Abstract
Levodopa is the most effective treatment in Parkinson’s disease and the association with COMT inhibitors widens its plasma bioavailability and effectiveness. Tolcapone is a potent COMT inhibitor whose utilization in PD is limited due to safety concerns on liver toxicity. However, recent data indicate that if liver function is actively monitored, tolerability is no worse than other currently available therapies. By contrast, administration of tolcapone is associated with significant clinical improvement and benefit involves also non-motor features. In this review we discuss the rationale for the use of tolcapone in association with levodopa and other treatments in PD, and we provide an indirect comparison of current strategies to reduce “off” time. We propose that future guidelines include a trial with tolcapone in all PD patients who continue to complain about motor fluctuations despite treatment with entacapone and/or MAO-B inhibitors. Moreover, we suggest that tolcapone should be considered before surgical or infusional strategies are applied.
Introduction
Management of motor fluctuations and dyskinesias is still a major challenge in the long-term treatment of patients with Parkinson’s disease (PD) as they affect daily life activities and in turn quality of life of patients and caregivers. Symptoms associated with the “off phase” not only include akinesia, rigidity, and tremor, but also depression, anxiety or even panic attacks (CitationLauterbach 2005). In the advanced stages of the disease both motor and non-motor fluctuations are present (CitationNutt and Holford 1996).
Dyskinesias are classified according to their temporal profile after drug administration, namely peak-dose dyskinesias (mainly choreic movements), biphasic dyskinesias, onset and end-of-dose (mainly dystonic and ballic movements), and finally “off”-period dyskinesias (dystonic movements) (CitationDefebvre 2004).
In general motor fluctuations and dyskinesia develop in about 10% of patients per year after starting levodopa treatment, reaching almost 100% of patients after 10 years’ treatment (Marsden and Parkes 1997; CitationVan Laar 2003). The DATATOP study reported high rates of motor fluctuations and dyskinesias (CitationPSG 1996), but this might be related to direct patient observation during the trial phase (CitationLang and Lozano 1998).
Recently, a retrospective analysis of the CALM-PD study reported an incidence of motor fluctuations within a range from 12% to 60%, and an incidence of dyskinesias from 8% to 64%, after 4–6 years of levodopa therapy (CitationHauser et al 2006).
Factors involved in the development of motor complications are not completely elucidated yet. Patients with motor fluctuations (wearing off, on-off phenomenon) may show akinetic-rigid onset, longer disease and treatment duration, higher intake of levodopa dose per day (>300 mg/day), and occasionally longer time latency since diagnosis. They also show greater disability according to the Schwab-England scale and disease severity. The finding of a family history of parkinsonism is also frequent, suggests that positive genetic may lead to greater propensity to development of motor complications. Similarly patients with dyskinesias tend to present higher Hoehn and Yahr score and longer treatment duration, while association with levodopa daily dose is less consistent. Gender as well as genetic factors related to dopamine D2 receptor gene may also play an important role and contribute to development of peak-dose dyskinesia in women (CitationSchrag and Quinn 2000; CitationZappia et al 2005).
Although fluctuations in response to levodopa are typically defined by changes in motor signs, autonomic and psychological fluctuations may also occur.
Non-motor fluctuations (NMF) are extremely disabling for PD patients; they affect activity of daily living and quality of life and have recently received growing attention by movement disorders experts. Similar to motor fluctuation, non-motor fluctuations also tend to increase with disease progression, and are more disabling in younger patients (CitationRiley and Lang 1993; CitationWitjas et al 2000).
Non-motor fluctuations may involve cognitive, psychiatric, sensory/pain, and autonomic domains (CitationChaudhuri et al 2006, Citation2007).
Assessment of frequency and disability caused by NMF was evaluated in 50 PD patients by the administration of a specific questionnaire (CitationMaricle et al 1998). All patients showed at least one type of NMF, mostly associated with the “off” state. The most represented were anxiety (66%), drenching sweats (64%), slowness of thinking (58%), fatigue (56%), and akathisia (54%). All NMF contributed to the worsening motor disability, and especially fluctuations linked to autonomic functions were also related to levodopa treatment.
Some degree of sadness and mood switching is also frequently reported during “on-off” periods (CitationMartinez-Martin et al 2007). Mood fluctuations may occur more frequently in patients with involvement of cortical or mesolimbic areas (CitationChaudhuri et al 2006).
The stringent relationship between levodopa response and non-motor fluctuations is also supported by the occurrence of “off period” depressive symptoms after discontinuation of levodopa intravenous infusion (CitationMaricle et al 1995, Citation1998).
Finally, a PET study in a small number of PD patients showed that rCBF response to levodopa in medial frontal gyrus and posterior cingulated cortex (PCC), was different between mood fluctuators and controls (CitationBlack et al 2005). As PCC is strongly linked to emotions and mood, and it is known to modulate dopaminergic innervations of caudate nucleus, anterior cingulated cortex, and orbital frontex, the authors speculate that mood fluctuations in PD might be related to dysfunction in levodopa responsiveness.
Levodopa administration and response to levodopa: addressing indication to starting therapy
Levodopa is the most effective treatment of PD cardinal symptoms (rigidity, akinesia, tremor, postural instability) (CitationRascol et al 2002). The onset of effect is rapid, and most clinical trial results suggest that initiating treatment provides superior motor and functional control compared with other dopaminergic substances. Levodopa is generally well tolerated, with lower incidence of gastrointestinal and neuropsychiatric adverse events (CitationHalkias et al 2007).
The mechanism of action is not simply characterized by a central conversion of levodopa to dopamine at surviving dopaminergic terminals, but also by a neuromodulation (CitationOpacka-Juffry and Brooks 1995), and likely internalization of D1 receptors, as demonstrated in rats (CitationMuriel et al 2002). Moreover, increasing motor fluctuations in the afternoon and evening in patients during long-term levodopa therapy may not relate to the pharmacokinetics of the drug but express tolerance to repeated doses of levodopa (CitationBonuccelli et al 2000).
Administration of levodopa is associated with negative perception on motor complications risk, leading to a delay in initiating treatment that has been defined as “levodopa phobia”.
The relevance of patient age at disease onset for starting levodopa therapy is still debated and current guidelines have not yet properly assessed this issue. The problem of starting levodopa treatment early arises in younger patients with mild motor dysfunction, in consideration of life expectancy and disease progression. Because of its greater efficacy than dopamine agonists, levodopa is recommended as first-line agent always in case of a rapid and sustained worsening of motor symptoms, regardless of age (CitationOlanow et al 2001).
The declining efficacy of levodopa therapy surely recognizes, at least in part, a progression of the disease, and this must be considered especially in terms of expectations on quality of life.
Some hypotheses around the mechanisms by which response fluctuations may occur with levodopa have been conceived (CitationVan Laar 2003):
presynaptic neuronal degeneration leading to insufficient buffering of released levodopa, which could be mainly related to the “wearing off” phenomenon.
postsynaptic changes in dopamine receptor sensitivity and number, partially caused by presynaptic changes , which could be related to the unpredictable fluctuations.
pharmacokinetic and pharmacodynamic influence of other dopaminergic agents or non-pharmacological agents (such as food).
Clinical response to levodopa can identify at least three different mechanisms
The best known is the short-duration response (SDR), measured in minutes to hours, with a steep concentration – response relationship such that the response appears “all or nothing”. The duration of the effect is dose-responsive. During chronic therapy, the SDR becomes shorter, as does the onset to effect, whereas the magnitude of the response becomes larger, probably because of a sensitization phenomenon (CitationNutt 1995). With regard to tolerance, experiments on levodopa holidays of 2–4 days, demonstrated that post-holiday infusion produced a longer response than did pre-holiday infusion, as measured by tapping and walking speed, tremor, and dyskinesias. The prolongation of the response was greater in patients receiving higher daily doses of levodopa (CitationNutt et al 1994).
The long-duration response (LDR) results from repetitive administrations of levodopa and develops over days or weeks after starting treatment (CitationZappia et al 1999a). It is independent of single intakes of levodopa which, in turn, determine SDRs associated with motor fluctuations and dyskinesia (CitationZappia et al 1997). On the contrary, the presence of the LDR may ensure a stable clinical benefit and its disappearance may be relevant for the development of a fluctuating condition (CitationZappia et al 1999b). The LDR is more evident in early PD stages, but it could be induced also in more advanced stages (CitationQuattrone et al 1995), by modulating the inter-dose intervals and the size of each dose (CitationZappia et al 2000). The rate of decay is proportional to the severity of illness, and therefore it might be related to the dopamine storage capacity of surviving nerve terminals. Finally in advanced stages of the disease, we can also identify a negative response (super-off), measured in minutes to hours, and consisting of motor function worsening below the basal level.
Strategies to widen levodopa therapeutic window
The management of motor and non-motor fluctuations aims at prolonging the effect of dopaminergic stimulation possibly reducing total levodopa load. The effects of a single dose of levodopa may shorten to less than 2–3 hours in advanced patients. Early symptoms of “wearing off” may be improved by redistributing levodopa dosing during the day and shortening the interval between doses (CitationWidnell and Cornella 2005). Alternatively, as modifications of LDR may underline motor fluctuations (CitationZappia et al 1999a, Citationb), strategies that involve increasing single levodopa dose rather than number of administrations might also be beneficial, if dyskinesia does not worsen (CitationZappia et al 2000).
There are different strategies aimed at stimulating dopamine receptors in a more continuous, less pulsatile manner, to slow levodopa metabolism, interacting at different levels, and consequently providing a more sustained central effect.
Continuous intraduodenal infusion produces stable levodopa plasma levels resulting in few off periods and dyskinesia as well as improved quality of life, but it can be applied only in limited cases (CitationAntonini et al 2007).
Slow-release preparations of levodopa (Sinemet CR®, Madopar HBS®), associated with peripheral dopa decarboxylase inhibitor (DDCI), may be helpful in smoothing out fluctuations, alleviating tremor and rigidity during the night, and morning akinesia as well. However, their absorption and effect is often erratic making their use in a clinical setting very limited.
The MAO-B inhibitors: effectiveness and rationale for use in clinical setting
MAO-B inhibitors (selegiline, rasagiline), may be an alternative to levodopa in the early treatment (CitationGoetz et al 2005). Although no medication has been proven to slow the progression of the disease, preclinical studies have demonstrated some neuroprotective effects of MAO-B blockers in animal experimental models. These drugs are generally well tolerated, although some concerns have been expressed with selegiline regarding cardiovascular effects (CitationLees 2005).
Three recent level 1 studies have provided new data on MAO-B rationale for clinical use. In 473 untreated early PD patients allocated to treatment with dopamine agonists (bromocriptine and lisuride), or to selegiline (CitationCaraceni and Musicco 2001), the authors found that the lower frequency of motor complications in patients assigned to selegiline was not statistically significant after a multivariate analysis including effects of age, disease duration, and baseline severity scores.
CitationShoulson et al (2002) performed an independent randomization of 368 patients from the DATATOP original cohort, who had been treated with selegiline and thereafter required levodopa, continuing with selegiline or switching to placebo under double-blind conditions. Patients were followed for 2 years, and primary outcome was the time to the first development of “wearing-off” “on-off” fluctuations, dyskinesia and freezing of gait; confusion and dementia were included in the analysis as well. The frequency of new occurrence in any primary variables was not significantly different between the two arms.
Rasagiline, a second-generation, selective, reversible MAO-B inhibitor, is associated with benefit in patients with early disease stage and reduced “off” time in patients with motor fluctuations (CitationOldfield et al 2007). According to the TEMPO study (CitationPSG 2002), rasagiline is beneficial as monotherapy in the early stage of the disease. Similar benefit was reported in the LARGO study that compared the effect of entacapone and rasagiline in advanced PD (CitationRascol et al 2005). Interestingly, a significant mean reduction in “off” time was reported only in patients with mild to moderate dyskinesia but no advantage compared to placebo was found in the most severe advanced PD cohort. These findings confirm the difficulties that current strategies encounter in the management of this specific group of patients.
COMT inhibition with entacapone and tolcapone
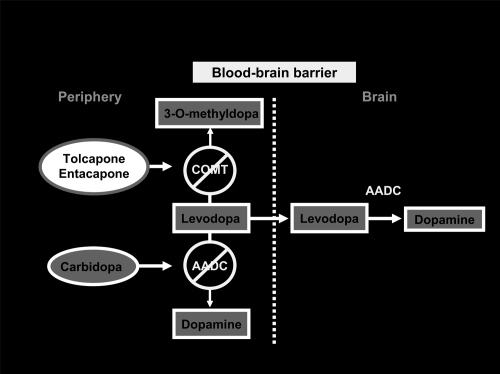
Association of DDCIs with levodopa increases its metabolism by catechol-O-methyltransferase to 3-O-methyl-dopa (3-OMD), in organs expressing COMT activity, such as liver, kidney, and gastrointestinal tract.
The 3-OMD metabolite has a long elimination half-life (approximately 15 hours), and may accumulate during long-term levodopa therapy. 3-OMD may compete with levodopa for passage across the BBB; moreover cerebral COMT metabolize levodopa to 3-OMD and dopamine to homovanillic acid (CitationKaakkola 2000). On the basis of this knowledge, the role of COMT inhibition seems crucial in PD therapy. Peripheral COMT inhibition reduces degradation of levodopa to 3-OMD, thus widening its therapeutic window, while a central COMT inhibition may further prolong levodopa effectiveness by reducing both levodopa and dopamine metabolism.
The new generation of COMT inhibitors entacapone and tolcapone are potent and selective and their role has become increasingly evident in the last decade ().
The two drugs show a number of pharmacologic differences and consequently, different clinical effectiveness. Tolcapone is a selective, reversible peripheral COMT inhibitor (CitationNapolitano et al 1999). Activity on central COMT has also been demonstrated both clinically and with functional imaging (CitationCeravolo et al 2002) and it can be observed for doses of 200 mg tid.
Data about efficacy and safety of these drugs have been examined recently by a Cochrane review (CitationDeane et al 2004). Comparing the efficacy of the two COMT inhibitors across 8 randomized controlled trials, tolcapone produced larger reductions in “off” time and levodopa requirements.
Tolerability profile was similar; some of the reported adverse events were related to the increased availability of levodopa (nausea, vomiting, dyskinesias, hallucination), and ameliorated reducing levodopa dose. Some other adverse events were more strictly related to COMT-inhibition, in particular the occurrence of diarrhea.
Role of tolcapone in PD management
Tolcapone, a potent selective reversible COMT inhibitor, was launched in Europe in 1997, and in the US in 1998 (CitationLeegwater-Kim and Waters 2006). Its efficacy as adjunct to levodopa therapy is attributed to the peripheral COMT inhibition, whereas there is still some debate around clinical relevance associated to the central activity. Results of clinical trials conducted in PD patients, confirm that tid administration of tolcapone magnifies levodopa motor benefit. Improvement had already been observed with a single 200 mg dose (CitationNapolitano et al 1999).
Compared with levodopa/DDCI alone, single or multiple doses of tolcapone 100 or 200 mg increased levodopa bioavailability by 1.3 to 2.1 fold, both in healthy volunteers and in PD patients (CitationLimousin et al 1995).
Pharmacological profile of tolcapone is characterized by a greater bioavailability, and so has a greater area under the curve, Tmax and COMT enzyme affinity, compared with entacapone. Tolcapone increases the levodopa half-life by 80% versus 40% for entacapone, the levodopa area under the curve by 80% versus 40% for entacapone, providing a greater decrease in the formation of O-methyl-dopa as well (CitationFactor et al 2001).
The results of a long-term comparative study between tolcapone and entacapone, suggest that tolcapone is more effective, and this difference in response would be predicted by the pharmacological differences between the two agents (CitationOnofrj et al 2001). The study, performed in patients with fluctuating PD, compared efficacy and tolerability of tolcapone and entacapone, evaluating the effects prospectively at 6, 12, 24, and 36 months. Fourteen patients were enrolled under tolcapone treatment, and eleven patients were enrolled under entacapone treatment. Tolcapone showed more effectiveness in lowering UPDRS motor score and complication subscores, duration of “off” periods, and levodopa doses requirement (UPDRS motor score change was −5.07 ± 1.48 in tolcapone group, and +2.46 ± 2.76 for entacapone (p < 0.02); duration of “off” period was −1 ± 0.23 for tolcapone, 0 ± 0.27 for entacapone (p < 0.03), lowering of L–DOPA dose was −400 ± 70.7 for tolcapone and +179.2 ± 160.8 for entacapone (p < 0.04).
Another study in 40 patients with severe PD, with open design and direct switch-over from tolcapone to entacapone, was prompted because of mandatory indications of the European drugs authority, who withdrew tolcapone from market in November 1998. “On” time was increased by 15% during tolcapone treatment (p < 0.05), and by 8% during entacapone treatment, whereas “off” time was decreased by 16% during tolcapone and by 7% during entacapone treatment. The reduction of L-DOPA was significantly improved by tolcapone compared with entacapone (p = 0.05) (CitationLees et al 2007). IGA (Investigator Global Assessment) for wearing off severity, indicated that 70–73% of the patients receiving tolcapone, and 56% of patients receiving entacapone, had a relevant improvement (p = 0.01).
A recent double blind study assessed patients receiving entacapone after random assignement to continue entacapone (n = 75) or switch to tolcapone (n = 75). The “on” time increased by ≥1 hour day in 43% of entacapone patients and in 53% of tolcapone patients and by ≥3 hours day in 13% and 25% of patients, respectively. The IGA indicated moderate/marked improvement in 25% of entacapone patients and 39% receiving tolcapone. No differences were observed in the rate of dyskinesias and adverse events leading to discontinuation of treatment (CitationPahwa et al 2006).
Safety and tolerability were evaluated with a focus on liver transaminase elevations and hepatotoxicity; 677 levodopa-naïve patients were randomized to receive placebo or tolcapone as add-on therapy to standard doses of levodopa (CitationThe Entacapone to Tolcapone Switch Study Investigators 2007). Liver transaminase elevations above the upper normal limit (ULN) occurred in 20.2% and 27.5% of patients in the placebo and tolcapone groups, respectively. Elevations of ULN ≥3 fold occurred in 1.2% and 1.8% of placebo and tolcapone patients, respectively. No serious hepatotoxicity events were reported.
Efficacy of oral tolcapone as adjunct therapy to levodopa/DDCI, was demonstrated in several trials, although only in the short period (3 weeks’ to 3 months’ duration), both in fluctuating and stable PD patients (CitationSuchowersky et al 2001).
Clinical trials conducted in PD patients with motor fluctuations (wearing off, predictable “on-off”), were analyzed by a Committee of the American Academy of Neurology. The Committee recognized tolcapone is useful in reducing the “off” time, the need of levodopa daily dose, and prolonging the “on” phase.
Recently, also data from a comparator study (the SWITCH study) were presented. The aim of the study was to determine if switching from entacapone to tolcapone improves motor features. In the per-protocol analysis, a statistically significant greater proportion of patients switched to tolcapone, had at least 3 hours/day of additional “on” time (p = 0.018). More patients on tolcapone were judged to have a moderate or marked improvement in the IGA, although not statistically significant. Also the number of responders was higher on tolcapone, which appeared to have greater long-term benefits compared to entacapone ().
Table 1 Efficacy of oral tolcapone 100 mg, entacapone, and rasagiline evaluated in terms of increasing hours of “on” time, decreasing hours of “off” time, and levodopa requirement
In patients with stable disease, tolcapone determines improvement from baseline in various end-points in a short-term study (CitationSuchowersky et al 2001), showing an overall global improvement in 77% of patients at the IGA score, after 4 weeks double-blind treatment.
These preliminary results suggest potential benefit on both motor and non-motor fluctuations. In this respect, patient-perceived benefit, obtained by smoother levodopa response, might be regarded also as an indirect effect of improved non-motor fluctuations.
With regard to safety, dopaminergic-related adverse events were the most frequently reported in patients receiving 200 mg tolcapone tid. Such events (nausea, vomiting, dyskinesia, orthostatic complaints, sleep disorders, dystonia, confusion, hallucination), are likely a consequence of the increase in levodopa concentrations, especially at the beginning of treatment, and are mostly resolved with a reduction in levodopa dose. New or worsening dyskinesias were one of the most commonly reported dopaminergic-related adverse events (25%–62% with tolcapone, 100 mg 3 times daily, 31%–66% with 200 mg 3 times daily); in any case they were not a reason for withdrawal (CitationDeane et al 2004). Some major concerns have been raised in the past over the emergence of liver toxicity (CitationWatkins 2000).
For this reason, marketing authorization for tolcapone was suspended in the EU (late 1998), and labeling was tightened in the US. However, suspension of the marketing authorization in the EU was later lifted following EMEA recommendation (CitationEMEA 2004).
The cause of hepatotoxicity has not been clearly established yet, although the current hypothesis suggests a possible interference with mithocondrial respiration in hepathocytes, by uncoupling oxidative phosphorilation (CitationBorges 2005).
Three cases of fatal fulminant hepatic failure were reported with tolcapone in 1998, but it should be noted that liver function had not been properly monitored in these patients and that the liver dysfunction emerged 2–6 months after starting treatment (CitationOlanow 2000). A recent review of tolcapone global safety (Valeant Pharmaceuticals International data on file), reported severe hepatocellular injury only in 0.04% of patients over the last 7 years, whereas the majority of patients experiencing liver toxicity, only showed an elevations in liver transaminases without clinical signs or symptoms.
In a review of clinical data on 1535 patients (CitationOlanow 2000), the expert panel also noted that about half the patients who continued tolcapone therapy experienced a normalization of ALT/AST levels within 1–3 months, whereas patients who discontinued tolcapone experienced the normalization after just 2–3 weeks. These data are crucial in the evaluation of tolcapone’s role in PD therapy; in fact, due to its fast onset of action, the identifications of responders can occur early, when the liver damage is probably still in a reversible initial phase, so non-responders may easily withdraw before liver damage is established, whereas responders should be strictly monitored, especially in the first 6 months.
In summary, tolcapone is a useful option for patients with idiopathic, fluctuating PD patients (CitationColosimo 1999). Clinical practice suggests that tolcapone is more effective than entacapone and it can be successfully associated to levodopa preparations. In addition it has central activity with potential benefits on mood and cognition although this might be observed only at doses of 200 mg tid. Data on tolcapone effect in patients who have not benefited from entacapone (this would be the target population according to EMEA guidelines) are still lacking. Randomized trials are warranted to demonstrate that adding tolcapone in these individuals is indeed beneficial.
Conclusions
Current evidence suggests that treatment strategies should target both motor and non-motor fluctuations, as the latter have been often neglected or underestimated in PD. COMT inhibitors are beneficial because they widen the therapeutic window in PD. In addition tolcapone may also have some central activity particularly at high dose. Given this evidence we suggest that tolcapone should be prescribed to all PD patients who continue to complain about motor fluctuations despite current or previous treatment with entacapone and/or MAO-B inhibitors. We believe that a tolcapone trial should be considered before other complex and more invasive procedures are proposed to the patient.
References
- AdlerCHSingerCO’BrienCRandomized, placebo-controlled study of tolcapone in patients with fluctuating Parkinson disease treated with levodopa-carbidopa. Tolcapone Fluctuator Study Group IIIArch Neurol1998551089959708959
- AntoniniAIsaiasIUCanesiMDuodenal levodopa infusion for advanced Parkinson’s disease: 12-month treatment outcomeMov Disord2007221145917661426
- BaasHBeiskeAGGhikaJCatechol-O-methyltransferase inhibition with tolcapone reduces the “wearing off” phenomenon and levodopa requirements in fluctuating parkinsonian patientsJ Neurol Neurosurg Psychiatry19976342189343116
- BlackKJHersheyTHartleinJMLevodopa challenge neuroimaging of levodopa-related mood fluctuation in Parkinson’s diseaseNeuropsychopharmacology20053059060115602502
- BonuccelliUDel DottoPLucettiCDiurnal motor variations to repeated doses of levodopa in Parkinson’s diseaseClin Neuropharmacol200023283310682228
- BorgesNTolcapone in Parkinson’s disease: liver toxicity and clinical efficacyExpert Opin Drug Saf20054697315709899
- CaraceniTMusiccoMLevodopa or dopamine agonists, or deprenyl as initial treatment for Parkinson’s disease. A randomized multicenter studyParkinsonism Relat Disord200171071411248591
- CeravoloRPicciniPBaileyDL18F-dopa PET evidence that tolcapone acts as a central COMT inhibitor in Parkinson’s disease. These findings are compatible with clinical doses of tolcapone having a significant blocking effect on peripheral and central COMT but not DDC activity in PDSynapse200243201711793426
- ChaudhuriKRMartinez-MartinPBrownRGThe metric properties of a novel non-motor symptoms scale for Parkinson’s disease: results from an international pilot studyMov Disord20072219011117674410
- ChaudhuriKRMartinez-MartinPSchapiraAHInternational multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson’s disease: the NMS Quest studyMov Disord2006219162316547944
- ColosimoCThe rise and fall of tolcaponeJ Neurol1999246880210552233
- DeaneKHSpiekerSClarkeCECatechol-O-methyltransferase inhibitors versus active comparators for levodopa-induced complications in Parkinson’s diseaseCochrane Database Syst Rev2004182005
- DefebvreLMotor complications in DOPA treatment of Parkinson’s disease: clinical description and evaluationTherapie20045993615199675
- European Medicines Evaluation AgencyEMEA public statement on the lifting of the suspension of the marketing authorisation for Tolcapone (Tasmar) (online) Accessed 2004. URL: http://www.emea.eu.int
- FactorSAMolhoESFeustelPJLong-term comparative experience with tolcapone and entacapone in advanced Parkinson’s diseaseClinic Neuropharmacol2001242959
- GoetzCGPoeweWRascolOEvidence based medical review update: pharmacological and surgical treatments of Parkinson’s disease: 2001 to 2004Mov Disord2005205233915818599
- HalkiasIAHaqIHuangZWhen should levodopa therapy be initiated in Patients with Parkinson’s disease?Drugs Aging2007246173
- HauserRAMcDermottMPMessingSFactors associated with the development of motor fluctuations and dyskinesias in Parkinson DiseaseArch Neurol20066317566017172616
- KaakkolaSClinical pharmacology, therapeutic use and potential of COMT inhibitors in Parkinson’s DiseaseDrugs20005912335010882160
- LangAELozanoAMParkinson’s disease. Second of two parts (Review)N Engl J Med19983391130439770561
- LauterbachECThe neuropsychiatry of Parkinson’s diseaseMinn Med20059615573
- Leegwater-KimJWatersCTolcapone in the management of Parkinson’s diseaseExpert Opin Pharmacother2006722637017059382
- LeesAJAlternatives to levodopa in the initial treatment of early Parkinson’s diseaseDrugs Aging2005227314016156677
- LeesAJRatziuVTolosaESafety and tolerability of adjunctive tolcapone therapy in early Parkinson’s disease patientsJ Neurol Neurosurg Psychiatry200778944817098835
- LimousinPPollakPPfefenJPAcute administration of levodopa-beserazide and Tolcapone, a COMT inhibitor, in Parkinson’s diseaseClin Neuropharmacol199518258658635184
- MaricleRANuttJGCarterJHMood and anxiety fluctuation in Parkinson’s disease associated with levodopa infusion: preliminary findingsMov Disord199510329327651451
- MaricleRAValentineRJCarterJMood response to levodopa infusion in early Parkinson’s DiseaseNeurology199850189029633754
- Martinez-MartinPChaudhuriKRStocchiFPrevalence of non-motor symptoms in Parkinson’s disease in an international setting; Study using non-motor symptoms questionnaire in 545 patientsMov Disord2007221623917546669
- MurielMPOrieuxGHirschECLevodopa but not ropinirole induces an internalization of D1 dopamine receptors in parkinsonian ratsMov Disord20021711747912465054
- NapolitanoADel DottoPPetrozziLPharmacokinetics and pharmacodynamics of L-Dopa after acute and 6-week tolcapone administration in patients with Parkinson’s diseaseClin Neuropharmacol19992224910047930
- NuttJGPharmacodynamics of levodopa in Parkinson’s diseaseClin Exp Pharmacol Physiol199522837408593740
- NuttJGCarterJHWoodwardWREffect of brief levodopa holidays on the short-duration response to levodopa: evidence for tolerance to the antiparkinsonian effectsNeurology1994441617227936285
- NuttJGHolfordNHGThe response to levodopa in Parkinsons’ disease: imposing pharmacological law and orderAnnals Neurol19963956173
- OlanowCWTolcapone and hepatotoxic effects. Tasmar Advisory PanelArch Neurol200057263710681087
- OlanowCWWattsRLKollerWCAn algorithm (decision tree) for the management of PD; 2001: treatment guidelinesNeurology200156Suppl 5S18811402154
- OldfieldVKeatingGMPerryCMRasagiline: a second generation monoamine oxidase type B inhibitor for the treatment of Parkinson’s diseaseDrugs20076717254717683172
- OnofrjMThomasAIaconoDSwitch-Over from tolcapone to entacapone in severe Parkinson’s disease patientsEur Neurol200146111611455177
- Opacka-JuffryJBrooksDJL-dihydroxyphenylalanine and its decarboxylase: new ideas on their neuroregulatory rolesMov Disord19951024197651438
- PahwaRFactorSALyonsKEPractice parameter: treatment of Parkinson Disease with motor fluctuations and dyskinesia (an evidence-based review): Report of the quality Standards Subcommittee of the American Academy of NeurologyNeurology2006669839516606909
- [PSG] Parkinson Study GroupImpact of deprenyl and tocopherol treatment on Parkinson’s Disease in DATATOP patients requiring levodopaAnn Neurol19963937458572664
- [PSG] Parkinson Study GroupA controlled trial of rasagiline in early Parkinson disease: the TEMPO StudyArch Neurol20025919374312470183
- [PSG] Parkinson Study GroupA randomized placebo-controlled trial of rasagiline in levodopa – treated patients with Parkinson disease and motor fluctuations: the PRESTO studyArch Neurol200562241815710852
- QuattroneAZappiaMAgugliaUThe subacute levodopa test for evaluating long-duration response in Parkinson’s diseaseAnn Neurol199538389957668824
- RajputAHMartinWSaint-HilaireMHTolcapone improves motor function in parkinsonian patients with the “wearing-off” phenomenon: a double-blind, placebo-controlled, multicenter trialNeurology1997491066719339691
- RascolOGoetzCKollerWTreatment interventions for Parkinson’s disease: an evidence based assessmentLancet200235915899812047983
- RascolOMelamedEOertelWRasagiline, as an adjunct to levodopa in patients with Parkinson’s disease and motor flucuations (LARGO, lasting effect in adjunct therapy with Rasagiline given once daily; study): a randomized, double-blind, parallel-group trialLancet20053659475415766996
- RileyDELangAEThe spectrum of levodopa-related fluctuations in Parkinson’s diseaseNeurology1993431459648350996
- SchragAQuinnNDyskinesias and motor fluctuations in Parkinson’s disease A community-based studyBrain2000123229730511050029
- ShanDELeeSJChaoLYGait analysis in advanced Parkinson’s disease – effect of levodopa and tolcaponeCan J Neurol Sci20012870511252300
- ShoulsonIOakesDFahnSImpact of sustained deprenyl (selegiline) in levodopa-treated Parkinson’s disease: a randomized placebo-controlled extension of the deprenyl and tocopherol antioxidative therapy of parkinsonism trialAnn Neurol2002516041212112107
- SuchowerskyOBaileyPPourcherEComparison of two dosages of Tolcapone added to levodopa in nonfluctuating patients with PDClin Neuropharmacol2001242142011479392
- The Entacapone to Tolcapone Switch Study InvestigatorsEntacapone to tolcapone switch: multicenter double blind, randomized, active controlled trial in Advanced Parkinson’s diseaseMov Disord200722141917089403
- Van LaarTLevodopa-induced response fluctuations in patients with Parkinson’s disease: strategies for managementCNS Drugs2003174758912751918
- WatkinsPCOMT inhibitors and liver toxicityNeurology20005511 SupplS51211147510
- WidnellKLComellaCRole of COMT Inhibitors and dopamine agonists in the treatment of motor fluctuationsMov Disord200520Suppl 11307
- WitjasTKaphanEAzulayJPNonmotor fluctuations in Parkinson’s disease: frequent and disablingNeurology2000594081312177375
- ZappiaMAnnesiGNicolettiGSex differences in clinical and genetic determinants of levodopa peak-dose dyskinesias in Parkinson disease: an exploratory studyArch Neurol200562601515824260
- ZappiaMBoscoDPlastinoMPharmacodynamics of the long-duration response to levodopa in PDNeurology1999a535576010449120
- ZappiaMColaoRMontesantiRLong-duration to levodopa influences the pharmacodynamics of short-duration response in Parkinson’s diseaseAnn Neurol19974224589266736
- ZappiaMOlivieriRLBoscoDThe long-duration response to L-dopa in the treatment of early PDNeurology2000542475010636162
- ZappiaMOlivieriRLMontesantiRLoss of long-duration response to levodopa over time i0Implications for wearing-offNeurology1999b52763710078724