randomized controlled trialAnn Clin Psychiatry20001251010798820
- KraepelinEManic-depressive insanity and paranoia1921EdinburghE and S Livingstone
- KrugerSTrevor YoungLBraunigPPharmacotherapy of bipolar mixed statesBipolar Disord200572051515898959
- LewekeFMBauerJElgerCEManic episode due to gabapentin treatmentBr J Psychiatry1999175291a10645343
- MacdonaldKJYoungLTNewer antiepileptic drugs in bipolar disorder: rationale for use and role in therapyCNS Drugs2002165496212096935
- MaidmentIDGabapentin treatment for bipolar disordersAnn Pharmacother2001351264911675857
- MargoleseHCBeauclairLSzkrumelakNHypomania induced by adjunctive lamotrigineAm J Psychiatry2003160183412505823
- MarkenPAPiesRWEmerging treatments for bipolar disorder: safety and adverse effect profilesAnn Pharmacother20064027985
- McElroySLKeckPEPopeHGClinical and research implications of the diagnosis of dysphoric or mixed mania or hypomaniaAm J Psychiatry19921491633441359799
- MootabiFPreparation and normalization of MMPI-2 questionnaire in Tehran city1995 Unpublished, personal communication
- MuzinaDJElhajOGajwaniPLamotrigine and antiepileptic drugs as mood stabilizers in bipolar disorderActa Psychiatr Scand Suppl200542621815833097
- PandeACCrockattJGJanneyCAGabapentin in bipolar disorder: a placebo-controlled trial of adjunctive therapy. Gabapentin Bipolar Disorder Study GroupBipolar Disord200023 pt 22495511249802
- PerugiGToniCRuffoloGClinical experience using adjunctive gabapentin in treatment-resistant bipolar mixed statesPharmacopsychiatry1999321364110505483
- PostRMKetterTADenicoffKThe place of anticonvulsant therapy in bipolar illnessPsychopharmacology (Berl)1996128115298956373
- SethiMAMehtaRDevanandDPGabapentin in geriatric maniaJ Geriatr Psychiatry Neurol2003161172012807075
- ShortCCookeLHypomania induced by gabapentinBr J Psychiatry1995166679807620760
- SokolskiKNGreenCMarisDEGabapentin as an adjunct to standard mood stabilizers in outpatients with mixed bipolar symptomatologyAnn Clin Psychiatry1999112172210596736
- SoutulloCACastoLSKeckPEGabapentin in the treatment of adolescent mania: a case reportJ Child Adolesc Psychopharmacol199888159639083
- SwannACSecundaSKKatzMMSpecificity of mixed affective states: clinical comparison of dysphoric mania and agitated depressionJ Affect Disord1993288178354772
- TohenMJacobsTGGrundySLEfficacy of olanzapine in acute bipolar mania: a double-blind, placebo-controlled studyArch Gen Psychiatry200057841910986547
- TohenMSangerTMMcElroySLOlanzapine versus placebo in the treatment of acute maniaAm J Psychiatry1999156702910327902
- YathamLNKusumakarVCalabreseJRThird generation anticonvulsants in bipolar disorder: a review of efficacy and summary of clinical recommendationsJ Clin Psychiatry2002632758312000201
- YoungLTRobbJCHaseyGMGabapentin as an adjunctive treatment in bipolar disorderJ Affect Disord19995573710512610
- VietaEMartinez-AranANietoEAdjunctive gabapentin treatment of bipolar disorderEur Psychiatry200015433711112936
- WangPWSantosaCSchumacherMGabapentin augmentation therapy in bipolar depressionBipolar Disord2002429630112479661