Abstract
Excessive sleepiness (ES) is a major but underestimated public health concern associated with significant impairments in alertness/wakefulness and significant morbidity. The term ES has been used in the sleep medicine literature for years, but due to its nonspecific symptoms (ie tiredness or fatigue), it frequently goes unrecognized or is misdiagnosed in primary care. In some cases ES arises due to poor sleep habits or self-imposed sleep deprivation; however, ES is also a key component of a number of sleep/wake disorders and multiple medical and psychiatric disorders. Identification and treatment of ES is critical to improve the quality of life and well-being of patients and for the safety of the wider community. The inability of patients to recognize the nature, extent, and symptomatic profile of sleep/wake disorders requires vigilance on the part of healthcare professionals. Interventions to address ES and its associated impairments, treatment of the underlying sleep/wake disorder, and follow-up are a priority given the potential for serious consequences if left untreated. Wakefulness-promoting agents are available that treat ES associated with sleep/wake disorders. This review examines current approaches for managing this debilitating and potentially life-threatening condition, focusing on the place of armodafinil as a wakefulness-promoting agent.
Introduction
Excessive sleepiness (ES) is a chronic inability to maintain alertness during waking episodes.Citation1 This impairment in wakefulness interferes with the activities of daily living and can increase the risk for sleep-related accidents at work, at home, and on the road.Citation2–Citation5 Moreover, ES can cause significant impairment to an individual’s work performance, overall well-being, clinical condition, psychological health and function, executive function, and social relationships.Citation2,Citation6–Citation9 ES is associated with many medical and psychiatric disorders; this review will focus on the sleep/wake disorders obstructive sleep apnea (OSA), narcolepsy, and shift-work disorder (SWD).Citation10–Citation12
Sleepiness is infrequently voiced as a presenting symptom for patients with ES, who instead report that they are experiencing tiredness, fatigue, or a lack of energy. Identifying and managing patients with ES requires a comprehensive approach to address any underlying condition and improve sleep practices. If ES arises from chronic self-imposed sleep restriction, efforts to improve sleep habits, such as increasing time in bed, may be sufficient. However when ES arises from an underlying disorder, interventions are typically required to address both the disorder as well as the ES. Wakefulness-promoting agents are proving particularly useful in addressing ES in the context of sleep/wake disorders. The introduction of armodafinil (Nuvigil®; Cephalon Inc., Frazer, PA), the R-enantiomer of the racemic wakefulness-promoting agent modafinil,Citation13 has expanded the pharmacologic options available for the management of ES. This agent is approved for the treatment of ES associated with OSA (as adjunctive therapy), SWD, and narcolepsy.
This review will explore practical ways in which the recognition of ES can be simplified in clinical practice and current approaches to managing this important symptom, focusing on the appropriate role of armodafinil in these patients.
Recognizing ES in clinical practice
Initial presentation
Vigilance for sleep/wake disorders and ES is necessary as clinical presentation can often be confounded by nondescript complaints such as tiredness, fatigue, lack of energy, apathy, lethargy, poor memory, and poor concentration. Patients presenting with ES may not recognize their daytime impairment, as their primary complaint may be disturbed sleep. For example, many patients with ES, including those with OSA, SWD, or narcolepsy, report disturbed sleep, and in one study, approximately half of patients diagnosed with OSA in a sleep center initially presented with the complaint of insomnia.Citation14
Patients presenting with symptoms or risk factors for disorders such as OSA, SWD, or narcolepsy often fail to recognize their ES or fail to relate cognitive impairments, such as an inability to focus or concentrate and memory problems, to ES and thus may not volunteer information about sleepiness unless specifically asked. A useful approach to differentiating ES from a generalized complaint such as fatigue is to ask patients if the presenting symptom (ie, “fatigue”) improves in sedentary situations. Most patients with ES will report an exacerbation of their complaint in soporific conditions, whereas patients with mental-, muscle-, or depression-related fatigue typically report an improvement during inactivity.
Comorbid conditions
The nonspecific nature of the complaints in patients with ES, such as tiredness and fatigue, may lead to a diagnosis of depression. This is of particular concern as it may result in the inappropriate prescription of antidepressant therapy and may lead to a delay in the recognition and treatment of ES. Moreover, while depression and sleep/wake disorders such as OSA can and frequently do coexist, it is important to follow up and screen such patients for the ongoing presence of ES. Hypertension, diabetes, and cardiovascular disease have a high comorbidity with OSA and associated ES.Citation15 Consideration of underlying sleep/wake disorders and ES in patients presenting with such comorbidities or generalized psychiatric complaints suggestive of depression is critical in ensuring prompt and appropriate intervention. presents a clinical checklist for the initial identification and assessment of ES.
Table 1 Identification and initial assessment of ES in the clinic
Assessment of ES
The Epworth Sleepiness Scale (ESS) is a useful screening tool for identifying ES ().Citation16 The ESS is a self-administered questionnaire that is used worldwide to quantify ES at baseline and to monitor ES at follow-up in patients with a variety of sleep complaints including OSA and narcolepsy.Citation17,Citation18 Respondents are asked to determine their likelihood of dozing in eight sedentary situations (including sitting and reading, watching TV, and in a car while stopped for a few minutes in traffic) on a 4-point scale (0 = never, 1 = slight, 2 = moderate, 3 = high). The total score ranges from 0 to 24, and a score ≥10 indicates an abnormal level of sleepiness. Objective laboratory assessments, such as the Multiple Sleep Latency Test (MSLT) and the Maintenance of Wakefulness Test (MWT), are useful in ruling out specific intrinsic sleep/wake disorders such as narcolepsy and in providing an objective assessment of sleepiness. While these laboratory measures are useful objective assays of sleepiness, the ESS has some important advantages in general clinical settings outside the sleep disorders center. First, it is easily administered. Second, the ESS is extremely valuable as a screening tool to identify and quantify sleepiness in at-risk patients. Third, the ESS can be used for patient follow-up and management as it is a useful indicator of treatment response.Citation17,Citation19
Table 2 The Epworth Sleepiness ScaleCitation16
Managing patients with ES
Treatment of ES
The initial steps in treating a patient with ES should be to identify and, if possible, address any underlying medical, psychiatric, or sleep/wake disorder that contributes to their ES and to ensure that the patient is taught good sleep hygiene practices. In narcolepsy, ES arises from an intrinsic dysfunction in sleep regulation and medication is required to enhance wakefulness. For a significant proportion of patients with sleep/wake disorders amenable to medical, mechanical, or other interventions, ES will persist even with appropriate and effective treatment for the underlying disorder. Continuous positive airway pressure (CPAP) therapy is the gold standard treatment for OSA and ensures that the upper airway remains open during sleep by acting as a pneumatic splint. However, despite treatment with CPAP therapy, 34% of patients with OSA have ongoing subjective sleepiness as measured by the ESS, and 65% of patients with OSA have persistent objective ES as measured by the MSLT.Citation20 The first goal of therapy for OSA is to ensure CPAP compliance, but adjunctive pharmacotherapy specifically aimed at improving wakefulness may be of benefit to improve the ongoing sleepiness that may persist despite CPAP therapy.
Stimulants and wakefulness-promoting medications
Stimulants, such as methylphenidate and dextroamphetamine, are approved for use in combatting ES in patients with narcolepsy but they have an associated potential for abuse and adverse cardiovascular and central nervous system (CNS) effects.Citation21 Sodium oxybate is approved for the treatment of ES and cataplexy in patients with narcolepsy.
The wakefulness-promoting agent modafinil has been available since 1999 and offers an alternative to traditional stimulants for the treatment of ES. Most recently, the R-enantiomer of modafinil – armodafinil – has been approved for the treatment of ES associated with SWD, narcolepsy, and as an adjunctive therapy for persistent ES in patients being treated for OSA. The remainder of this review will focus on the role of armodafinil in the management of ES in these approved indications.
Armodafinil
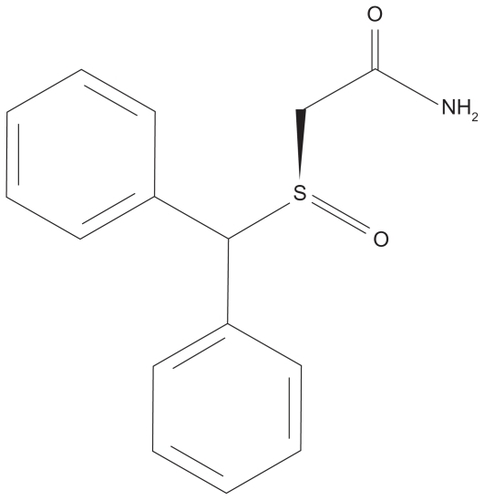
Armodafinil (2-[(R)-(diphenylmethyl)sulfinyl]acetamide) is an oral wakefulness-promoting agentCitation13 (). Plasma concentrations of the R-enantiomer remain elevated for a prolonged period of time relative to the S-enantiomer (10 to 14 hours compared with 3 to 4 hours).Citation22–Citation24 To date there have been no adequate head to head studies of armodafinil versus modafinil in any of its approved indications.
The Food and Drug Administration-approved dose of armodafinil for patients with narcolepsy or ES associated with OSA is 150 mg or 250 mg taken as a single dose in the morning or 150 mg taken 1 hour before the start of the night shift for patients with SWD.Citation25
Pharmacology and mode of action
The precise mechanism by which armodafinil and modafinil promote wakefulness is unknown but it is likely to involve the selective potentiation of CNS catecholaminergic signaling.Citation26 Specifically, modafinil may exert its effect by increasing electrical coupling across gap junctions between neurons.Citation27 Potentiation of glutaminergic synapses on hypocretin/orexin neurons in the lateral hypothalamusCitation28 together with effects on the GABA and monoaminergic systems are thought to mediate the wakefulness-promoting effects of modafinil and may also account for the observed clinical effect of armodafinil.Citation29,Citation30 In addition, although armodafinil is not a direct or indirect dopamine receptor agonist, it has been shown to bind to the dopamine transporter and inhibit dopamine reuptake.Citation31,Citation32
Pharmacokinetics of armodafinil
Armodafinil displays linear pharmacokinetics over the 50 mg to 400 mg dose range.Citation33 The peak plasma concentration is reached ~2 hours after dosing, and steady state is reached within ~7 days with once-daily dosing, with no gender effects.Citation33 Food slows the rate but not the extent of absorption of armodafinil.Citation33 Elimination of armodafinil is monophasic with a mean elimination half-life of ~15 hours.Citation33
Armodafinil dose reductions should be considered for patients with severe hepatic impairment based on an observed reduction in oral clearance and an increase in the steady-state concentration of modafinil in this patient group.Citation25 Similar reductions should be considered for elderly patients in whom elimination may be slowed. Armodafinil is metabolized via amide hydrolysis followed by sulfone formation via the cytochrome P450 (CYP) 3A4/5 enzymes.Citation25 Dosage adjustment to sustain clinical effect may be necessary if patients are receiving other agents that are also substrates for these enzymes, eg, steroidal contraceptives, cyclosporine, midazolam, and triazolam.Citation25 Armodafinil has also displayed reversible inhibition of CYP2C19, and drugs such as diazepam that are metabolized via this enzyme may display prolonged elimination and thus lower doses of these agents may be required if coadministered.Citation34
Armodafinil for ES in OSA
OSA is a condition characterized by recurrent complete and partial obstruction of the airway, resulting in repeated arousal from sleep. OSA is the most common of the sleep-related breathing disorders.Citation1 In the USA, 9% of women and 24% of men meet minimum diagnostic criteria for OSA (≥5 obstructive events per hour), while at least 2% and 4% of women and men, respectively, are thought to have OSA with associated ES and resultant daytime impairment.Citation35 Patients with OSA may also present initially with the complaint of insomnia.Citation14
The efficacy of armodafinil as a therapy to address ES in patients who are receiving treatment for OSA has been evaluated in >650 individuals who took part in one of two phase III studies ().Citation36,Citation37 Patients recruited into these studies had a diagnosis of OSA and also residual ES (ESS score ≥10 points and a Clinical Global Impression of Severity of Illness score ≥4 points) despite effective regular CPAP therapy. The MWT was a primary efficacy endpoint and this test was used to assess participants at 09:00, 11:00, 13:00, 15:00, 17:00, and 19:00 at baseline and every 4 weeks during treatment with armodafinil (150 or 250 mg daily) or placebo until study completion at 12 weeks. Armodafinil significantly improved patients’ mean sleep latency at the first four MWT assessments at all visits in each studyCitation36,Citation37 (P < 0.001) (),Citation39 and a post hoc analysis of pooled data from both studies also demonstrated that the beneficial effects of armodafinil were sustained late in the waking day, with a significant improvement relative to placebo observed from the first assessment point at week 4 and at the final visit ().Citation39 However, differences between armodafinil and placebo in terms of MWT assessments at timepoints later in the day (15:00, 17:00, and 19:00) did not reach statistical significance at the final visit in the individual studies.Citation36,Citation37
Figure 2 Improvements in maintenance of wakefulness with armodafinil versus placebo among adults with residual ES during effective management of OSA.Citation39 Panel A: early assessments (09:00 to 15:00). Panel B: late assessments (15:00 to 19:00).
Abbreviations: ES, excessive sleepiness; MWT, maintenance of wakefulness test; OSA, obstructive sleep apnea; SEM, standard error of the mean.
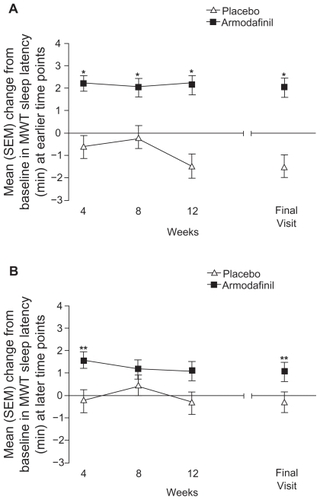
Table 3 Efficacy of armodafinil in the treatment of ES associated with OSA, SWD, and narcolepsy
Clinical condition measured using the Clinical Global Impression of Change (CGI-C) was a second primary endpoint in both of the studies that evaluated armodafinil in patients with ES associated with OSA. In the study conducted by Roth and colleagues,Citation36 a significantly greater proportion of armodafinil-treated patients experienced at least minimal improvement on the CGI-C at all time points (P < 0.001) compared with patients receiving placebo. The proportion of patients in the armodafinil group with at least minimal improvement on the CGI-C was also significantly higher versus the placebo group at the final visit in the study conducted by Hirshkowitz and colleagues (71% vs 53%, respectively; P = 0.0069).Citation37
Patients in these studies also completed the 9-item Brief Fatigue Inventory. Patients treated with armodafinil 150 or 250 mg experienced significant reductions in fatigue (−1.2 and −1.3 points, respectively) compared with those who received placebo (−0.6 points; P < 0.01 for both doses vs placebo) ().Citation38 Cognitive performance was assessed with a battery of tests aimed at evaluating aspects of memory and attention. Both studies individually and the pooled analysis of data confirmed that armodafinil significantly improved the quality of secondary memory (the ability to store and retrieve information) compared with placebo (P < 0.05) ().Citation39 Armodafinil also significantly improved the overall ESS score as well as patients’ ability to participate in activities of daily living from the first assessment at 4 weeks (P < 0.0001 vs placebo). These improvements were sustained throughout the 12-week study period ().
In summary, armodafinil relieves residual ES associated with treated OSA and improves ES-related sequelae, such as fatigue and memory deficits, allowing patients to re-engage with their normal daily lives.
Armodafinil for ES in SWD
Shift work, including night and rotating shifts, is a regular component of the working pattern for millions of individuals worldwide, including almost 22 million Americans.Citation43 These individuals are at risk of SWD, a circadian rhythm sleep disorder that arises from misalignment with the endogenous circadian sleep/wake cycle as a result of the artificial sleep/wake pattern imposed by shift work.Citation44 SWD (defined by a diagnosis of insomnia and/or ES) has been reported to affect 32% of night-shift workers and 26% of rotating-shift workers.Citation2 Such individuals are at increased risk for a variety of potentially serious medical sequelae, including gastrointestinal and cardiovascular disease, and also may experience detrimental effects to their social, economic, and personal quality of life.Citation2,Citation8,Citation45–Citation47 The persistent ES and insomnia that are the defining symptoms of SWD result in elevated rates of work absenteeism and place affected individuals at considerably increased risk for accidents compared with shift workers without SWD.Citation2
In a 12-week phase III study of 216 permanent or rotating night-shift workers randomized to receive armodafinil 150 mg 30 to 60 minutes before the start of their scheduled work period, active treatment significantly improved sleep latency on the MSLT compared with placebo at the final study assessment (P < 0.0001).Citation41 Importantly, the wakefulness benefit for armodafinil was sustained throughout the work period () but did not affect scheduled sleep.Citation40 CGI-C ratings improved in a greater proportion of patients treated with armodafinil than in the placebo group (75% vs 59%, respectively; P = 0.001).Citation40 Patients treated with armodafinil also reported a statistically significant reduction in the maximum level of sleepiness during the work period compared with those who received placebo (2.0 vs 1.1 points on the Karolinska Sleepiness Scale; P < 0.0001) and a statistically significant twofold reduction in sleepiness during the commute home (1.2 vs 0.6 points; P = 0.0027), a key risk period for sleepiness-related accidents.Citation40
Armodafinil for ES in narcolepsy
Narcolepsy is an intrinsic sleep/wake disorder with a primary presenting symptom of ES. Although the estimated prevalence of this condition is low (<1% of the US population),Citation48 there are variants of narcolepsy that also warrant clinical attention.Citation49 The cognitive impact of this disorder is considerable, with patients typically experiencing impaired concentration as well as learning and memory difficulties. ES is the hallmark of narcolepsy and this symptom has a significant impact on patients’ ability to function safely and effectively in their daily lives.
A phase III study has confirmed the benefit of armodafinil in addressing the ES component of narcolepsy.Citation42 This 12-week study evaluated 194 men (aged 18 to 65 years) with narcolepsy diagnosed according to the International Classification of Sleep Disorders Criteria.Citation1 Patients received either armodafinil (150 mg or 250 mg daily) or placebo for up to 12 weeks. Daytime sleep tendency (assessed using the MWT) during the period 09:00 to 15:00 improved significantly with active treatment (+1.9 minutes for both armodafinil treatment groups combined) compared with a reduction of 1.9 minutes among those who received placebo (P < 0.01 vs armodafinil). As reported for OSA, the beneficial effects of armodafinil were sustained late in the waking day (15:00 to 19:00), with sleep latency improvements from baseline of 1.6 minutes for both armodafinil treatment groups combined compared with a reduction of 1.2 minutes for those who received placebo (P < 0.05 vs armodafinil) (; ). CGI-C assessments indicated that the proportion of patients with at least minimal improvement in clinical condition at the final visit was significantly higher in the armodafinil groups versus the placebo group (P < 0.0001).Citation42
Figure 3 Improvements in MWT with armodafinil versus placebo among adults with narcolepsy. Reproduced with permission from Harsh JR, Haydak R, Rosenberg R, et al. The efficacy and safety of armodafinil as treatment for adults with excessive sleepiness associated with narcolepsy. Curr Med Res Opin. 2006;22(4):761–774.Citation42 Copyright © 2006 Informa Healthcare.
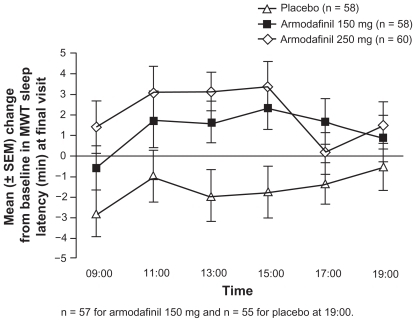
Baseline ESS scores were high (~17 points) but improved significantly compared with placebo for those patients treated with armodafinil (P < 0.01) (). Patients treated with armodafinil also experienced significant improvements in a variety of cognitive parameters including power of attention (as derived from improvements in choice reaction time and digit vigilance) (P < 0.05), quality of episodic secondary memory (P < 0.05), and speed of memory (P < 0.05) at the final study assessment.Citation42 Patients also reported significant relief from the fatigue associated with this disorder (P = 0.0002 vs placebo at the final assessment).Citation42
Safety of armodafinil
The most commonly reported adverse events with armodafinil were similar across the patient populations for all three approved indications, however, detailed analyses of the safety of armodafinil in patients with SWD were recently published ().Citation39,Citation42 Headache was the most commonly reported event and nausea, dizziness, and insomnia were the next most commonly reported events.Citation39,Citation42 Less than 12% of active-treatment patients in any study withdrew from study treatment due to an emergent adverse event compared with <6% in the placebo group; headache was the adverse event most frequently associated with treatment withdrawal.Citation36,Citation37,Citation39,Citation42 A dose-dependent effect was noted for headache and a number of the less frequently encountered adverse events including rash, depression, dry mouth, insomnia, and nausea.Citation25
Table 4 The most commonly reported adverse events (>5% of patients) among adults with narcolepsy or OSA treated with armodafinil (150 mg or 250 mg daily)Citation39,Citation42
Further safety data concerning armodafinil have recently been reported in two open-label, 12-month studies, one of which recruited patients from one of the earlier armodafinil trials concerning ES associated with OSA, SWD, or narcolepsy, while patients in the other study were new to treatment.Citation50,Citation51 These studies enrolled a total of 1071 patients and confirmed that headache is the most prevalent adverse event experienced when taking armodafinil (reported by 25% and 17% of armodafinil-treated patients in the two trials).Citation50,Citation51 Nasopharyngitis and insomnia were experienced by 17% and 14% of armodafinil-treated patients, respectively. Citation50 No clinically meaningful changes from baseline in laboratory variables or clinical examination findings were reported.Citation51
Serious rash and multi-organ hypersensitivity reactions have been reported in association with the use of modafinil. No serious skin rashes or hypersensitivity reactions have been reported in clinical trials of armodafinil.Citation25 However, because armodafinil is the R-isomer of modafinil, the risk of serious rash/hypersensitivity reaction with armodafinil remains possible. Similarly, psychiatric adverse experiences have been reported in patients treated with modafinil. The incidence and type of psychiatric symptoms associated with armodafinil are expected to be similar to those for modafinil.Citation25
Abuse potential
Armodafinil is classified as a schedule IV medication, as it has a lower potential for abuse compared with schedule II agents, such as methylphenidate and amphetamines, or schedule III agents such as sodium oxybate. A recent studyCitation32 reported that modafinil increased dopamine levels in the nucleus accumbens region of the brain – such increases are associated with the potential for abuse – and for this reason modafinil and armodafinil should be used with caution in patients with a history or at risk of drug abuse.
Therapeutic implications and future developments
The personal health and public safety implications of ES and its associated impairments have been under-recognized and under-treated to date. ES-associated detriments in wakefulness and alertness are linked with an increased risk for occupational and automobile accidents.Citation2–Citation5
There is a need to identify and treat ES to potentially improve the safety and well-being of patients and the wider community. Treatment of ES also has the potential to improve work productivity and to enhance patients’ quality of life and ability to engage with their daily lives. Patients with sleep/wake disorders may not always be able to fully comprehend the severity or impact of this debilitating and potentially dangerous symptom. It is also possible that a lack of awareness of the availability of interventions for ES may also contribute to under–reporting of this symptom. Physician vigilance for ES as a core component of the clinical profile of patients with sleep/wake disorders is a fundamental aspect of its identification and treatment. Clinical recognition of ES should extend to patients at risk for sleep/wake disorders, including those with cardiac disease, which is often comorbid with OSA, as well as those presenting with generalized somatic symptoms for whom a differential diagnosis from a mood disorder should be sought.
Armodafinil has been shown to improve wakefulness throughout the day in patients with the sleep/wake disorders OSA and narcolepsy, and throughout the working period/shift in patients with SWD. In addition, a randomized, placebo-controlled phase III study in 427 subjects traveling between the USA and France who had previously experienced jet lag demonstrated that armodafinil 150 mg significantly improved wakefulness compared with placebo, as measured using the MSLT (+11.7 minutes vs +4.8 minutes, respectively; P < 0.0001).Citation52 A number of studies are ongoing that may better define and expand the clinical utility of armodafinil.
Effective and well-tolerated pharmacotherapeutic options are now available for the treatment of ES associated with sleep/wake disorders. It is important that physicians include the recognition and treatment of ES as a part of their management program.
Disclosures
Editorial support was provided by Jane Bryant of Anthemis Consulting Ltd, Aaron Henley, and support from Cephalon Inc., Frazer, PA who provided a medical accuracy review. The authors were not compensated and retained full editorial control over the content of the paper.
Dr Schwartz has served as a consultant and/or speaker for AstraZeneca, Boeringer Ingelheim, Cephalon, Inc., GlaxoSmithKline, Jazz, Pfizer, Schering-Plough, Sepracor, and Takeda.
Dr Roth has received grants from Aventis, Cephalon, Inc., GlaxoSmithKline, Neurocrine, Pfizer, Sanofi, Schering-Plough, Sepracor, Somaxon, Syrex, Takeda, TransOral, Wyeth, and Xenoport, and has acted as a consultant for Abbott, Acadia, Acoglix, Actelion, Alchemers, Alza, Ancile, Arena, AstraZeneca, Aventis, AVER, Bristol-Myers Squibb, BTG, Cephalon, Inc., Cypress, Dove, Elan, Eli Lilly, Evotec, Forest Labs, GlaxoSmith-Kline, Hypnion, Impax, Intec, IntraCellular, Jazz, Johnson and Johnson, King, Lundbeck, McNeil, MediciNova, Merck, Neurim, Neurocrine, Neurogen, Novartis, Orexo, Organon, Prestwick, Proctor and Gamble, Pfizer, Purdue Pharma, Resteva, Roche, Sanofi, Schering-Plough, Sepracor, Servier, Shire, Somaxon, Syrex, Takeda, TransOral, Vanda, Vivometrics, Wyeth, Yamanuchi, and Xenoport. Dr Roth has also been a speaker for Cephalon, Inc., Sanofi, and Takeda.
Dr Drake has received research support from Cephalon, Inc., Pfizer Inc., and Neurocrine, and has participated in speaking engagements supported by Sepracor and Sanofi-Aventis.
References
- American Academy of Sleep MedicineInternational Classification of Sleep Disorders2nd EditionDiagnostic and Coding ManualWestchester, ILAmerican Academy of Sleep Medicine2005
- DrakeCLRoehrsTRichardsonGWalshJKRothTShift work sleep disorder: prevalence and consequences beyond that of symptomatic day workersSleep20042781453146215683134
- HorstmannSHessCWBassettiCGuggerMMathisJSleepiness-related accidents in sleep apnea patientsSleep200023338338910811382
- KingshottRNCowanJOJonesDRThe role of sleep-disordered breathing, daytime sleepiness, and impaired performance in motor vehicle crashes – a case control studySleep Breath200482617215211390
- LindbergECarterNGislasonTJansonCRole of snoring and daytime sleepiness in occupational accidentsAm J Respir Crit Care Med2001164112031203511739131
- DauvilliersYAPaquereauJBastujiHDronotXWeilJJViot-BlancVPsychological health in central hypersomnia: The French Harmony StudyJ Neurol Neurosurg Psychiatry200980663664119211597
- MulgrewATRyanCFFleethamJAThe impact of obstructive sleep apnea and daytime sleepiness on work limitationSleep Med200791425317825611
- PucaFMPerrucciSPrudenzanoMPQuality of life in shift work syndromeFunct Neurol19961152612689119269
- SforzaEJanssensJPRochatTIbanezVDeterminants of altered quality of life in patients with sleep-related breathing disordersEur Respir J200321468268712762357
- KeamSWalkerMCTherapies for narcolepsy with or without cataplexy: evidence-based reviewCurr Opin Neurol200720669970317992092
- SchwartzJRLRothTShift work sleep disorder. Burden of illness and approaches to managementDrugs200666182357237017181377
- SmithIEQuinnellTGPharmacotherapies for obstructive sleep apnoea. Where are we now?Drugs200464131385139915212557
- HauckWAdamPBobierCLandmesserNUse of large-scale chromatography in the preparation of armodafinilChirality200820889689918506833
- KrakowBMelendrezDFerreiraEPrevalence of insomnia symptoms in patients with sleep-disordered breathingChest200112061923192911742923
- HirshkowitzMThe clinical consequences of obstructive sleep apnea and associated excessive sleepinessJ Fam Pract200857SupplS9S1618687238
- JohnsMWA new method for measuring daytime sleepiness: the Epworth sleepiness scaleSleep19911465405451798888
- HardingeFMPitsonDJStradlingJRUse of the Epworth Sleepiness Scale to demonstrate response to treatment with nasal continuous positive airways pressure in patients with obstructive sleep apnoeaRespir Med19958996176207494915
- JohnsMWSensitivity and specificity of the multiple sleep latency test (MSLT), the maintenance of wakefulness test and the Epworth Sleepiness Scale: failure of the MSLT as a gold standardJ Sleep Res20009151110733683
- HeatonKAndersonDA psychometric analysis of the Epworth Sleepiness ScaleJ Nurs Meas200715317718818232617
- WeaverTEMaislinGDingesDFRelationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioningSleep200730671171917580592
- DarkeSKayeSMcKetinRDuflouJMajor physical and psychological harms of methamphetamine useDrug Alcohol Rev200827325326218368606
- RobertsonPJrHellriegelETClinical pharmacokinetic profile of modafinilClin Pharmacokinet200342212313712537513
- WongYNKingSPSimcoeDOpen-label, single-dose pharmacokinetic study of modafinil tablets: influence of age and gender in normal subjectsJ Clin Pharmacol199939328128810073328
- WongYNSimcoeDHartmanLNA double-blind, placebo-controlled, ascending-dose evaluation of the pharmacokinetics and tolerability of modafinil tablets in healthy male volunteersJ Clin Pharmacol199939130409987698
- Food and Drug AdministrationFDA-approved Labeling Text for NDA 21-875/NUVIGIL™ (armodafinil) Tablets2007 URL: http://www.accessdata.fda.gov/drugsatfda_docs/label/2007/021875lbl.pdfAccessed June 30, 2009
- WisorJPDementWCAimoneLWilliamsMBozyczko-CoyneDArmodafinil, the R-enantiomer of modafinil: wake-promoting effects and pharmacokinetic profile in the ratPharmacol Biochem Behav200685349249917134745
- BeckPOdleAWallace-HuittTSkinnerRDGarcia-RillEModafinil increases arousal determined by P13 potential amplitude: an effect blocked by gap junction antagonistsSleep200831121647165419090320
- RaoYLiuZWBorokEProlonged wakefulness induces experience-dependent synaptic plasticity in mouse hypocretin/orexin neuronsJ Clin Invest2007117124022403318060037
- MitchellHABogenpohlJWLilesLCBehavioral responses of dopamine beta-hydroxylase knockout mice to modafinil suggest a dual noradrenergic–dopaminergic mechanism of actionPharmacol Biochem Behav200891221722218703079
- QuWMHuangZLXuXHMatsumotoNUradeYDopaminergic D1 and D2 receptors are essential for the arousal effect of modafinilJ Neurosci200828348462846918716204
- MignotENishinoSGuilleminaultCDementWCModafinil binds to the dopamine uptake carrier site with low affinitySleep19941754364377991954
- VolkowNDFowlerJSLoganJEffects of modafinil on dopamine and dopamine transporters in the male human brain: clinical implicationsJAMA2009301111148115419293415
- DarwishMKirbyMHellriegelETYangRRobertsonPJrPharmacokinetic profile of armodafinil in healthy subjects: pooled analysis of data from three randomized studiesClin Drug Investig200929287100
- DarwishMKirbyMRobertsonPJrHellriegelETInteraction profile of armodafinil with medications metabolized by cytochrome P450 enzymes 1A2, 3A4 and 2C19 in healthy subjectsClin Pharmacokinet2008471617418076219
- YoungTPaltaMDempseyJSkatrudJWeberSBadrSThe occurrence of sleep-disordered breathing among middle-aged adultsN Engl J Med199332817123012358464434
- RothTWhiteDSchmidt-NowaraWEffects of armodafinil in the treatment of residual excessive sleepiness associated with obstructive sleep apnea/hypopnea syndrome: a 12-week, multicenter, double-blind, randomized, placebo-controlled study in nCPAP-adherent adultsClin Ther200628568970616861091
- HirshkowitzMBlackJEWesnesKNieblerGAroraSRothTAdjunct armodafinil improves wakefulness and memory in obstructive sleep apnea/hypopnea syndromeRespir Med2007101361662716908126
- HullSLankfordAVinceBNieblerGEAroraSArmodafinil improves fatigue in patients with excessive sleepiness due to obstructive sleep apnea/hypopnea syndromeAmerican Psychiatric Association Annual MeetingToronto, CanadaMay 25, 2006 [Abstract No. 127]
- RothTRipponGAAroraSArmodafinil improves wakefulness and long-term episodic memory in nCPAP-adherent patients with excessive sleepiness associated with obstructive sleep apneaSleep Breath2008121536217874255
- RothTCzeislerCAWalshJKRandomized, double-blind, placebo-controlled study of armodafinil for the treatment of excessive sleepiness associated with chronic shift work sleep disorderNeuropsychopharmacology200530SupplS140 [Abstract No. 161]
- CzeislerCAWalshJKWesnesKAAroraSARothTArmodafinil for the treatment of excessive sleepiness associated with shiftwork disorder: A randomized controlled studyMayo Clinic Proceedings2009841195897219880686
- HarshJRHaydakRRosenbergRThe efficacy and safety of armodafinil as treatment for adults with excessive sleepiness associated with narcolepsyCurr Med Res Opin200622476177416684437
- McMenaminTMA time to work: recent trends in shift work and flexible schedulesMonthly Labor Review122007315 URL: http://www.bls.gov/opub/mlr/2007/12/art1full.pdfAccessed April 2, 2009
- AkerstedtTShift work and disturbed sleep/wakefulnessOccup Med (Lond)2003532899412637592
- BoggildHKnutssonAShift work, risk factors and cardiovascular diseaseScand J Work Environ Health1999252859910360463
- KnutssonAHealth disorders of shift workersOccup Med (Lond)200353210310812637594
- CzeislerCAWalshJKWesnesKAAroraSARothTArmodafinil for the treatment of excessive sleepiness associated with shiftwork disorder: A randomized controlled studyMayo Clinic Proceedings2009841195897219880686
- LongstrethWTJrTonTGKoepsellTGersukVHHendricksonAVeldeSPrevalence of narcolepsy in King County, Washington, USASleep Med200910442242619013100
- MignotESleep, sleep disorders and hypocretin (orexin)Sleep Med20045Suppl 1S2S815301991
- BlackJHullSGTillerJYangRHarshJRMaintenance of efficacy, safety and tolerability of armodafinil: An open-label extension study [Abstract 0419]Sleep200932SupplA139
- SchwartzJRKhanAMcCallVWeintraubJTillerJA 12 month or more open-label study of the efficacy and tolerability of armodafinil [Abstract 0148]Sleep200932SupplA50A51
- BoganRTillerJYangRYouakimJRothTArmodafinil for excessive sleepiness associated with jet lag disorder [Abstract 0153]Sleep200932SupplA52