Abstract
Tourette’s syndrome (TS) is a neurological disorder characterized by motor and vocal tics that typically begin in childhood and often are accompanied by psychiatric comorbidities. Symptoms of TS may be socially disabling and cause secondary medical complications. Pharmacological therapies remain the mainstay of symptom management. For the subset of patients in whom TS symptoms are medically recalcitrant and do not dissipate by adulthood, neurosurgery may offer an alternative treatment strategy. Greater understanding of the neuroanatomic and pathophysiologic basis of TS has facilitated the development of surgical procedures that aim to ameliorate TS symptoms by lesions or deep brain stimulation of cerebral structures. Herein, the rationale for the surgical management of TS is discussed and neurosurgical experiences since the 1960s are reviewed. The necessity for neurosurgical strategies to be performed with appropriate ethical considerations is highlighted.
Introduction
Historical perspective
In 1825, Jean-Marc Gaspard Itard, the Chief Physician at the National Institute for Deaf Mutes in Paris, provided careful clinical observations of stereotypic movements and vocalizations in the 26-year-old Marquise de Dampierre:
[she] began to have convulsive contractions in her hand and arm muscles, which manifested themselves especially at the moments in which this [woman as a] child tried to write as she spread her hand over the characters that she traced. After this deviation, her hand movements returned to normal once more and were subject to her will until another jolt interrupted the operation of her hand once more…soon it became certain that these movements were involuntary and convulsive, as the muscles of the shoulders, the neck and the face participated in them. The malady continued to progress, spasms were propagated in the organs of the voice and of the speech, and this young woman uttered bizarre cries and words which made no sense (CitationItard 1825).
The socially disabling effects of the disorder were also clear to Itard:
Among the continuous and disordered movements which lead to these morbid contractions, those imparted by the organs of voice and speech are the only ones deserving all our attention as presenting the more rare phenomenon as well as constituting the most disagreeable inconvenience, one which prevents the person from attaining all the kindnesses society offers; because the disorder that she bears is proportional to the pleasure of which it deprives her…the more others seem revolted by her uncivilized statements, the more she is tormented by the fear of uttering them (CitationItard 1825).
Although George Beard (1880–1881) later described an additional fifty patients with motor tics and echolalia, the disorder was eponymously named by Jean-Martin Charcot after his student Georges Gilles de la Tourette, who had written a two-part manuscript in 1885 describing nine affected patients, including the Marquise (CitationGilles de la Tourette 1885).
Clinical features of Tourette’s syndrome
The clinical features of Tourette’s syndrome (TS) as described by those original reports remain the basis for current diagnostic criteria. More specifically, both the Tourette Syndrome Classification Study Group and the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) (CitationThe Tourette Syndrome Classification Study Group 1993; CitationMüller-Vahl 2002) currently require the presence of multiple motor and vocal tics (stereotyped, purposeless, and involuntary movements or sounds) daily or intermittently over the course of over one year, and with onset in childhood (prior to eighteen years of age for the DSM-IV). An additional essential diagnostic criterion is tic variability; that is, their severity, frequency, and affected anatomic location must vary over the course of the disorder (CitationThe Tourette Syndrome Classification Study Group 1993).
Categorization of TS into subtypes according to the patient’s symptoms has been proposed (CitationShapiro et al 1988; CitationRobertson et al 1990; CitationJankovic 2001). Simple TS is characterized by the predominance of motor and vocal tics, whereas more complex subtypes feature coprolalia, copropraxia, echolalia, echopraxia, palilalia, and/or palipraxia (CitationRobertson et al 1990; CitationJankovic 2001). Patients with TS may also commonly exhibit other behavioral symptoms (CitationGilbert 2006), such as attention deficit-hyperactivity disorder (CitationRobertson 2000), obsessive compulsive disorder (CitationFrankel et al 1986; CitationComo 1995), self-injurious behaviors (CitationRobertson et al 1989; CitationKurlan et al 1990; CitationLeckman et al 1993; CitationHoueto et al 2005; CitationAnandan et al 2004; CitationAckermans et al 2007), and/or anxiety disorders (CitationRobertson 2000).
Treatment of TS symptoms is pursued if the symptoms adversely affect quality of life or cause medical complications (CitationSinger 2001). Patients who exhibit mild symptoms may require only psychobehavioral therapies and the support of family, teachers, and TS support groups (CitationKurlan 1997; CitationMüller-Vahl 2002; CitationVisser-Vanderwalle 2007). Some patients may be able to avoid the use of pharmacological therapies since TS is unique as a hyperkinetic movement disorder in that patients describe the ability to suppress their tics (CitationDemirkiran and Jankovic 1995), at least in part. Moreover, although patients typically demonstrate a chronic disease course, symptom remission may occur spontaneously in up to 40% of patients by late adolescence and an additional 30% of patients may experience later symptom improvement (CitationBruun and Budman 1992; CitationLeckman et al 1998; CitationBabel et al 2001). Pharmacological therapies may be beneficial for patients whose tics do not respond sufficiently to conservative treatments or are functionally incapacitating (CitationGilbert 2006). The association of tics with medical or psychiatric conditions may necessitate more aggressive tic management. For example, TS has been associated with self-injurious behaviors requiring urgent medical attention (CitationRobertson et al 1989; CitationKurlan et al 1990; CitationLeckman et al 1993; CitationHoueto et al 2005; CitationAnandan et al 2004; CitationAckermans et al 2007), such as corneal and orbital injuries (CitationRobertson et al 1990) and stab wound- inflicted injuries (CitationAnandan et al 2004), or tic-induced medical complications, such as cervical myelopathy (Krauss and Jankovic 1996), limb fractures, and retinal detachments (CitationFlaherty et al 2005). The variety of medications used to manage TS has included dopaminergic antagonists, benzodiazepines, calcium channel antagonists, and atypical neuroleptics (CitationKurlan 1997; CitationRobertson and Stern 2000; CitationJiménez-Jiménez and García-Ruiz 2001; CitationMüller-Vahl 2002). Botulinum toxin injections have been used to treat painful dystonic tics (CitationJankovic 1994). Medical therapies may be particularly useful even during the waning phase of tics in early adolescence since tics have been reported to worsen at that time before they may ultimately resolve (CitationLeckman et al 1998). However, medical therapies may cause detrimental and intolerable side effects (CitationLeckman 2002; CitationFlaherty et al 2005) and, for a subset of patients with TS, are ineffective (CitationMüller-Vahl 2002; CitationVisser-Vandewalle 2007). As CitationKopell and colleagues (2003) note, the development of additional treatment strategies for this subset of patients is particularly important given that the endpoint for unsatisfactorily managed neuropsychiatric disease is “dismal”.
Pathophysiology of Tourette’s syndrome
Anatomical studies
The development of surgical strategies for medically recalcitrant TS was enabled by efforts to localize an anatomical source of the symptoms. Psychodynamic theories prior to the 1970s sought to explain tics as resulting from suppressed aggression and “impaired functioning of the ‘motility controlling function of the ego’ ” (Mahler and Luke 1946;Flinn et al 1983). Autopsy findings from patients with TS that demonstrated no specific anatomical pathologies or only subtle changes in the striatum seemed to support conceptions that tics had a psychogenic origin (CitationDeWulf and van Bogaert 1941; CitationBalthasar 1957). The search for an anatomical basis for TS using autopsy material is thought to be problematic, however, both because few post-mortem brains have been studied and most of those available have come from patients with long-standing disease and treatment that might confound the histological analyses (CitationMink et al 2001; CitationFrey et al 2006). Nevertheless, more recent studies have found abnormalities particularly in the basal ganglia and frontal cortex. For example, studies have reported decreased levels of dynorphin-like immunoreactivity in the striatum and globus pallidus (CitationHaber et al 1986; CitationHaber and Wolfer 1992), decreased subcortical levels of serotonin (CitationAnderson et al 1992), increased numbers of neurons expressing parvalbumin in the globus pallidus pars internus (GPi) and decreased numbers of neurons expressing parvalbumin in the caudate nucleus and globus pallidus pars externus (GPe) (CitationKalanithi et al 2005). Interestingly, Itard had speculated that the symptoms of TS had a cerebral origin in his 1825 case report:
What is the nature of these strange convulsions, or to pose the question in a clearer manner, the seat of the irritation which provokes them? Considering that the muscles which move against the will belong to different motor apparatuses which are not managed by the same nerves, this irritation is not caused by any of them but at their common centre which is the brain.
Neuroimaging studies
Modern imaging studies lend further evidence for a neuroanatomic basis of TS. Asymmetric striatal volumes have been observed in magnetic resonance images (MRI) of patients with TS, with smaller striatal volumes suggesting abnormalities in striatal development or the loss of GABAergic interneurons that are thought to play a role in gating sensory information (CitationPeterson et al 1993; CitationSinger et al 1993). Abnormally smaller caudate volumes are thought to be predictive of the development of more severe tics in early adulthood (CitationBloch et al 2005) and have been associated with more severe symptoms in twin studies (CitationHyde et al 1995). Abnormalities of volume also have been observed in the lenticular nuclei (CitationPeterson et al 1993; CitationSinger et al 1993). These findings are inconsistent, however, since other studies have found no significant differences in basal ganglia nuclei volumes compared to those of normal control patients (CitationMoriarty et al 1997; CitationZimmerman et al 2000), or have found increased caudate volumes in children with TS (CitationDenkla et al 1991). Functional MRI has revealed activity in the neocortex, striatum, thalamus, parietal operculum, supplementary motor area, insular cortex, and cerebellum in association with tic generation (CitationPeterson 2001; CitationBohlhalter et al 2006; CitationLerner et al 2007), while increased activity in the caudate and frontal and temporal cortices and decreased activity in the ventral GP, putamen and thalamus have been observed during tic suppression (CitationPeterson 2001). Increased activation of the substantia nigra, ventral tegmental area, and basal ganglia structures of the direct pathway has also been associated with increased tic severity in nonmedicated children with TS (CitationBaym et al 2008). Positron emission tomography (PET) studies using 18F-fluorodeoxyglucose in patients with TS demonstrate increased metabolic activity in the premotor and supplementary cortices and midbrain and concomitant decreased activity in the caudate and thalamus (CitationStoetter et al 1992; CitationEidelberg et al 1997) while in those studies using 15O-water found increased activity in cerebral regions associated with sensorimotor, language, executive and paralimbic functions, which was temporally related to both the expression of motor and phonic tics and their premonitory urges (CitationStern et al 2000). Finally, consistent with PET findings of decreased glucose utilization, single photon emission tomography (SPECT) has revealed decreased regional blood perfusion of the basal ganglia, thalamus, and frontal and temporal cortices in TS (CitationPeterson et al 1993).
Anatomic localization of TS symptom generation to the basal ganglia is consistent with current understanding of basal ganglia physiology. In a general sense, disordered cortico-striato-pallido-thalamo-cortical circuitry is thought to be etiologically-related to TS, although the specific nuclei and circuits responsible for the various symptoms remain a matter of continued investigation (CitationMüller-Vahl 2002; CitationMink et al 2006). It has been suggested, for example, that simple motor tics could be due to abnormal activation of the motor cortex via thalamocortical pathways while involvement of premotor, supplementary motor, and cingulate cortices may be related to more complex motor tics (CitationStern et al 2000; CitationMink et al 2001). Inappropriate activity in Broca’s area, the frontal operculum, and the caudate nucleus could elicit vocal tics (CitationStern et al 2000; CitationMink et al 2001). Abnormal activation of the orbitofrontal region, which has been observed in obsessive compulsive disorder, may underlie the compulsions and urges that patients with TS experience (CitationStern et al 2000; CitationMink et al 2001). Alternatively, tics in TS may be due to abnormal activity within subsets of neurons within the caudate and putamen (CitationGraybiel et al 1994; CitationMink et al 2001); indeed, microstimulation of discrete areas of the putamen in monkeys induces stereotypic movements akin to tics (CitationAlexander et al 1985).
Neurotransmitter studies
Given the diversity of nuclei and circuits that may be involved in TS pathogenesis, the variety of neurotransmitter systems that are also implicated is not surprising (CitationJankovic 2001). In the frontal subcortical circuits alone, abnormalities in glutamate, dopamine, serotonin, GABA, acetylcholine, noradrenaline, opioid, and cannabinoid receptors are thought to be involved (CitationLavenstein et al 2003). The serotonergic system has been studied in particular: patients with TS have been found to have decreased levels of serotonin and its metabolite 5-hydroxyindoleacetic acid in their serum (CitationComings 1990) and cerebrospinal fluid (CitationButler et al 1979), respectively, and post-mortem studies have found decreased serotonin levels in the brain stem (CitationSwerdlow and Young 2001). This latter finding is particularly relevant since serotonergic projections from the medial raphe nucleus are known to project to regions implicated in tic generation, such as the prefrontal cortex, substantia nigra pars compacta, ventral tegmental area, striatum, and nucleus accumbens (CitationAlex et al 2005; CitationPehek et al 2006). These target structures are components of the dopaminergic system and, thus, it has been hypothesized that it is not serotonergic dysfunction, per se, that induces tics, but rather its effects on the dopaminergic system (CitationHarris et al 2006). Serotonin influences dopaminergic release via a number of receptors, including serotonin heteroreceptors and inhibitory and stimulatory somatodendritic receptors, and is involved in dopamine reuptake (CitationSershen et al 2000; CitationAlex et al 2005; CitationPehek et al 2006; CitationCarta et al 2007).
Indeed, the most widely purported hypothesis to explain the etiology of TS concerns dopaminergic circuitry dysfunction. The ability of dopaminergic antagonists to suppress tics suggests that the dopaminergic system is hyperactive in TS (CitationJankovic 2001). The direct dopaminergic basal ganglia pathway is thus facilitated, and the indirect pathway inhibited, which results in thalamocortical over-activity (CitationVisser-Vandewalle 2007; ). Dopaminergic hyperactivity could arise due to alterations in dopamine release (CitationSnyder et al 1970; CitationSinger et al 1982; CitationHarris et al 2006). Imaging studies In TS suggest an “overactive DAT [dopamine transporter] system” (CitationHarris et al 2006): for example, SPECT has demonstrated increased dopamine transporter binding in the striatum in patients with TS (CitationCheon et al 2004; CitationSerra-Mestres et al 2004), suggesting an elevated density of presynaptic dopamine terminals and postsynaptic D2 dopamine receptors, particularly in the ventral striatum (CitationWong et al 1989; CitationErnst et al 1999; CitationWolf et al 1996; CitationAlbin et al 2003). In further study of D2 receptors using 11C raclopride PET, amphetamine challenge caused a 21% increase in intrasynaptic dopamine levels that was not observed in control patients (CitationSinger et al 2002). Although D2 receptor density has correlated with TS symptom intensity in monozygotic twins (CitationWolf et al 1996), this has not been consistently observed (CitationWong et al 1997). The net result of increased DAT density is decreased tonic extracellular levels of dopamine, increased dopamine levels in the axon terminal, and dopamine receptor supersensitivity (reviewed in CitationHarris et al 2006). Alternatively, TS may result not from abnormalities of dopaminergic transmission but from changes in the resting membrane potentials of striatal neurons whose response to dopamine is consequently affected (CitationMink et al 2001).
Figure 1 Schematic representation of basal ganglia circuitry (modified from Visser-Vandewalle et al 1997), with excitatory (glutamatergic) projections

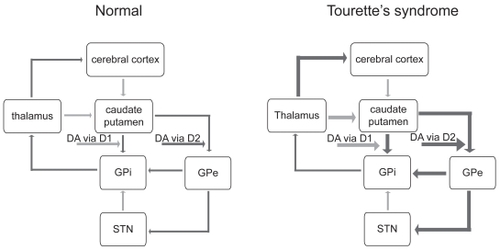
Animal studies
Animal models have been developed to better understand the pathophysiologic basis of TS (CitationSwerdlow and Sutherland 2005, Citation2006). Normally, a prestimulus can prevent a startle response elicited by a stimulus, but this has been found to be deficient in TS (CitationCastellanos et al 1996; CitationFreudenberg et al 2007). This is thought to be due to deficient “gating” of sensorimotor information and has been modelled in rodents, pigs, and nonhuman primates (CitationSwerdlow et al 2001; CitationSwerdlow and Sutherland 2005; CitationFreudenberg et al 2007; CitationHadamitzky et al 2007). In order to model the hyperactive basal ganglia and limbic circuitry thought to underlie TS, transgenic mice have been developed that have tonically hyperactive cortical and limbic circuits (CitationCampbell et al 2000; CitationMcGrath et al 2000; CitationNordstrom and Burton 2002; CitationSwerdlow and Sutherland 2005,2007). These rodents display tics as well as compulsive behaviors (CitationCampbell et al 2000; CitationMcGrath et al 2000; CitationNordstrom and Burton 2002; CitationSwerdlow and Sutherland 2005, 2007). Micro- injection of the GABAA antagonist bicuculline in the limbic area of the GPe has also been shown to induce complex tics and compulsions in African green monkeys and may therefore provide an additional primate model of TS (CitationGrabli et al 2004). In addition to enhancing understanding of abnormal TS neural circuitries, animal models could be used to test surgical therapies before they are introduced to the clinical setting.
Neurosurgical lesions
Frontal lobe disconnection
Surgical disruption of basal ganglia circuits began to be employed for the treatment of TS in 1955 (CitationStevens 1964; , ). The earliest studies focused on surgical disconnection of the frontal lobe. CitationStevens (1964) reported the details of the prefrontal lobotomy that James Watts had performed nine years earlier in a 37-year-old man who had become incapacitated due to his motor and vocal tics. Two years post-operatively, the patient demonstrated improvement in his symptoms, which persisted for at least an additional six years. His beneficial clinical course could not be attributed entirely to the surgery, however, since he also began to use neuroleptics. CitationBaker (1962) reported the apparently successful management of tics and panic attacks in a 22-year-old man with bimedial frontal leucotomy, but CitationMoldofsky and colleagues’ (1974) found that these benefits did not persist. The longest reported duration of symptom control attributed to prefrontal leucotomy was that experienced by a 29-year-old man with TS in whom there was complete resolution of motor and vocal tics beginning five years post-operatively and for thirty-three years thereafter (CitationKulisevsky et al 1995). Measurement of regional cerebral blood flow using 99Tc-hexamethlypropylene amine oxide SPECT showed bilateral frontal hypoperfusion (CitationKulisevsky et al 1995).
Figure 2 Sagittal magnetic resonance image demonstrating the sites of lesions for TS.
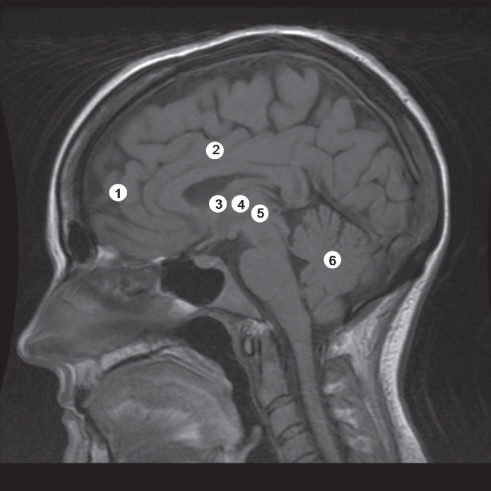
Table 1 Clinical studies of neurosurgical lesions for Tourette’s syndrome
Cingulotomy
Demonstration of the efficacy of frontal lobotomy procedures for TS was not clear, and as a result procedures that targeted the cingulate cortex were investigated as an alternative. Anterior cingulotomy had been used since 1948 to treat a variety of psychiatric disorders to disconnect neural circuits involving the cortex, limbic system, and basal ganglia (CitationWhitty et al 1952; CitationBallantine et al 1987). Targeting the cingulate cortex for the treatment of TS seemed appropriate given its recognized role in mediating emotional experiences and language and coordinated movements (CitationRobertson et al 1990). Cingulotomy decreased tics by 75% by 2 years post-operatively in one patient (CitationAnandan et al 2004) and provided enduring improvement in another (CitationSawle et al 1993). In an imaging study that found normalization of preoperative hypermetabolism in the caudate nucleus and thalamus, the beneficial effects of cingulotomy were ascribed nonspecifically to the disruption of abnormal basal ganglia-thalamocortical circuitry (CitationSawle et al 1993). Other case reports have not confirmed those beneficial effects of cingulotomy and found that the procedure had little effects on reducing either tic severity or frequency or seemed more efficacious for other comorbid symptoms, such as SIB and OCD (CitationKurlan et al 1988, Citation1990; CitationRobertson et al 1990; CitationBaer et al 1994). CitationBeckers (1973) has recommended against the use of cingulotomy because of its side effects, which have included the development of apathy, intellectual impairment, and difficulties with concentration.
Thalamotomy
Attenuation of the corticostriatal hyperactivity that is thought to underlie TS symptomatology has also been attempted by thalamic lesions. Cooper’s performance of a right and then, one year later, left chemothalamectomy in a 16-year-old girl with TS significantly improved her tic frequency (Cooper 1962) and he later described his experience with thalamic lesions in six other patients with TS (Cooper 1969). Details for only one representative case were provided, in which surgery had no effect on the tics. The results of CitationHassler and Dieckeman (1970) were more promising: up to 14 coagulations were made stereotactically in each hemisphere in the medial, intralaminar, and ventrolateral thalamic nuclei and between 70% and 100% subjective improvement of tic symptomatology was observed. The same nuclei have been targeted in 4 other reported patients, but complete tic resolution was observed in only one patient, and in the others tic improvement was only transient at best (Citationde Divitiis et al 1977; CitationCappabianca et al 1987). A case report in which bilateral cingulotomies and infrathalamic lesions were made highlights the potential devastating complications of thalamic lesions (CitationLeckman et al 1993). A 40-year-old man who underwent these procedures demonstrated improvement of obsessive compulsive symptoms but persistence of motor and vocal and the left lesions were therefore repeated (CitationLeckman et al 1993). The infrathalamic lesion was extended inferiorly within the red nucleus and included as well the H fields of Forel, the basal intralaminar nucleus of the thalamus, the subthalamic nucleus, efferent fibres from the VTA and SNr, and the dentatorubrothalamic efferent fibres from the cerebellum (CitationLeckman et al 1993). Post-operatively the patient developed a number of severe neurological deficits including dysarthria, dysphagia, gait and hand-writing difficulties, mild hemiparesis, abnormal extraocular movements, axial rigidity, and bradykinesia (CitationLeckman et al 1993).
Lesions of other structures
Stereotaxy has facilitated the targeting of other deep-seated cerebral structures for the treatment of TS. In combination with lesions of the lamella medialis and ventrolateral nuclei of the thalamus, the zona incerta has been lesioned with significant reduction of tic severity (CitationBabel et al 2001). Transient and permanent deficits were experienced by 66% and 27% of patients (CitationBabel et al 2001), with bilateral lesions carrying a higher risk of neurological morbidity, including hemiballism, dystonia, dysarthria (CitationBabel et al 2001), and quadriplegia (CitationAsam and Karrass 1981). Other structures targeted include the anterior limb of the internal capsule (CitationSun et al 2005), within which striatal circuits and circuits that connect orbitofrontal cortical and medial thalamic nuclei pass. Lesions of the posterior third of the anterior internal capsule provided more durable improvement of symptoms, which was thought to be due the greater efficacy of posterior lesions to disrupt neural pathways connecting the basal ganglia and frontal cortex (CitationSun et al 2005). In one case report, lesions of the dentate nucleus reportedly attenuated motor and vocal tics, but neither rationale for targeting this cerebellar structure nor a proposed mechanism of beneficial action were discussed (CitationNádvorník et al 1972).
Deep brain stimulation
Although neurosurgical lesions for TS have ameliorated symptoms for some patients, for others, benefits have not been apparent and lesions have been associated with a number of permanent and disabling side effects. Deep brain stimulation (DBS) emerged as a promising alternative therapeutic strategy to stereotactic lesions after its efficacy in the treatment of tremor was demonstrated in 1987 (CitationBenabid et al 1987). The similar clinical effects of lesions and high frequency stimulation (HFS) suggest that DBS may inhibit the activity of target structures, by depolarization blockade, synaptic inhibition, and/or the release of inhibitory neurotransmitters (CitationBenazzouz and Hallet 2000; CitationDostrovsky et al 2002; CitationFilali et al 2004; CitationLozano and Mahat 2004; CitationMcIntyre et al 2004; CitationChang et al 2008; CitationLiu et al 2008; CitationMontgomery and Gale 2008). Other studies suggest, however, that DBS has an excitatory effect on neurons and axonal pathways in the vicinity of the electrode (CitationMcIntyre et al 2004; CitationChang et al 2008; CitationLiu et al 2008; CitationMontgomery and Gale 2008), or that DBS works by modulating the pattern of neuronal firing (CitationFukada et al 2001; CitationGarcia et al 2005;Change et al 2008; CitationLiu et al 2008; CitationMontgomery and Gale 2008). Regardless of its mechanism of action, compared to lesion procedures DBS has the advantage of being both reversible (since the electrodes can be removed if HFS ineffectively treats symptoms) and adjustable (since the stimulation parameters can be varied to elicit the most optimal clinical response). CitationBenabid (2007) has thus referred to DBS as a “flexible scalpel.”
Thalamic stimulation
Based upon the reported success of thalamic lesions in the control of TS symptoms, CitationVisser-Vandewalle and colleagues (1999) performed HFS of the thalamus in a 42-year-old man with medically recalcitrant TS (). They found that HFS of the thalamic targets of CitationHassler and Dieckemann (1970) significantly reduced the number of tics by 4 months post-operatively, with complete resolution of the tics after 1 year. Clinical benefits were ascribed to deactivation of frontal cortical areas (via stimulation of the nucleus ventro-oralis internus) and corticostriatal circuits that involve the ventral (limbic) and dorsal (sensorimotor) striatum (via stimulation of intralaminar and medial thalamic nuclei). In a subsequent study in which three patients were followed up to five years post-operatively, thalamic DBS was associated with a 72%, 83%, and 90% decrease in tics with complete resolution of all major vocal and motor tics (CitationVisser-Vandewalle et al 2003). Side effects of HFS included a slight sedative effect in all three patients and changes in sexual behavior in two (CitationVisser-Vandewalle et al 2003). CitationServello and colleagues (2008) confirmed the therapeutic efficacy of thalamic DBS, albeit using targets different than those used by Visser-Vandewalle and colleagues, in their case series of eighteen patients in whom the centromedian-parafascicular and ventral oral thalamic nuclei were targeted. All patients demonstrated improvement in all four components of the Yale Global Tic Severity Rating Scale (YGTSS). By six months post-operatively, 3 patients’ tics had improved to the extent that they no longer required any adjunctive medical therapy. None of the patients experienced any disabling permanent side effects, but twelve patients required HFS adjustments. The ability for the effects of DBS to be tested in a double-blind manner was utilized in a recent prospective crossover trial (CitationMaciunas et al 2007). Patients in whom bilateral thalamic DBS electrodes were implanted were assessed over four weeks, with one week spent in each of the following DBS states: both stimulators off, unilateral stimulation on the left or right, or bilateral stimulation (CitationMaciunas et al 2007). The study’s primary and secondary outcome measures were met in that all patients demonstrated significant improvements in Rush Video-Based Rating Scale (RVBRS) scores as well as YGTSS and TS Symptom List scores (CitationMaciunas et al 2007). CitationAckermans and colleagues’ (2007) case report highlights that significant adverse effects can occur due to the thalamic DBS. Their 39-year-old patient developed a vertical gaze paralysis due to hemorrhage in the upper mesencephalon at the caudal tip of one of the electrodes.
Table 2 Clinical studies of deep brain stimulation for Tourette’s syndrome
Pallidal stimulation
Attenuation of thalamic activity, indirectly, has also been attempted by HFS of the GPi in order to inhibit its pathological over-activity that is thought to ultimately lead to disinhibition of the thalamus and, thus, tics (Citationvan der Linden et al 2002; CitationDiederich et al 2005; CitationHoueto et al 2005; CitationAckermans et al 2006; CitationGallagher et al 2006; CitationShahed et al 2007). Citationvan der Linden and colleagues (2002) found that bilateral GPi DBS decreased tics by 95% in a 27-year-old man with long-standing TS, which was greater than the suppression of tics induced by medial thalamic DBS (80%), an effect that lasted at least six months. The efficacy of thalamic or pallidal DBS was found to be similar in another case report, although tic attenuation occurred more abruptly with pallidal stimulation (CitationAckermans et al 2006). CitationHoueto and colleagues (2005) also directly compared the efficacy of pallidal versus thalamic DBS in their prospective double blind study. In one patient in whom electrodes were placed in the anteromedial GPi and centromedian-parafascicular complex of the thalamus, either thalamic or pallidal stimulation significantly reduced tic severity and SIB to a similar extent. Symptoms relating to mood and impulsivity were better controlled by thalamic DBS, however. CitationDiederich and colleagues (2005) provide up to fourteen months of follow-up data regarding their patient with bilateral DBS of the posteroventrolateral GPi. Tic frequency and intensity improved, as did the patient’s concomitant anxious and depressive symptoms. These beneficial effects have been observed in two other case reports (CitationGallagher et al 2006; CitationShahed et al 2007).
Other targets for stimulation therapy
Other less frequently targeted structures include the anterior limb of the internal capsule and the nucleus accumbens (CitationFlaherty et al 2005; CitationKuhn et al 2007). Using a more lateral and anterior target than has been used for the surgical treatment of OCD, DBS of the anterior limb of the internal capsule has been shown to decrease tic severity and frequency, but not to the extent that has been observed with thalamic DBS (CitationFlaherty et al 2005). Stimulation of the electrode’s middle contacts resulted in the greatest improvement of tic symptomatology (CitationFlaherty et al 2005). Interestingly, variation in voltage parameters affected the patient’s mood: HFS in the middle or dorsal-most contacts, which lay in the body of internal capsule, produced euthymia and hypomania, respectively, whereas stimulation of the ventral-most contacts, which was located near the nucleus accumbens, caused depression (CitationFlaherty et al 2005). CitationKuhn and colleagues (2007) did not report any alteration of mood in their case report of HFS of the anterior limb of the internal capsule and fundus subventricularis of the nucleus accumbens, and found that it decreased their 26-year-old patient’s YGTSS and RVBRS scores by 41% and 50%, respectively.
DBS caveats
Despite their therapeutic promise for TS and apparent advantages compared with neurosurgical lesion procedures, DBS strategies also can be associated with a number of deleterious side effects and complications. While certain adverse events are common to both lesion and DBS procedures, such as intracerebral hemorrhageFootnote1 and the development of surgery-related infection or seizures (CitationHardesty and Sackheim 2007), others are unique to DBS. For example, patients with TS in whom DBS systems have been implanted have experienced poor healing, infections, or hardware malfunctions due to compulsions to repeatedly touch their incisions or push on the subcutaneously-buried hardware (CitationServello et al 2008; CitationShahed et al 2007; CitationSillay et al 2008). CitationLimousin-Downey and Tisch (2005) suggest that patients with comorbid OCD symptoms may become obsessed with their DBS systems in terms of thoughts of “mind control”. Moreover, DBS requires ongoing surgical maintenance to replace pulse generators (CitationBin-Mahfoodh et al 2003), which last between several months to five years depending upon stimulation parameters, manage hardware-related complications (CitationOh et al 2002; CitationTemel et al 2004; CitationConstantoyannis et al 2005; CitationHamani and Lozano 2006), which have been noted to occur in up to 26% of cases, or adjust the stimulation settings. As has been reported for patients who have received DBS for Parkinson’s disease, HFS for TS may induce or exacerbate pre-existing psychiatric symptoms (CitationAppleby et al 2007).
Surgical considerations
With DBS emerging as a promising therapeutic strategy for the management of TS, guidelines have been proposed regarding its use (CitationMink et al 2006; CitationHardesty and Sackeim 2007; CitationOkun et al 2008). Since TS symptoms, especially motor symptoms, may dissipate in late adolescence (CitationLeckman et al 1998), it has been suggested that surgical candidates be at least twenty-five years of age, although CitationMink and colleagues (2006) acknowledge that selection of that age is somewhat arbritary. Patient selection should occur in a standardized manner. Patients should have their diagnosis of TS confirmed by two independent clinicians and demonstrate recalcitrance to nonsurgical therapies (CitationSinger et al 2001; CitationMinks et al 2006; CitationVisser-Vandewalle 2007). CitationVisser-Vandewalle (2007) suggests that this entails a lack of, or at best only partial, response to three different classes of medications (antidopaminergic, anti-psychotic, and experimental agents) and behavioral therapies. Patients should have tics severe enough to significantly impair quality of life and/or pose medical risk, and this is suggested to correspond to a YGTSS total tic severity score of >35/50 for at least one year (which corresponds to tics that are frequent and noticeable in most situations most of the time) (CitationMink et al 2006). Patients unsuitable for surgical management include those who have a disorder other than TS, other severe psychiatric co-morbidities, structural cerebral abnormalities, or who have medical contraindications to undergoing surgery (CitationMink et al 2006; CitationVisser-Vandewalle 2007). It is mandatory that an ethics review board oversee DBS clinical trials and that a multidisciplinary team provides ongoing input into clinical trial design and evaluation (CitationFins 2003; CitationNuttin and Gybels 2003; CitationGilbert 2006; CitationHamani and Moro 2007). Patients should be assessed using validated and standardized tests (CitationMüller-Vahl 2002), such as the YGTSS (CitationHarcherik et al 1984), RVBRS, Shapiro TS Severity Scale (CitationShapiro et al 1988), and TS Symptom List (CitationLeckman et al 1988), as well as a battery of neuropsychological tests (CitationMink et al 2006; CitationVisser-Vandewalle 2007). Preoperatively, MRI should be performed to facilitate stereotactic planning and post-operatively, imaging is mandatory to confirm electrode placement (CitationMink et al 2006). This would additionally provide visualization of the centre of the active contact of stimulation, the positioning of which is crucial to avoid DBS failure (CitationOkun et al 2005). Since insertion of DBS electrodes can cause a microlesion effect (CitationHoueto et al 2005), post-operative assessments of DBS efficacy should be performed after these possible effects have resolved and after stimulation parameters have stabilized (CitationVisser-Vandewalle 2007). Assessment of DBS effects is recommended to be performed in a double-blind fashion. Since DBS for TS remains an experimental therapy, its performance should be limited to neurosurgical centres with DBS experience in the treatment of movement disorders and with collaborations with neurological and psychiatric services (CitationVisser-Vandewalle 2007; CitationVisser-Vandewalle et al 2006). As is standard with other therapies, patients must be able to provide informed consent to participate in clinical trials and should be allowed to withdraw from DBS management at any time (CitationMink et al 2006; CitationNuttin and Gybels 2003). The aim of DBS for TS is solely to relieve patients of their symptoms and not for political, law enforcement, or social purposes (CitationKopell et al 2004).
Conclusions
In any scientific treatment which is not fully understood there is the serious danger of not being able to predict possible damage. It should be an inflexible duty to become thoroughly familiar with the drug or procedure to be employed. This implies a thorough understanding of the physiological and psychobiological functions which may have a bearing on the proposed treatment (CitationDiethelm 1939).
Oskar CitationDiethelm’s (1939) advice at that 1938 Annual Meeting of the American Psychiatric Association bears relevance even seventy years later. Neurosurgical lesion procedures and DBS have demonstrated therapeutic promise for a subset of patients with TS but have not yet provided sufficient evidence to justify their use as standard therapies for TS. Lesion studies provided the proof-of-principle that TS symptoms could be treated using surgery to target specific cerebral structures, but are limited by methodologic issues in study design, such as their open-label nature (see ), unclear patient inclusion and exclusion criteria, and lack of post-operative assessment of the size and location of the lesions performed. To date, most surgical experiences with DBS also have been described in open-label case reports or series with varying degrees of success and with inconsistency in the types of post-operative assessments performed (CitationTemel and Visser-Vandewalle 2004; CitationNeimat et al 2006). Ultimately, improvement of the efficacy of surgical strategies for TS will require identification of the patients whose tics are most likely to respond to HFS, performing comprehensive pre-and post-operative blinded and standardized clinical assessments of tic symptoms, and determination of the most appropriate surgical target(s) for resolution of tic symptomatology. It is anticipated that the development of animal models of TS as well as further advances in anatomical and functional imaging technologies will enhance understanding of TS pathophysiology and so lead to the refinement of current surgical strategies (CitationSachdev and Sachdev 1997; CitationSakas et al 2007).
Acknowledgments
KM acknowledges the generous support of a Killam Scholarship. Work in the Cell Restoration Laboratory is supported by funding from the Stem Cell Network and Atlantic Innovation Fund.
Notes
1 The reported incidence of symptomatic intracerebral hemorrhage associated with stereotactic procedures ranges from 0.6% to 2.1%, with age, male sex, Parkinson’s disease, and hypertension found to be significant risk factors (CitationBeric et al 2001; CitationFavre et al 2002; CitationLyons et al 2004; CitationBinder et al 2005; CitationGorgulho et al 2005; CitationSansur et al 2007). It has been found that the incidence of intracerebral hemorrhage following either thalamic DBS or ablative thalamic lesions for parkinsonian tremor is not significantly different (CitationBlomstedt and Hariz 2006).
References
- AckermansLTemelYBauerNJC2007Vertical gaze palsy after thalamic stimulation for Tourette syndrome: case reportNeurosurgery61E110018091260
- AckermansLTemelYCathD2006Deep brain stimulation for Tourette’s syndrome: two targets?Mov Disord217091316463374
- AlbinRLKoeppeRABohnenNI2003Increased ventral striatal monoaminergic innervations in Tourette syndromeNeurology61310512913189
- AlexKDYavanianGJMcFarlaneHG2005Modulation of dopamine release by striatal 5-HT2C receptorsSynapse552425115668911
- AlexanderGEDeLongMR1985Microstimulation of the primate striatum. II. Somatotopic organization of striatal microexcitable zones and their relation to neuronal response propertiesJ Neurophysiol531417304009227
- AnandanSWiggCLThomasCR2004Psychosurgery for self-injurious behaviour in Tourette’s disorderJ Child Adolesc Psychopharmacol14531815662144
- AndersonGPollakEChatterjeeD1992Postmortem analysis of subcortical monamines and amino acids in Tourette syndromeAdv Neurol58123331414615
- ApplebyBSDugganPSRegenbergA2007Psychiatric and neuropsychiatric adverse events associated with deep brain stimulation: a meta-analysis of ten years’ experienceMov Disord221722817721929
- AsamUKarrassW1981Gilles de la Tourette syndrome and psychosurgeryActa Paedopsychiatr4739487020328
- BabelTBWarnkePCOstertagCBImmediate and long term outcome after infrathalamic and thalamic lesioning for intractable Tourette’s syndrome2001J Neurol Neurosurg Psychiatry706667111309463
- BaerLRauchSLJenikeMA1994Cingulotomy in a case of concomitant obsessive-compulsive disorder and Tourette’s syndromeArch Gen Psychiatry517348279932
- BakerEFW1962Gilles de la Tourette syndrome treated by bimedial frontal leucotomyCan Med Assoc J867467
- BallantineHTBoukomsAJThomasEK1987Treatment of psychiatric illness by stereotactic cingulotomyBiol Psychiatry2280793300791
- BalthasarK1957Über das anatomische Substrat de generalisierten Tic-Krankheit (maladie des tics, Gilles de la Tourette): Entwicklungshemmung des corpus striatumArchiv für Psychiatrie und Nervenkrankheiten19553149
- BaymCLCorbettBAWrightSB2008Neural correlates of tic severity and cognitive control in children with Tourette syndromeBrain1311657918056159
- BeardGM1880–1881Experiments with the ‘jumpers’ or ‘jumping Frenchmen’ of MainePop Sci Month (NY)18170
- BeckersW1973Gilles de la Tourette’s disease based on five own observationsArch Psychiatr Nervenkr217169864145826
- BenabidALPollakPLouveauA1987Combined (thalamotomy and stimulation) stereotactic surgery of the VIM thalamic nucleus for bilateral Parkinson diseaseAppl Neurophysiol5034463329873
- BenabidAL2007What the future holds for deep brain stimulationExpert Rev Med Devices489590318035954
- BenazzouzAHalletM2000Mechanism of action of deep brain stimulationNeurology55Suppl 6S131611188968
- BericAKellyPJRezaiA2001Complications of deep brain stimulation surgeryStereotact Funct Neurosurg7773812378060
- BinderDKRauGMStarrPA2005Risk factors for hemorrhage during microelectrode-guided deep brain stimulator implantation for movement disordersNeurosurgery567223215792511
- Bin-MahfoodhMHamaniCSimeE2003Longevity of batteries in internal pulse generators used for deep brain stimulationStereotact Funct Neurosurg80566014745210
- BlochMHLeckmanJFZhuH2005Caudate volumes in childhood predict symptom severity in adults of Tourette syndromeNeurology651253816247053
- BlomstedtPHarizMI2006Are complications less common in deep brain stimulation than in ablative procedures for movement disorders?Stereotact Funct Neurosurg84728116790989
- BohlhalterSGoldfineAMattesonS2006Neural correlates of tic generation in Tourette syndrome: an event-related functional MRI studyBrain12920293716520330
- BruunRDBudmanCL1992The natural history of Tourette syndromeAdv Neurol58161414612
- ButlerIJKoslowSHSeifertWEJr1979Biogenic amine metabolism in Tourette syndromeAnn Neurol274436
- CampbellKMVeldmanMBMcGrathMJ2000TS+OCD-like neuropotentiated mice are supersensitive to seizure inductionNeuro-report1123358
- CappabiancaPSpazianteRCarrabsG1987Surgical stereotactic treatment for Gilles de la Tourette’s syndromeActa Neurol (Napoli)9273803324652
- CartaMCarlssonTKirikD2007Dopamine released from 5-HT terminal is the cause of L-DOPA-induced dyskinesias in parkinsonian ratsBrain13018193317452372
- CastellanosFXFineEJKaysenDL1996Sensorimotor gating in boys with Tourette’s Syndrome and ADHD: preliminary resultsBiol Psychiatry3933418719124
- ChangJ-YShiL-HLuoF2008Studies of the neural mechanisms of deep brain stimulation in rodent models of Parkinson’s diseaseNeurosci Biobehav Rev323526618035416
- CheonKARyuYHNamkoongK2004Dopamine transporter density of the basal ganglia assessed with [123I]IPT-SPECT in drug-naive children with Tourette’s disorderPsychiatry Res130859514972371
- ComingsDE1990Blood serotonin and tryptophan in Tourette syndromeAm J Med Genet36418302389798
- ComoP1995Obsessive-compulsive disorder in Tourette’s syndromeAdv Neurol65281917872146
- ConstantoyannisCBerkCHoneyCR2005Reducing hardware-related complications of deep brain stimulationCan J Neurol Sci3219420016018154
- de DivitiisED’ErricoACerilloA1977Stereotactic surgery in Gilles de la Tourette syndromeActa Neurochir (Wien), Suppl2473
- DemirkiranMJankovicJ1995Paroxysmal dyskinesias: clinical features and classificationAnn Neurol3857197574453
- DenklaBMHarrisELAylwardEH1991Executive function and volume of the basal ganglia in children in with Tourette syndrome and attention deficit hyperactivity disorderAnn Neurol30476
- DeWulfAvan BogaertL1941Etudes anatomo-cliniques des syndromes hypercinétiques complexes, III. Une observation anatomo-clinique de maladie des tics (Gilles de la Tourette)Monatsschrift für Psychiatrie und Neurologie1045361
- DiederichNJKalteisKStamenkovicM2005Efficient internal pallidal stimulation in Gilles de la Tourette Syndrome: a case reportMov Disord20149652016037913
- DiethelmO1939An historical review of somatic therapy in psychiatryAm J Psychiatry95116579
- DostrovskyJOLozanoAM2002Mechanisms of deep brain stimulationMov Disord17Suppl 1S63S6811948756
- EidelbergDMoellerJRAntoniniA1997The metabolic anatomy of Tourette’s syndromeNeurology48927349109879
- ErnstMZametkinAJJonsPH1999High presynaptic dopaminergic activity in children with Tourette’s disorderJ Am Acad Child Adolesc Psychiatry3886949893421
- FavreJTahaJMBurchielKJ2002An analysis of the respective risks of hematoma formation in 361 consecutive morphological and functional stereotactic proceduresNeurosurgery50485711844234
- FilaliMHutchisonWDPalterVN2004Stimulation-induced inhibition of neuronal firing in human subthalamic nucleusExp Brain Res1562748114745464
- FinsJJ2003From psychosurgery to neuromodulation and palliation: history’s lessons for the ethical conduct and regulation of neuropsychiatric researchNeurosurg Clin N Am143031912856496
- FlahertyAWWilliamsZMAmirnovinR2005Deep brain stimulation of the anterior internal capsule for the treatment of Tourette syndrome: technical case reportNeurosurgeryONS4E40316234657
- FrankelMCummingsJRobertsonM1986Obsessions and compulsions in Gilles de la Tourette’s syndromeAdv Neurol3637882
- FreudenbergFDieckmannMWinterS2007Selective breeding for deficient sensorimotor gating is accompanied by increased perseveration in ratsNeuroscience1486122217693035
- FreyKAAlbinRLNeuroimaging of Tourette syndrome2006J Child Neurol21672716970868
- FukadaMMentisMJMaY2001Networks mediating the clinical effects of pallidal brain stimulation for Parkinson’s disease: a PET study of resting-state glucose metabolismBrain1241601911459751
- GallagherCLGarellPCMontgomeryEBJr2006Hemi tics and deep brain stimulationNeurology66E1216476922
- GarciaLD’AlessandroGBioulacB2005High-frequency stimulation in Parkinson’s disease: more or less?Trends Neurosci222091615808356
- GilbertD2006Treatment of children and adolescents with tics and tourette syndromeJ Child Neurol2169070016970870
- Gilles de la TouretteGAEB1885Etude sur une affection nerveuse caracterisee par de l’incoordination motrice accompagnee d’echolalie et de copralalieArch Neurol91942
- GorgulhoADe SallesAAFrighettoL2005Incidence of hemorrhage associated with electrophysiological studies performed using macroelectrodes and microelectrodes in functional neurosurgeryJ Neurosurg1058889615926715
- GrabliDMcCairnKHirschEC2004Behavioural disorders induced by external globus pallidus dysfunction in primates: I. Behavioural studyBrain12720395415292053
- GraybielAMAosakiTFlahertyAW1994The basal ganglia and adaptive motor controlScience2651826318091209
- HaberSKowallNVonsattelJ1986Gilles de la Tourette syndrome: a post-mortem neuropathological and immunohistochemical studyJ Neurol Sci75225412428943
- HaberSNWolferD1992Basal ganglia peptidergic staining in Tourette syndrome: a follow-up studyAdv Neurol48145501414617
- HadamitzkyMHarichSKochM2007Deficient pre-pulse inhibition induced by selective breeding of rats can be restored by the dopamine D2 antagonist haloperidolBehav Brain Res177364717182114
- HamaniCLozanoAM2006Hardware-related complications of deep brain stimulation: a review of the published literatureStereotact Func Neurosurg8424851
- HamaniCMoroE2007Surgery for other movement disorders: dystonia, ticsCurr Opin Neurol20470617620884
- HarcherikDFLeckmanJFDetlorJ1984A new instrument for clinical studies of Tourette’s syndromeAm J Acad Child Psychiatry2315360
- HardestyDESackeimHA2007Deep brain stimulation in movement and psychiatric disordersBiol Psychiatry61831517126303
- HarrisKSingerHS2006Tic disorders: neural circuits, neurochemistry, and neuroimmunologyJ Child Neurol216788916970869
- HasslerRDieckmannG1970Traitement stéréotaxique des tics et cris inarticulés ou coprolaliques considérés comme phenomena d’obsession motrice au cours de la maladie de Gilles de la TouretteRevue Neurologique (Paris)12389100
- HouetoJLKarachiCMalletL2005Tourette’s syndrome and deep brain stimulationJ Neurol Neurosurg Psychiatry76992515965209
- HydeTMStaceyMECoppolaR1995Cerebral morphometric abnormalities in Tourette’s syndrome: a quantitative MRI study of monozygotic twinsNeurology451176827783885
- ItardJMG1825Mémoire sure quelques fonctions involontaires des appareils de la locomotion, de la préhension et de la voixArchives Generales de Médecine8385407
- JankovicJ1994Botulinum toxin in the treatment of dystonic ticsMov Disord934798041378
- JankovicJ2001Tourette’s syndromeN Engl J Med34511849211642235
- Jiménez-JiménezFJGarcía-RuizPJ2001Pharmacological options for the treatment of Tourette’s disorderDrugs6122072011772131
- KalanithiPSAZhengWKataokaY2005Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndromeProc Natl Acad Sci U S A102133071216131542
- KopellBHGreenbergBRezaiAR2004Deep brain stimulation for psychiatric disordersJ Clin Neurophysiol21516715097294
- KopellBHRezaiAR2003Psychiatric neurosurgery: a historical perspectiveNeurosurg Clin N Am141819712856487
- KraussJKJankovicJSevere motor tics causing cervical myelopathy in Tourette’s syndromeMov Disord1156368866498
- KuhnJLenartzDMaiJK2007Deep brain stimulation of the nucleus accumbens and the internal capsule in therapeutically refractory Tourette-syndromeJ Neurol254963517410328
- KulisevskyJBerthierMLAvilaA1995Longitudinal evolution of prefrontal leucotomy in Tourette’s syndromeMovement Disorders1034587651455
- KurlanR1997Treatment of ticsNeurol Clin1540399115470
- KurlanRCaineELichterD1988Surgical treatment of severe obsessive-compulsive disorder associated with Tourette’s syndromeNeurology38Suppl 12034
- KurlanRKersunJBallantineHTJr1990Neurosurgical treatment of severe obsessive-compulsive disorder associated with Tourette’s syndromeMov Disord515252325677
- LavensteinBL2003Treatment approaches for children with Tourette’s syndromeCurr Neurol Neurosci Rep3143812583843
- LeckmanJFde LotbinièreAJMarekK1993Severe disturbances in speech, swallowing, and gait following stereotactic infrathalamic lesions in Gilles de la Tourette’s syndromeNeurology4389048492943
- LeckmanJFTowbinKEOrtSICohenDJBruunRDLeckmanJF1988Clinical assessment of tic disorder severityTourette’s Syndrome and Tic DisordersNew YorkWiley5578
- LeckmanJFZhangHVitaleA1998Course of tic severity in Tourette syndrome: the first two decadesPediatrics1021499651407
- LeckmanJF2002Tourette’s syndromeLancet36057786
- LernerABagicABoudreauFA2007Neuroimaging of neuronal circuits involved in tic generation in patients with Tourette syndromeNeurology6819798717548547
- Limousin-DowseyPTischS2005Surgery for movement disorders: new applications?J Neurol Neurosurg Psychiatry7690415965193
- LiuYPostupnaNFalkenbergJ2008High frequency deep brain stimulation: what are the therapeutic mechanisms?Neurosci Biobehav Rev323435117187859
- LozanoAMMahatN2004Deep brain stimulation surgery for Parkinson’s disease: mechanisms and consequencesParkinsonism Relat Disord10S49S5715109587
- LyonsKEWilkinsonSBOvermanJ2004Surgical and hardware complications of subthalamic stimulation: a series of 160 proceduresNeurology63612615326230
- MaciunasRJMadduxBNRileyDE2007Prospective randomized double-blind trial of bilateral thalamic deep brain stimulation in adults with Tourette syndromeJ Neurosurg10710041417977274
- MahlerMLukeJOutcome of the tic syndromeJ Nerv Ment Dis10343345
- McGrathMJCampbellKMParksCR2000Glutamatergic drugs exacerbate symptomatic behaviour in a transgenic model of comorbid Tourette’s syndrome and obsessive-compulsive disorderBrain Res877233010980239
- McIntyreCCSavastaMWalterBJ2004How does deep brain stimulation work? Present understanding and future questionsJ Clin Neurophysiol21405015097293
- MinkJW2001Basal ganglia dysfunction in Tourette’s syndrome: a new hypothesisPediatr Neurol25190811587872
- MinkJWWalkupJFreyKA2006Patient selection and assessment recommendations for deep brain stimulation in Tourette syndromeMov Disord211831816991144
- MoldofskyHTullisCLamonR1974Multiple tic syndrome (Gilles de la Tourette’s syndrome)J Nerv Ment Dis159282924529247
- MontgomeryEBJrGaleJT2008Mechanisms of action of deep brain stimulationNeurosci Biobehav Rev3238840717706780
- MoriartyJVarmaARStevensJ1997A volumetric MRI study of Gilles de la Tourette’s syndromeNeurology4941059270569
- Müller-VahlKR2002The treatment of Tourette’s syndrome: current opinionsExpert Opin Pharmacother389991412083990
- NádvorníkPŠramkaMLisýL1972Experiences with dentatotomyConfin neurol3432044566501
- NeimatJSPatilPGLozanoAM2006Noval surgical therapies for Tourette syndromeJ Child Neurol21715816970873
- NordstromEJBurtonFH2002A transgenic model of comorbid Tourette’s syndrome and obsessive-compulsive disorder circuitryMol Psychiatry76172552412140785
- NuttinBGybelsJ2003Deep brain stimulation for psychiatric disordersNeurosurg Clin N Am14xvxvi12856486
- OhMYAboschAKimSH2002Long-term hardware-related complications of deep brain stimulationNeurosurgery5012687612015845
- OkunMSFernandezHHFooteKD2008Avoiding deep brain stimulation failures in Tourette syndromeJ Neurol Neurosurg Psychiatry79111218202200
- OkunMSTagliatiMPourfarM2005Management of referred deep brain stimulation failuresArch Neurol621250515956104
- PehekEANocjarCRothBL2006Evidence for preferential involvement of 5-HT2A serotonin receptors in stress-and drug-induced dopamine release in the rat medial prefrontal cortexNeuropsychopharmacology312657715999145
- PetersonBRiddleMACohenDJ1993Reduced basal ganglia volumes in Tourette’s syndrome using three-dimensional reconstruction techniques from magnetic resonance imagesNeurology4394198492950
- PetersonBSCohenDJJankovicJGoetzCG2001Neuroimaging studies of Tourette syndrome: a decade of progressTourette syndrome. Volume 85 of Advances in neurologyPhiladelphiaLippincott Williams and Wilkins17996
- RobertsonMDoranMTrimbleM1990The treatment of Gilles de la Tourette syndrome by limbic leucotomyJ Neurol Neurosurg Psychiatry5369142213047
- RobertsonMMSternJS2000Gilles de la Tourette syndrome: symptomatic treatment based on evidenceEur Child Adolesc Psychiatry9I/60I/75
- RobertsonMMTrimbleMRLeesAJ1989Self-injurious behaviour and the Gilles de la Tourette syndrome: a clinical study and review of the literaturePsychol Med19611252678199
- RobertsonMM2000Tourette syndrome, associated conditions and the complexities of treatmentBrain1234256210686169
- SachdevPSachdevJ1997Sixty years of psychosurgery: its present status and its futureJ Aust N Z J Psychiatry3145764
- SakasDEKouyialisATBoviatsisEJ2007Technical aspects and considerations of deep brain stimulation surgery for movement disordersActa Neurochir Suppl971637017691301
- SansurCAFrysingerRCPouratianN2007Incidence of symptomatic hemorrhage after stereotactic electrode placementJ Neurosurg107998100317977273
- SawleGVLeesAJHymasNF1993The metabolic effects of limbic leucotomy in Gilles de la Tourette syndromeJ Neurol Neurosurg Psychiatry56101698410025
- Serra-MestresJRingHACostaDA2004Dopamine transporter binding in Gilles de la Tourette syndrome: A [123I]FP-CIT/SPECT studyActa Psychiatr Scand109140614725596
- SershenHHashimALajthaA2000Serotonin-mediated striatal dopamine release involves the dopamine uptake site and the serotonin receptorBrain Res Bull53353711113592
- ServelloDPortaMSassiM2008Deep brain stimulation in 18 patients with severe Gilles de la Tourette syndrome refractory to treatment: the surgery and stimulationJ Neurol Neurosurg Psychiatry791364217846115
- ShahedJPoyskyJKenneyC2007GPi deep brain stimulation for Tourette syndrome improves tics and psychiatric comorbiditiesNeurology681596017210901
- ShapiroAKShapiroESYoungJGShapiroAKShapiroESYoungJG1988Signs, symptoms, and clinical courseGilles de la Tourette Syndrome2nd edNew YorkRaven Press12793
- SillayKALarsonPSStarrPA2008Deep brain stimulator hardware-related infections: incidence and management in a large seriesNeurosurgery62360618382313
- SingerHSButlerIJTuneLE1982Dopaminergic dysfunction in Tourette syndromeAnn Neurol1236166184010
- SingerHSReissALBrownJE1993Volumetric MRI changes in basal ganglia of children with Tourette syndromeNeurology4395068492951
- SingerHSSzymanskiSGiulianoJ2002Elevated intrasynaptic dopamine release in Tourette’s syndrome measured by PETAm J Psychiatry15913293612153825
- SingerHS2001The treatment of ticsCurr Neurol Neurosci Rep119520211898516
- SnyderSHTaylorKMCoyleJT1970The role of brain dopamine in behavioural regulation and the actions of psychotropic drugsAm J Psychiatry1271992074319649
- SternESilbersweigDACheeK-Y2000A functional neuroanatomy of tics in tourette syndromeArch Gen Psychiatry57741810920461
- StevensH1964The syndrome of Gilles de la Tourette and its treatmentMed Ann Dist Columbia33277930414155608
- StoetterBBraunARRandolphC1992Functional neuroanatomy of Tourette syndrome. Limbic-motor interactions studied with FDG PETAdv Neurol58213261414626
- SunBKrahlSEZhanS2005Improved capsulotomy for refractory Tourette’s syndromeStereotact Func Neurosurg83556
- SwerdlowNRGeyerMABraffDL2001Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challengesPsychopharmacology15619421511549223
- SwerdlowNRSutherlandAN2005Using animal models to develop therapeutics for Tourette SyndromePharmacol Ther1082819315970330
- SwerdlowNRSutherlandAN2006Preclinical models relevant to Tourette syndromeAdv Neurol99698816536353
- SwerdlowNRYoungABCohenDJJankovicJGoetzCG2001Neuropathology in Tourette syndrome: an updateTourette syndrome, Volume 85 of Advances in neurologyPhiladelphiaLippincott Williams and Wilkins17996
- TemelYAckermansLCelikH2004Management of hardware infections following deep brain stimulationActa Neurochir (Wien)1463556115057529
- TemelYVisser-VandewalleV2004Surgery in Tourette syndromeMov Disord1931414743354
- The Tourette Syndrome Classification Study Group1993Definitions and classifications of tic disordersArch Neurol50101368215958
- van der LindenCColleHVandewalleV2002Successful treatment of tics with bilateral internal pallidum (GPi) stimulation in a 27-year-old male patient with Gille de la Tourette’s syndrome (GTS)Mov Disord17Suppl 5S341
- Visser-VandewalleVAckermansLvan der LindenC2006Deep brain stimulation in Gilles de la Tourette’s syndromeNeurosurgery58E59016528175
- Visser-VandewalleVTemelYBoonP2003Chronic bilateral thalamic stimulation: a new therapeutic approach in intractable Tourette syndromeJ Neurosurg99109410014705742
- Visser-VandewalleVvan der LindenCGroenewegenHJ1999Stereotactic treatment of Gilles de la Tourette syndrome by high frequency stimulation of thalamusLancet353724
- Visser-VandewalleV2007DBS in Tourette syndrome: rationale, current status and future prospectsActa Neurochir Suppl972152217691307
- WhittyCWMDuffieldJETowPM1952Anterior cingulectomy in the treatment of mental diseaseLancet14758114898782
- WolfSSJonesDWKnableMB1996Tourette syndrome: prediction of phenotypic variation in monozygotic twins by caudate nucleus D2 receptor bindingScience273122578703056
- WongDFPearlsonGDYoungLT1989D2 dopamine receptors are elevated in neuropsychiatric disorders other than schizophreniaJ Cereb Blood Flow Metab9Suppl 1S593
- WongDFSingerHSBrandtJ1997D2-like dopamine receptor density in Tourette syndrome measured by PETJ Nucl Med38124379255158
- ZimmermanAMAbramsMTGiulianoJD2000Subcortical volumes in girls with Tourette syndromeNeurology542224910881244