Abstract
Oxidative stress has been implicated in the progression of a number of neurodegenerative diseases, including Alzheimer’s disease (AD), Parkinson’s disease and amyotrophic lateral sclerosis. We carried out an in-depth study of cognitive impairment and its relationships with oxidative stress markers such as ferric-reducing ability of plasma (FRAP), plasma malondialdehyde and total antioxidative capacity (TAC), as well as cholesterol parameters, in two subsets of subjects, AD patients (n = 59) and a control group of neurologically normal subjects (n = 29), attending the University Hospital Salvador in Santiago, Chile. Cognitive impairment was assessed by a set of neuropsychological tests (Mini-Mental State Examination, Boston Naming Test, Ideomotor Praxia by imitation, Semantic Verbal Fluency of animals or words with initial A, Test of Memory Alteration, Frontal Assessment Battery), while the levels of those oxidative stress markers and cholesterol metabolism parameters were determined according with standard bioassays in fresh plasma samples of the two subgroups of patients. No significant differences were observed when the cholesterol parameters (low-, high-density lipoprotein, total cholesterol) of the AD group were compared with normal controls. Interestingly, a correlation was evidenced when the levels of cognitive impairment were analyzed with respect to the plasma antioxidant capacity (AOC) of patients. In this context, the subset of subjects exhibiting cognitive impairment were divided into two subgroups according with their Global Dementia Scale performance: a subgroup with mild AD and a subgroup with moderate to severe AD. Significant differences in AOC were found between subgroups. The different correlations between cognitive impairment of subgroups of subjects with the oxidative stress profile are discussed in the context of AD pathogenesis.
Introduction
Alzheimer’s disease (AD) is the most common cause of dementia nowadays. This disease is characterized by the formation of protein aggregates in the human brain, namely paired helical filaments composed of hyperphosphorylated tau and senile plaques of Aβ (CitationMaccioni et al 2001). The pathogenesis of AD and other neurodegenerative disorders has been related with oxidative stress, which might be responsible for the resulting dysfunction and death of neuronal cells. Oxidative stress is the result of the unregulated production of reactive oxygen (ROS) or nitrogenated species, which alters the structure of proteins, lipids and nucleic acids (CitationPerry et al 2000; CitationBarnham et al 2004). These investigations have suggested several hypotheses on the mechanisms of pathogenesis potentially implicated in AD as related with oxidative damage. Studies have also pointed to relationships between progression of dementia and neurodegenerative disorders with the increase in oxidative stress biomarkers (CitationKikuchi et al 2002; CitationNunomura et al 2007).
Moreover, evidence exists that cholesterol is linked to the development of AD (CitationRojo et al 2006). Hypercholesterolemia is an early risk factor for the development of amyloid pathology, and longitudinal, population-based studies demonstrated that cholesterol is associated with AD in the later lifespan (CitationKivipelto et al 2001). On the other hand, ApoE participates in the transport of cholesterol and other lipids in the blood stream and in the cerebrospinal fluid (CSF). This protein is expressed by three major alleles: ε2, ε3, and ε4, being the homozygozity for Apoε4, is the most relevant risk factor for AD (CitationCorder et al 1993; CitationLavados et al 2005). However, its mode of action is still unknown (CitationGoedert and Spillantini 2006). The levels of peroxidation in the brains of AD patients depended on the ApoE genotype, and is higher when Apoε4 allele is present, while the level of peroxidation was inversely proportional to the concentration of ApoE (CitationRammassamy et al 1998). This lipid-transport protein is a target for the attack of free radicals, and a correlation exists between ApoE peroxidation and AD (CitationLeininger-Muller et al 1998). Prior studies with patients had confirmed that oxidative stress is clearly associated with cognitive decline (CitationBerr et al 2000), and an oxidative imbalance in AD patients has been proven with different oxidative stress biomarkers (CitationPulido et al 2005; CitationGuidi et al 2006; CitationZafrilla et al 2006).
In this article, we analyze the oxidative stress status and some of the cholesterol metabolism parameters from a group of AD patients with different stages of dementia, as compared with neurologically normal controls. Moreover, we have also attempted to correlate cognitive impairment with changes in cholesterol homeostasis.
Materials and methods
Subjects
Eighty-eight subjects in total were included in this study. All the recruited participants were more than 60 years-old and recruited from the Metropolitan area population of Santiago, Chile, genetically composed by around 40% Amerindians and 60% Caucasians. The AD patients subset included fifty-nine subjects (mean age 76.4 years, SD 6.1, 32 women and 27 men) diagnosed with probable Alzheimer-type dementia according to NINCDS/ADRDA criteria (CitationMcKhann et al 1984). This subset was compared with twenty-nine control subjects (mean age 70.7 years, SD 6.9, 20 women and 9 men). The subjects in the control subset were recruited from among elderly relatives with the indicated ages and gender distributions. All patients and control subjects were incorporated into the study after informed consent. Only subjects giving informed consent for themselves or from caregivers, and able to be evaluated neuropsychologically were recruited, thus concentrating the population in the study in 88 subjects. They did not exhibit psychiatric and neurological clinical antecedents. Moreover, none of the participants had any other major medical illness or were taking any medication known to affect oxidative stress markers or the cholesterol parameters. Moreover, caution was taken that individuals recruited in the study were not taking any supplement of vitamin E or C, nor extracts with high content of polyphenols (Vitis vinifera, Ginkgo biloba, Camellia sinensis). The education level of the entire population was also analyzed. For evaluation of cholesterol and oxidative markers parameters, venous blood samples were obtained from each subject under study. Subjects were under fasting condition early in the morning, and determinations were carried our in the separated plasma samples. All the experimental protocols were approved by the Committee on Ethical Issues of the Faculty of Medicine, University of Chile, and all subjects provided informed consent prior to the initiation of the study. In the cases of demented subjects, the informed consent for participation in the study was obtained from their caregivers.
Neuropsychological battery of tests
Once the subjects were included in the AD or control group, they were subjected after informed consent of their caregivers, a neuropsychological battery of tests. The neuropsychological evaluation consisted of the neuropsychological battery that includes Mini Mental State Examination (MMSE), Grober and Buschke, Global Dementia Scale (GDS) (CitationReisberg et al 1998), Ideomotor Praxia by imitation (PRAXIAS), Boston Naming Test (BNT) of 12 images, Frontal Assesment Battery (FAB), and Semantic Verbal Fluency of animals or words with initial A (FLU Animals and FLU A). Thus the 88 subjects in the study were properly evaluated and included in all measurements.
Measurement of cholesterol parameters
The total plasma cholesterol levels were determined using the cholesterol assay (Abbott Diagnostics, Huechuraba, Chile) according to the manufacturer’s instructions. The low-density lipoprotein (LDL) cholesterol in human plasma was measured using a MULTIGENT Direct LDL assay (Abbott Diagnostics). In the same way, the quantitation of high-density lipoprotein (HDL) cholesterol in human plasma was employed the Ultra HDL assay (Abbott Diagnosis). The VLDL cholesterol was calculated as the difference between total cholesterol and both HDL and LDL. The index total cholesterol/HDL was calculated with the data obtained of the experimental measurements.
FRAP assay
The ferric-reducing ability of plasma (FRAP assay) was adapted from the original protocol (CitationBenzie and Strain 1996) with minor modifications introduced by CitationPulido and colleagues (2000). This method is based on a single electron transfer reaction between an oxidant and the antioxidants, ie, plasma antioxidants are oxidized by the oxidant Fe(III). Thus, a single electron is transferred from the antioxidant molecule to the oxidant. Briefly, 900 μL of FRAP reagent, prepared freshly was mixed with 90 μL of distilled water and 30 μL of plasma sample. Water was used for the reagent blank. The FRAP reagent contained 2.5 mL of a 10 mmol/L TPTZ solution in 40 mmol/L HCl plus 2.5 mL of 20 mmol/L FeCl3*6H2O and 25 mL of 0.1 mol/L acetate buffer, pH 3.6. The change of absorbance of the Fe-(TPTZ)2(III) complex (FRAP reagent), due to the action of an antioxidant in the plasma, was measured at 593 nm by a spectrophotometer after twenty minutes of reaction-stabilization period. The absorbance value is used as the measurement for the reducing capability of the antioxidant. Aqueous solutions of known Fe(II) concentrations in the range of 100–2000 μmol/L (FeSO4*7H2O) were used for calibration. All measurements were performed in triplicates. FRAP analysis is widely used in the evaluation of antioxidant capacity in biological fluids including plasma, since it is a simple, reliable, and fast technique. FRAP measurements were complemented with TAC-crocin that allows determinations of the protecting capacity of blood plasma against peroxidative agents, as indicated below.
Serum lipid peroxide measurement
Levels of lipid peroxidation were measured as the formation of a thiobarbituric acid (TBA) adduct of malondialdehyde (MDA). The serum lipid peroxide measurement was used with minor modifications in the original protocol (CitationSatoh 1978). The standard procedure was established as follows: To 0.25 ml of plasma sample, 1.25 ml of 1.23 M trichloroacetic acid (TCA) were added and the mixture was left to react for 10 min at room temperature. After centrifugation at 1500g for 10 min, the supernatant was decanted and 1.25 mL of sulfuric acid 5 mM was aggregated to the precipitate, and then ultrasonicated three times for 15 sec immersion in ice-cool water. Next, we added 1.5 mL of TBA 16 mM, and the coupling reaction of the lipid peroxide with TBA was carried out by heating in a boiling water bath for 60 min. After cooling in cold water, the resulting chromogen was extracted with 1.0 ml of n-butanol by vigorous shaking in vortex. The organic phase was separated by centrifugation at 1500 g for 5 min. Then, the optical density was determined at 532 nm. The standard curves were performed with MDA solutions (0.075–2.25 μmol/L), and 5 mM sulfuric acid was used as a blank. All measurements were performed by triplicate.
Total antioxidant capacity by the crocin bleaching assay
Crocin bleaching assay was used with minor modifications to the original protocols (CitationTubaro et al 1996; CitationMalliaraki et al 2003). Briefly, crocin was extracted and purified from saffron according with the method of CitationOrdoudi and Tsimidou (2006). A stock solution of crocin was prepared in methanol and was diluted with 0.1 mol L-1 phosphate buffer, pH 7.0 in order to obtain a 1.35 × 10−5 mol L-1 crocin solution (the absorption coefficient of crocin at 443 nm is 1.33 × 105 mol-1 cm-1). As initiator, a stock solution of AAPH (250 mmol L-1) was prepared in PBS 10 mmol L-1 pH 7.0. The assay was performed in the cuvettes adding the reactants in the following order: 20 μL of plasma sample or standard, 80 μL of AAPH (250 mmol L-1) and finally 1000 μL of crocin solution. Bleaching rate of crocin at 443 nm was monitored at 40 °C during 25 minutes using a V-500 spectrophotometer (Jasco, Easton, MD, USA). The blank reference cuvette contained only phosphate buffer solution. Antioxidant capacity was calculated using similar expressions adapted from ORAC methodology (CitationCao et al 1999; CitationOu et al 2001). The corrected area under the relative absorbance curve (AUC) of each sample was compared with those obtained from the standard curve made from Trolox (3.5–60 μmol L-1), and results were expressed as μmol of Trolox equivalent per milliliter of plasma (TEAC). These TEAC values refer to the net protection (AUC) of crocin in presence of an antioxidant. All measurements were performed in triplicates. Thus, in all the studies corrected values of TAC were obtained after substracting circularing antioxidant agents such as uric acid, bilirubin, and lipoproteins (CitationKampa et al 2002).
Statistical analysis
Statistical analysis was performed with GraphPad Prism4 program (GraphPad Software, La Jolla, CA, USA). Comparisons were made by student t-test for normally distributed variables. One-way ANOVA and Bonferroni’s Multiple Comparison Test were used to test differences in mean values. All contrasts were made considering alpha lower than 5% to be an error. Correlations were assessed by Pearson’s method.
Results
Demographic and neuropsychological data among the two groups in the study are given in . No significant differences were found in the educational levels between the subsets of subjects (p > 0.05). A significant difference (p = 0.0002) in the mean ages of AD patients as compared with ages of the control group was obtained in the recruited population. AD patients were from moderately to significantly impaired in their cognitive performance, on the basis of all neuropsychological tests relative to control performance. As expected, the means of AD group were smaller than the control group in all the tests.
Table 1 Demographic data and cognitive performance differences between groups in the study
Mean values of cholesterol parameters and SD are reported in . No significant differences were found between the two AD and control subsets in the study. This data also agreed with other authors (CitationCorzo et al 2007).
Table 2 Comparison of the cholesterol parameters (mg/dL) of AD subjects and neurologically normal controls
The oxidative stress biomarkers analyzed in this article are summarized in . In all the assays applied, no statistical differences were observed between the AD subset and the control subjects. However, when subjects exhibiting cognitive impairment were divided into subgroups according with their GDS performance score, significant differences in FRAP were found between the controls and the moderate and severe AD subgroups, and between mild and moderate/severe AD ().
Figure 1 Ferric reducing ability of plasma (eq. Fe(II) μM) of patients with Alzheimer’s disease at different stages of the disease, as evaluated by GDS. Other details in Methods. The number of subjects in every group (n) was: n = 23 in the mild AD (GDS = 3–4);n = 24 in the moderate AD (GDS 5), and n = 12 for severe AD; n = 29 for the control group.
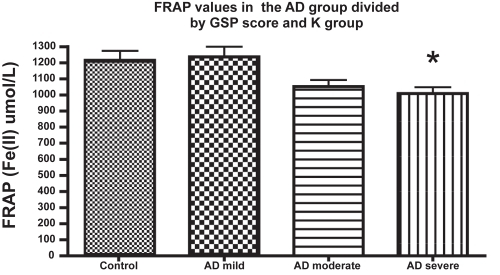
Table 3 FRAP, MDA levels, and TAC-crocin mean values and SD in both AD and control groups
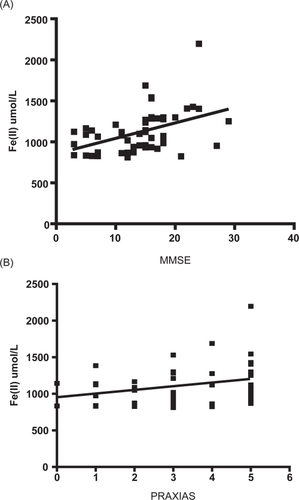
However, differences between AD and controls were not observed for the MDA levels and TAC-crocin when the subgroups were classified by GDS scores. Then, we tried to find correlations between the cognitive performance and oxidative stress biomarkers. Antioxidant capacity values measured by FRAP methods were positively correlated with MMSE (r = 0.443, P < 0.01) and PRAXIAS (r = 0.301, P < 0.05) (). We could not find another correlation between oxidative stress status and the cognitive impairment.
Figure 2 (A) Correlation between FRAP (eq Fe(II) μM) and cognitive impairment in AD patients (r = 0.443, P < 0.01). (B) Correlation between FRAP (eq Fe(II) μM) and PRAXIAS test (r = 0.301, P < 0.05).
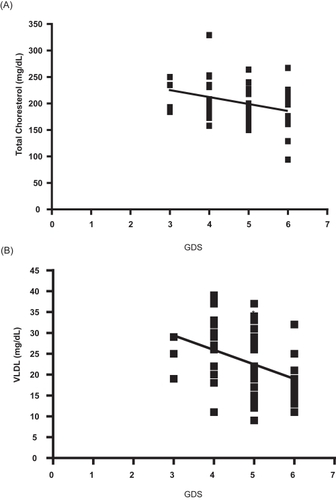
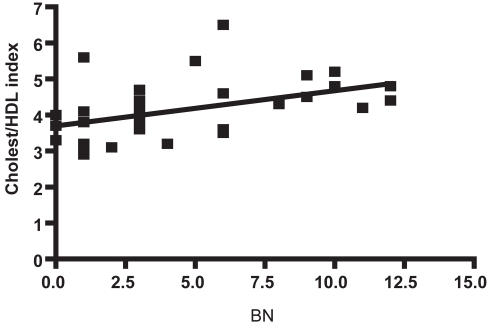
Finally, we investigated the correlations between cholesterol parameters and the results of the neuropsychological tests. In this case, a negative correlations between total cholesterol (r = −0.298, P < 0.05) and VLDL (r = −0.382, P < 0.01) versus GDS was found (). Instead, a positive correlation (r = 0.464, P < 0.01) was found between the cholesterol/HDL index and the BNT or semantic memory (). No correlations were found with the other cholesterol metabolism parameters like HDL, LDL, or triglycerides.
Discussion
We did not find statistically significant differences in determinations of plasma FRAP and TAC-crocin in AD subjects as compared with controls. Similar results are reported in the literature concerning plasma TAC measurement in AD patients (CitationSinclair et al 1998; CitationFeillet-Coudray et al 1999; CitationPulido et al 2005), however other studies have shown a lower plasma TAC in AD patients (CitationReppetto et al 1999; CitationSerra et al 2001; CitationGuidi et al 2006).
However, when the subset of cognitively impaired subjects were stratified by their GDS status, significant differences were found in the FRAP average values between the subgroups of patients. In our study, those patients with moderate/severe AD displayed the lowest anti-oxidant capacity by FRAP assay. This result is consistent with CitationZafrilla and colleagues (2006) that characterized the total antioxidant plasmatic status of the AD patients. Both the light-moderate AD subjects and patients with an advanced phase of AD exhibited lower FRAP values than in the controls. This is a novel and interesting observation. Previous reports that investigated this specific aspects, did not find this type of relationship in the plasma of AD patients (CitationCeballos-Picot et al 1996). Interestingly, CitationPulido and colleagues (2005) discovered a lower antioxidant capacity of AD plasma samples by FRAP method only in subjects with APOE genotype ε4/ε4, which is the group with the highest incidence of AD. We have to point out that FRAP should be analyzed in a global context together with other biochemical parameters, and considering some nutritional conditions of subjects.
On the other hand, we observed no modification in plasma malondialdehyde (MDA) levels when AD patients were compared with control subjects. This observation is different from previous publications that reported significant differences in MDA levels of erytrocytes of AD patients as compared with the controls (CitationBermejo et al 1997). In other studies, the ex - vivo susceptibility to lipid peroxidation was higher in mild and moderate AD with respect to controls, but not in severe AD patients (CitationGalbusera et al 2004). Peroxidation was higher in patients in the advanced stage of the disease than in the control group. However, no significant differences were observed between the different stages of the disease (CitationZafrilla et al 2006).
We explored the association of plasma oxidative components to cognitive impairment in mild and severe conditions of AD. We found that cognitive impairment parameters MMSE and PRAXIAS were both positively correlated with the FRAP, this results indicates that oxidative damage in demented patientes can be promoted in part by a reduced ability of plasma to scavenge free radicals. Interestingly, in a previous report it has been suggested that a decrease in TAC of plasma is negatively correlated with the duration of moderate/severe AD and mild AD, and significant increases in plasma total homocysteine are also present in AD patients (tHcy) (CitationGuidi et al 2006).
Previous studies on the links between plasma levels of lipids and cholesterol with AD exihibit a marked controversy. When we explored the association of plasma lipids to cognitive impairment in these groups we found no significant differences in LDL, HDL, VLDL and total cholesterol levels between AD patients and healthy controls. This is consistent with recent reports decribing no association between lipids and the risk of amnesiac or nonamnesiac mild AD (CitationReitz et al 2008). However, we found a slightly negative correlation between GDS against total cholesterol, also GDS status against VLDL levels in AD group respect to controls. These clinical data may seem controversial, in the light of the current hypothesis that links cholesterol levels with amyloid pathology and tau hyperphosphorylation based on in vitro studies (CitationRojo et al 2006), but they are consistent with recent reports describing the lipid profile of demented patients (CitationDimopoulos et al 2007). An important contribution of our work to elucidate the association of lipids with cognitive impairment is the finding of a positive correlation between the cholesterol/HDL ratio and the impairment of semantic memory (BNT test) (). This is a very interesting finding in the light of recent reports decribing correlations between metabolic syndrome and high risk of cognitive decline (CitationKomulainen et al 2007; CitationPanza et al 2007).
Studies of the changes in cholesterol levels have shown that thotal cholesterol tends to increase with age in young or middle-aged adults but later decreases as individuals get older (CitationAbbott et al 1997; CitationFerrara et al 1997). Another study shows that total cholesterol levels in plasma were lower in patients with AD than in controls at least five years before diagnosis (CitationMielke et al 2005), and decreased more rapidly among people who subsequently developed dementia (CitationNotkola et al 1998). CitationSolomon and colleagues (2007) have proposed that high midlife serum TC is a risk factor for subsequent dementia/AD, but decreasing serum total cholesterol after midlife may reflect ongoing disease processes and may represent a risk marker for late-life cognitive impairment. Apparently, several years before clinical evidence of dementia, total cholesterol begins to decline, possibly as a results of the ongoing AD pathology (CitationPanza et al 2007). On the other hand, the decrease in plasma total cholesterol in the development of disease may be partially explained by the physiological aging process. However, no correlation was evidenced between age and the total cholesterol, VLD-cholesterol and cholesterol/HDL index in the AD patients under our study. The minor decrease detected with the time progression of AD might be explained by unintentional or voluntary changes in lifestyle-related factors because of the reduced quality-of-life of AD patients.
In summary, in a study on human subjects we corroborated the findings that the degree of oxidative stress is directly related to the advance of cognitive impairment and AD. Thus, high correlations between the oxidative stress marker FRAP and cognitive impairment indicate that oxidative stress should be considered a risk factor in the development of cognitive disorders (CitationPerry et al 2000). Oxidative stress is critical in the pathogenesis of AD, and is involved in biological damage, thus one might think that the administration of antioxidants may be useful in the prevention and treatment of AD and other neurodegenerative disorders.
In the meantime, we demonstrated that plasmatic levels of cholesterol also decrease with the progression of dementia in the AD population as evaluated by changes in GDS measurements (). This might be a result of the pathologic processes leading to dementia, at least during the early stages of the disease. The negative correlations between cholesterol parameters and the cognitive impairment open new questions about the role of cholesterol and oxidative markers in AD progression. Interestingly, in a recent study on the levels of brain redox-iron overload, a positive correlation between cerebral spinal fluid redox-iron and AD progression was evidenced at the early stages of the disease (mild AD cases), but this particular iron compartment decreased at advanced stages of the disease (CitationLavados et al 2008).
Acknowledgements
Research was supported by grants 1050198 and 1080254 from FONDECYT, the International Center for Biomedicine (to RBM) and by the Bicentennial Project IPC-79 of PBCT-CONICYT (to AS). The authors report no conflicts of interest in this work.
References
- AbbottRDSharpDSBurchfieldCMCross-sectional and longitudinal changes in total and high-density-lipoprotein cholesterol levels over a 20-year period in elderly men: the Honolulu Heart ProgramAnn Epidemiol19977417249279451
- BarnhamKJMastersCLBushAINeurodegenerative diseases and oxidative stressNat Rev Drug Discov200432051415031734
- BenzieIFFStrainJJThe ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assayAnal Biochem19962397068660627
- BermejoPGómez-SerranillosPSantosJDetermination of malonaldehyde in Alzheimer’s disease: a comparative study of high-performance liquid chromatography and thiobarbituric acid testGerontology199743218229222750
- BerrCBalansardBArnaudJCognitive decline is associated with systemic oxidative stress: the EVA study, Etude du Vieillissement ArteirelJ Am Geriatr Soc20004812859111037017
- CaoGPriorRMeasurement of oxygen radical absorbance capacity in biological samples. Oxidants and antioxidantsMethods Enzymol199929950629916196
- Ceballos-PicotIMansouriaMBNicoleAPeripheral antioxidant enzyme activities and selenium in elderly subjects and in dementia of Alzheimer’s type place of the extracellular glutathione peroxidaseFree Radical Biol Med199620579878904299
- CorderEHSaundersAMStrittmatterWJGene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset familiesScience199326192138346443
- CorzoLZasRRodríguezSDecreased levels of nitric oxide in different forms od dementiaNeurosci Lett2007420263717556102
- DimopoulosNPiperiCSaloniciotiACharacterization of the lipid profile in dementia and depression in the elderlyJ Geriatr Psychiatry Neurol2007201384417712096
- Feillet-CoudrayCTourtauchauxRNiculescuMPlasma levels of 8-epiPGF2a, an in vivo marker of oxidative stress, are not affected by aging or Alzheimer’s diseaseFree Radic Biol Med199927463910468223
- FerraraABarrett-ConnorEShanJTotal, LDL, and HDL cholesterol decrease with age in older men and women. The Rancho Bernardo Study 1984–1994Circulation19979637439236414
- GalbuseraCFacherisMMagniFIncreased susceptibility to plasma lipid peroxidation in Alzheimer’s disease patientsCurr Alzheimer Res20041103915975074
- GoedertMSpillantiniMGA century of Alzheimer’s diseaseScience20063147778117082447
- GuidiIGalimbertiDLonatiSOxidative imbalance in patients with mild cognitive impairment and Alzheimer’s DiseaseNeurobiol Aging200627262916399211
- KampaMNistikakiATsausisVA new authomated method for determination of the total antioxidant capacity (TAC) of human plasma based on crocin bleaching assayBMC Clin Pathol2002211611882256
- KikuchiATakedaAOnoderaHSystemic increase of oxidative nucleic acid damage in Parkinson’s disease and multiple atrophyNeurobiol Dis20029448
- KivipeltoMHelkalaELLaaksoMPMidlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based studyBMJ200132214475111408299
- KomulainenPLakkaTAKivipeltoMMetabolic syndrome and cognitive function: a population-based follow-up study in elderly womenDement Geriatr Cogn Disord200723293417068394
- LavadosMFariasGRothhammerFApoE alleles and tau markers in pateints with different levels of cognitive impairmentArch Med Res200536474916099324
- LavadosMGuillonMMujicaMCMild cognitive impairment and Alzheimer’s patients disk’lay different levels of redox CSF ironJ Alz Dis20081322532
- Leininger-MullerBJolivaltCBertrandPPackerLChristenYOxidation of human apolipoprotein E: isoforms susceptibility and protection with Ginkgo biloba EGb 761 extractGinkgo biloba Extract (Egb761) Study: Lesson from Cell Biology1998ParisElsevier5768
- MaccioniRBMunozJPBarbeitoLThe molecular bases of Alzheimer’s disease and other neurodegenerative disordersArch Med Res2001323678111578751
- MalliarakiNMpliampliasDKampaMTotal and corrected antioxidant capacity in hemodialyzed patientsBMC Nephrol20034412837136
- McKhannGDrachmanDFolsteinMClinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA work group under the auspices of the Department Of Health and Human Services Task Force on Alzheimer’s DiseaseNeurology198434939446610841
- MielkeMMZandiPPSjogrenMHigh total cholesterol levels in late-life associated with a reduced risk of dementiaNeurology20056416899515911792
- NotkolaILSulkavaRPekkanenJSerum total cholesterol, apolipoprotein E epsilon 4 allele, and Alzheimer’s diseaseNeuroepidemiology19981714209549720
- NunomuraAMoreiraPILeeHGNeuronal death and survival under oxidative stress in Alzheimer diseaseCNS Neurol Dis Drug Targets2007641123
- OrdoudiSATsimidouMZCrocin bleaching assay step by step: observations and suggestions for an alternative validated protocolJ Agric Food Chem2006516637116506817
- OuBHampsch-WoodillMPriorRDevelopment and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probeJ Agric Food Chem20014946192611599998
- PanzaFCapursoCD’IntronoATotal cholesterol levels and the risk of mild cognitive impairment and Alzheimer’s diseaseJ Am Geriatr Soc200755133517233704
- PerryGNunomuraAJonesPKOxidative imbalance is a major feature of Alzheimer diseaseCurr Biochem Res200031516
- PulidoRBravoLSaura-CalixtoFAntioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assayJ Agric Food Chem200048339640210956123
- PulidoRJiménez-EscrigAOrensanzLStudy of plasma antioxidant status in Alzheimer’s diseaseEur J Neurol200512531515958093
- RamassamyCKrzywokowskiPBastianettoSPackerLChristenYApolipoprotein E, oxidative stress and EGb 761 in Alzheimer’s disease brainGinkgo biloba Extract (EGb 761) Study: Lesson from Cell Biology1998ParisElsevier6983
- ReisbergBFranssenEHSourenLEProgression of Alzheimer’s disease: variability and consistency: ontogenic models, their applicability and relevanceJ Neural Transm Suppl1998549209850911
- ReitzCTangMXManlyJPlasma lipid levels in the elderly are not associated with the risk of mild cognitive impairmentDement Geriatr Cogn Disord200825232718264008
- RepettoMGReidesCGEvelsonPPeripheral markers of oxidative stress in probable Alzheimer patients (1999)Eur J Clin Invest199929643910411672
- RojoLSjöbergMKHernándezPRoles of cholesterol and lipids in the etiopathogenesis of Alzheimer’s diseaseJ Biomed Biotechnol200620067397617047312
- SatohKSerum lipid peroxide in cerebrovascular disorders determined by a new colorimetric methodClin Chim Acta1978903743719890
- SerraJADominguezROde LustigESParkinson’s disease is associated with oxidative stress: comparison of peripheral antioxidant proles in living Parkinson’s, Alzheimer’s and vascular dementia patientsJ Neural Transm200110811354811725816
- SinclairAJBayerAJohstonJOAltered plasma antioxidant status in subjects with Alzheimer’s disease and vascular dementiaInt J Geriat Psychiatry1998138405
- SolomonAKåreholtINganduTSerum cholesterol changes after midlife and late-life cognition: Twenty-one-year follow-up studyNeurology200768751617339582
- TubaroFMicossiEUrsiniFThe antioxidant capacity of complex mixtures by kinetic analysis of crocin bleaching inhibitionJ Am Oil Chem Soc1996731739
- ZafrillaPMulcroJXandriJMOxidative stress in Alzheimer patients in differents stages of the diseaseCurr Med Chem20061310758316611085