Abstract
Restless legs syndrome (RLS) is a sleep-related movement disorder commonly involving an unpleasant urge to move the limbs, typically the legs. Dopaminergic agents represent the first-line therapy for RLS; however, long-term use of such drugs results in worsening symptoms due to “augmentation” or other adverse events. Gabapentin, an analog of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA), is an anticonvulsant/analgesic agent. Gabapentin is only mildly effective in relieving RLS symptoms, perhaps a result of its poor absorption from the gastrointestinal (GI) tract. Gabapentin enacarbil is a prodrug of gabapentin specifically designed to enhance absorption via the GI tract, and hence provide improved circulating levels of gabapentin on metabolism. Clinical trials to date have demonstrated favorable safety and (compared to traditional gabapentin) improved pharmacokinetics and efficacy in treating RLS symptoms. Thus, gabapentin enacarbil may prove to be a useful drug in treating RLS. An application of gabapentin enacarbil for treatment of RLS is currently pending with FDA for approval.
Introduction
Restless legs syndrome (RLS) is a common, distressing disease that affects a significant percentage of the adult population. In the US, 2% to 3% of the population experience clinically bothersome symptoms severe enough to warrant treatment.Citation1–Citation3 RLS is characterized by discomfort of the legs during rest or inactivity and involves an urge to move the legs to relieve the symptoms. It can occasionally also affect the arms. RLS symptoms are usually present or worsen in the evening.Citation4 Because of the discomfort of the limbs during rest, RLS can reduce sleep quality, daily function, and overall quality of life. Several drugs are currently used to treat RLS: dopaminergic agents such as levodopa (in combination with carbidopa or benserazide), dopamine agonists (such as ropinirole, and pramipexole), and nondopaminergic medications such as gabapentin, clonazepam and oxycodone.Citation5,Citation6 Common side effects of these drugs are sedation, dizziness, fatigue, nausea, and vomiting.
Gabapentin is an anticonvulsant drug and is approved in the United States for the treatment of postherpetic neuralgiaCitation7 and partial seizures.Citation7–Citation10 Gabapentin has also been shown effective in improving RLS symptoms, reducing the frequency of periodic leg movements and improving sleep quality, suggesting a potential role in treating RLS.Citation11–Citation15 Gabapentin is not approved for the indication of RLS treatment, although it is often used off-label for this purpose.Citation5,Citation16,Citation17 Additional placebocontrolled, double-blind clinical trials have also suggested potential uses of gabapentin in the treatment of painful neuropathies in patients with diabetes mellitusCitation18 and anxiety disorders.Citation19
However, gabapentin has inherent pharmacokinetic deficiencies that limit its clinical effectiveness: variable bioavailability and short half-life. In healthy volunteers as well as epilepsy patients, clinical pharmacokinetic studies demonstrate limited absorption and high inter-patient variability.Citation20–Citation23 Bioavailability of orally administered gabapentin is dose-dependent. Surprisingly, bioavailability actually decreases with increasing dosages. Specifically, the oral bioavailability of gabapentin is approximately 60%, 47%, 34%, 33%, and 27% following 900, 1200, 2400, 3600, and 4800 mg/day given in 3 divided doses, respectively.Citation24 Importantly, the range of doses where bioavailability decreases coincides with doses reported to be useful to treat neuropathic pain.Citation20
Saturation of the gabapentin transport pathway may account for the observed dose-dependent decrease in bio-availability. Absorption of gabapentin is mediated by a low-capacity solute transporter, probably an L-type amino acid transporter, which is located mainly in the upper small intestine.Citation25,Citation26 It has been suggested that the high inter-patient variability due to non-responders in clinical trialsCitation18,Citation27 could be because of lower abundance of transporters in these patients,Citation28 resulting in unpredictable plasma levels of gabapentin that may not reach the rapeutically meaningful levels.
Gabapentin has a short half-life of just 5 to 7 hours. After oral administration and absorption, gabapentin is rapidly excreted via urine; therefore gabapentin must be dosed 3 to 4 times a day to maintain therapeutic levels. The requirement of frequent administration can lead to noncompliance and missed doses which can reduce clinical effectiveness.Citation29
Enhancing the effectiveness of gabapentin has long been a goal, and pregabalin was developed in this direction. Pregabalin is a structural analog of gabapentin, which does not bind to gamma-aminobutyric acid (GABA) receptors, and is approved as an add-on medication for epilepsy and neuropathic pain. In a small clinical trial, pregabalin was efficacious in treating primary RLS as well as secondary RLS, and further investigation with randomized, placebo-controlled trials was recommended.Citation30
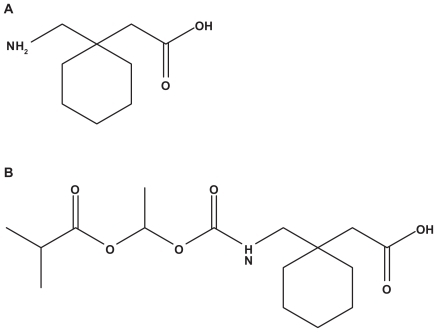
In an attempt to address the pharmacokinetic limitations of gabapentin, Cundy and coworkers at Xenoport Inc. designed and synthesized a series of analogs of gabapentin and selected one candidate for further development based on its physico-chemical properties, enzymatic stability, transport, and in vivo pharmacokinetics. This compound was designated as gabapentin enacarbil or XP13512Citation28 (). XP13512 is being further developed by XenoPort Inc/Astellas Pharma/GlaxoSmithKline. A New Drug Application (NDA) has been filed with the Food and Drug Administration (FDA) and the current Prescription Drug User Fee Act (PDUFA) goal date for its review is February 2010.
Drug design
Gabapentin enacarbil (XP13512) is a gabapentin prodrug. Specifically, it is an acyloxyalkylcarbamate analog with an efficient enzymatic conversion to gabapentin in tissues. In addition, gabapentin enacarabil is designed to be recognized as a substrate for two high-capacity nutrient transporters with broad distribution through the human intestinal tract. One of the two transporters, monocarboxylate transporter type I (MCT-1), regulates the absorption of small-chain fatty acids and may transport pharmaceutical agents across the intestine.Citation31 The second transporter is a sodium-dependent multivitamin transporter (SMVT). SMVT regulates absorption of the essential cofactors biotin, lipoate and pantothenate.Citation32
Pharmacology
Gabapentin enacarbil is administered as an oral formulation. The pharmacology was evaluated as immediate-release (IR) and extended release (XR) formulations.Citation33,Citation34
Improved bioavailability
Gabapentin enacarbil is rapidly absorbed and undergoes efficient enzymatic hydrolysis to yield gabapentin in vivo. In contrast to gabapentin, which uses lower capacity transport receptors located only in the upper intestinal area, gabapentin enacarbil uses alternative, higher-capacity transport receptors found across the whole length of the gastrointestinal tract. Increased transport capacity allows the delivery of higher doses of gabapentin in good dose proportionality, avoiding uptake saturation at clinically relevant doses.
No saturation of transport was observed in a dose escalation study up to 2800 mg using immediate release formulation; the plasma concentration of gabapentin was observed to increase proportionally in accordance with an orally administered dose of gabapentin enacarbil.Citation33 In contrast, gabapentin transport was saturated at 1400 mg oral dose levels. In the same study, similar observations were made with extended release tablet formulations of gabapentin enacarbil, with no sign of transport saturation up to 2100 mg oral dose levels.
Improved oral bioavailability for gabapentin enacarbil compared to gabapentin has been demonstrated in animal and human pharmacokinetic studies. Cundy et alCitation35 reported improved oral bioavailability, dose proportionality, and colonic absorption for the prodrug compared to gabapentin in rats and monkeys. Compared with intracolonic gabapentin, intracolonic gabapentin enacarbil exhibited a 17-fold and 34-fold higher exposure in rats and monkeys, respectively. In addition, in monkeys, gabapentin enacarbil capsules resulted in 84.2% oral bioavailability compared with 25.4% bioavailability after a similar oral gabapentin dose. Importantly, this improvement in bioavailability was also observed in a human study, specifically, it was reported that the oral bioavailability of gabapentin enacarbil compared to gabapentin was 74.5% vs 36.6%.Citation33 Based on the molecular weights and milligram-equivalent of gabapentin relative to the gabapentin enacarbil, a 365 mg dose of gabapentin as prodrug (700 mg) achieved higher or comparable plasma concentrations of gabapentin compared to a 1200 to 1400 mg dose of free gabapentin in immediate release formulation.Citation33 Efficient oral absorption of gabapentin enacarbil and conversion to achieve higher and dose-dependent concentration of gabapentin in plasma will allow preparation of longer-acting extended release formulations.
Mode of action
After intestinal absorption, gabapentin enacarbil is rapidly converted to gabapentin and the latter is the active form. Although gabapentin is well established as an effective antiepileptic and antineuropathic pain drug, the underlying molecular mechanism of gabapentin is still not entirely clear.Citation36 Gabapentin is a structural analog of GABA; however gabapentin does not interact significantly with any GABA subtype receptors,Citation37,Citation38 for instance, several studies reported no evidence that gabapentin binds substantially to any subtype of GABAB receptors.Citation37,Citation39,Citation40 Even so, one study suggested that gabapentin acts as an agonist for a subset of GABAB receptors,Citation41 which may negatively regulate voltage-gated Ca2+ channelsCitation42 and activate inwardly rectifying K+ channels.Citation43 Gabapentin has shown a direct effect on the excitability of sensory neurons by blockade of Ca2+ and Na+ channels or activation of K+ channels.Citation44 Lee and coworkers demonstrated that gabapentin upregulated renal outer medullary potassium channel, thus reducing neuronal excitability, suggestive of an important role in gabapentin’s antiepileptic effect.Citation45
D-serine, an agonist for N-methyl-D-aspartate (NMDA) receptors, reverses some of gabapentin’s actions.Citation46 However, gabapentin does not directly interact with the glycine-NMDA complex,Citation37,Citation47 and a rat study concluded that D-serine and gabapentin do not act at the same site.Citation48 Gabapentin also exhibits a profound synergistic anti-allodynic action with the AMPA receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX).Citation49 A recent study concluded that after peripheral nerve injury, gabapentin acts directly in the brainstem via a glutamate-dependent mechanism to stimulate descending inhibition to produce antihypersensitivity.Citation50
Suman-Chauhan and coworkers identified a high-affinity binding site for gabapentin in rat brain homogenate.Citation38 Binding to this receptor was dependent on the system L transporter, indicating that gabapentin likely crosses the cell membrane and binding occurs intracellularly. There is no clear relationship between system L transporter binding and clinical efficacy of gabapentin.Citation51 The binding site for gabapentin was subsequently identified as the α2δ subunit of voltage-gated Ca2+ channel using pig cerebral cortex membranes.Citation52 Hendrich and coworkers provided a definitive proof that gabapentin acts primarily at an intracellular location.Citation53
Pharmacokinetics, safety and tolerability
Preclinical studies were conducted to establish the efficiency and conversion rate of gabapentin enacarbil to gabapentin in various tissues. Gabapentin enacarbil was chemically stable at physiological pH range as indicated by >95% recovery of the prodrug after incubation for 1 hour at 37°C in buffers over the pH range of 2 to 8.Citation28 However, the prodrug was rapidly converted to gabapentin by non-specific esterases in intestinal and liver tissues from rats, dogs, monkeys, and humans. Rates of conversion to gabapentin in human intestinal and liver tissue preparations at 1 mg protein/mL were 196 and 146 pmol/min/mg protein. Similar rates of conversion were observed in rats and monkeys. After 1 hour of incubation at 37°C in tissue preparations of pancreatin, Caco-2 cells, rat plasma, human plasma, rat liver, and human liver, 52%, 18%, 47%, 96%, 25%, and 4% of gabapentin enacarbil was recovered with release rates of gabapentin of 43%, 75%, 45%, 5%, 71%, and 81%, respectively.Citation28 The conversion to gabapentin was quantitative in these studies and no formation of gabapentin lactam was observed. Both isomers of gabapentin enacarbil showed similar cleavage rates in human tissues. Gabapentin enacarbil also did not show substrate or inhibitor properties for major P450 isoforms in human liver homogenates.Citation28 Therefore, gabapentin enacarbil is likely to have significantly low drug–drug interaction following administration.
To understand the mechanism of transport, in vitro transport studies across artificial lipid membranes were carried out. Gabapentin enacarbil showed pH-dependent passive permeability across the membrane suggesting that the prodrug should have some ability to passively diffuse across cells.Citation28 However, passive diffusion is likely a minor component because pKa of gabapentin enacarbil is 5.0. Using in vitro cell culture assays, the transport of gabapentin enacarbil was shown to be dependent on MCT-1 and SMVT.
To understand the biodistribution and oral bioavailability, pharmacokinetic studies were performed in rats and monkeys. A dose-proportional exposure to gabapentin was observed after oral administration of gabapentin enacarbil, while exposure to the intact prodrug was low. More than 95% of radio labeled [14C]-gabapentin enacarbil was excreted as gabapentin in urine in rats. In monkeys 84.2% of gabapentin enacarbil was excreted as gabapentin compared to 25.4% when gabapentin was delivered orally. Less than 1% of the intact prodrug was recovered in feces.Citation53 In healthy human subjects, using supratherapeutic doses gabapentin enacarbil was converted rapidly to gabapentin after absorption. Blood levels of gabapentin were proportional to gabapentin enacarbil dose over the range of 2400 to 6000 mg (1250–3125 mgequivalent gabapentin). Blood concentrations of intact gabapentin enacarbil were low and transient (≤0.5% of the released gabapentin concentration at all doses).Citation35 These studies indicate that gabapentin enacarbil is readily absorbed after oral delivery.
In addition to one molecule of gabapentin, hydrolysis of gabapentin enacarbil also produces one molecule each of carbon dioxide, acetaldehyde, and isobutyric acid. Both acetaldehyde and isobutyric acid are generally regarded as safe (GRAS) molecules by the FDA. Given that gabapentin enacarbil is converted into gabapentin, with a known safety profile, and also converted into GRAS compounds, it is likely that gabapentin enacarbil should exhibit a favorable safety profile. Consistent with this hypothesis, no significant toxicities were observed with gabapentin enacarbil in monkeys following repeated oral administration of 2000 mg/kg/day doses. In addition no detectable accumulation was observed after 2 weeks.Citation35
In a randomized-sequence, double-blind, placebo-controlled crossover study in healthy human subjects, gabapentin enacarbil was well tolerated up to supratherapeutic doses.Citation54 In this study 600 mg extended-release tablets of gabapentin enacarbil were administered as a single oral dose of 2400, 3600, 4800, or 6000 mg. In a 32 healthy volunteer study, the most commonly reported adverse events were dizziness and nausea (50% and 25% of subjects, respectively). Two subjects experienced treatment-emergent adverse events rated as severe: psychomotor retardation, vertigo, and sedation (4800 mg dose) and somnolence (6000 mg dose). All treatment-emergent adverse events were resolved without medical intervention. None of the adverse events led to study withdrawal. These data support that gabapentin enacarbil is safe and well tolerated with minimal side effects.
To study the bioavailability of gabapentin enacarbil from immediate release formulation, pharmacokinetic studies were conducted in healthy volunteers in phase I clinical trials.Citation34,Citation55–Citation57 Single doses of IR gabapentin enacarbil (350, 700, 1400, 2100, and 2800 mg orally; n = 40) or placebo (n = 10) were evaluated. After 1 week, approximately equimolar doses of gabapentin (200, 800, 1200, and 1400 mg) were given to participants. Gabapentin exposure with prodrug was dose-proportional; bioavailability was 82.9% for the 350 mg dose and 79.7% for the 2800 mg dose. In contrast, with gabapentin parent the bioavailability was 65.2% and 26.5% with the lowest and the highest doses respectively. Multiple doses of gabapentin enacarbil (350, 400, 800, 1200 and 1400 mg orally; twice daily, n = 29), administered over 7 days, resulted in dose-proportional gabapentin. The bioavailability of gabapentin ranged from 73.1% to 93.2%.
Bioavailability from sustained release formulation of gabapentin enacarbil (1200 mg orally) was evaluated either with (n = 10) or without food (n = 12). Gabapentin (600 mg orally; n = 11) taken without food served as a reference. Exposure to gabapentin from gabapentin enacarbil was higher in the with food group compared to the fasted group. Exposure to gabapentin was higher in prodrug groups than the gabapentin group. Bioavailability of gabapentin was 46.5% and 73.7% for the fasted and fed gabapentin enacarbil groups respectively, while the bioavailability was 37.7% in parent drug group.Citation58
The effect of fat content in food on gabapentin enacarbil absorption was evaluated under fasted, low-fat (200–300 kcal, 6% from fat), moderate-fat (500–600 kcal, 30% from fat), and high-fat (1000 kcal, 50% from fat) conditions. The different fat contents did not appear to affect the absorption of the prodrug. The bioavailability of gabapentin was 42%, 64%, 65%, and 76% in fasted, low-, medium-, and high-fat food conditions respectively.Citation34
Potential for drug interactions between gabapentin enacarbil, naproxen (an MCT-1 substrate) and cimetidine (an organic cation transporter 2 substrate) was evaluated to assess the alterations in drug absorption when the prodrug is used in combination with these and other transporter substrates. In healthy volunteers, gabapentin enacarbil did not affect the bioavailability of naproxen or cimetidine. The AUC values of gabapentin enacarbil, when co-administered in combination with naproxen or cimetidine, increased up to 12% and 24%, respectively. The results indicate that gabapentin enacarbil can be used together with MCT-1, organic cation transporter 2, or other transporter substrates without the need to alter the dose regimes.Citation59,Citation60
Efficacy
Efficacy of gabapentin enacarbil was evaluated in several phase II and phase III trials. Because various phase I, II and III trials were recently summarized by Merlino et al 2009,Citation34 this review will focus on key phase II and III studies in conjunction with drug efficacy. Readers are encouraged to refer the review by Merlino et al for a summary of clinical trials prior to the publication of this article.
Efficacy of gabapentin enacarbil in relieving RLS symptoms was evaluated over a two week period in a multicenter, randomized, double-blind, placebo-controlled, crossover, polysomnographic phase IIa clinical trial in RLS patients. Patients were treated for two weeks with either gabapentin enacarbil (600 mg orally at 5 PM and 1200 mg orally 1 hour before bed) or placebo, with a 1-week washout period between treatments. At the end of the treatment period, a significant improvement was observed in RLS symptoms such as quality of sleep, number of awakenings per night and hours awake per night. Compared to 14.7% patients reporting improvement of RLS symptoms in placebo group, 79.5% of patients in the gabapentin enacarbil group reported either much improved or very much improved symptoms.Citation61 Efficacy and tolerability of 1800 mg dose of gabapentin enacarbil was evaluated in 38 naïve subjects with RLS, over 9 clinical sites.Citation62 Patients were treated with 1800 mg/day for 14 days, with a 7-day washout period between treatment periods. After 14 days, significantly reduced RLS symptoms and improved sleep were observed in subjects with moderate-to-severe primary RLS while dose levels were well tolerated.
In a 12-week, multicenter, randomized, double-blind, placebo controlled phase III clinical trial, efficacy of gabapentin enacarbil (1200 mg/day orally at 5 PM) was evaluated in patients with primary RLS. Patients treated with gabapentin enacarbil showed an improved international RLS (IRLS) total score (−13.2) in contrast to placebo group (−8.8) at 2 weeks. On the investigator-rated CGI (Clinical Global Impression), significantly more gabapentin enacarbil-treated patients (76.1%) responded than placebo (38.9%). At the end of study more than 50% of gabapentin enacarbil-treated patients showed no sign of RLS over the 24-hour assessment period compared with 18% placebo patients.Citation63,Citation64
Long-term safety and efficacy of gabapentin enacarbil was evaluated in a second multicenter, placebo-controlled clinical trial involving 327 patients with primary moderate-to-severe RLS symptoms.Citation65 Patients received gabapentin enacarbil (1200 mg/day orally) at 5 PM for 24 weeks in a single-blind phase of trial. The initial 24 weeks phase was completed with 221 patients, out of which 194 (88%) responded either “much improved” or “very much improved” CGI scores. These patients were further entered into a 12- week randomized, double-blind phase. Patients were given gabapentin enacarbil for either 12 weeks (1200 mg/day po) or for 2 weeks (600 mg/day po) and then placebo for 10 weeks. For the extended trial, the primary end-point was number of patients who relapsed or had worsening symptoms. Gabapentin enacarbil treated group showed a statistically significant lower proportion of relapses than placebo, 9% versus 23% respectively. A third phase III trial evaluated the safety and efficacy of 600 and 1200 mg/day over 12 weeks.Citation66 Both dose groups of gabapentin enacarbil resulted in significantly improved IRLS and CGI scores than with placebo.
In addition to RLS, other indications are being investigated for gabapentin enacarbil.Citation67 The additional indications include post-herpetic neuralgia, painful diabetic neuropathy, and migraine prophylaxis. Recently, top-line results were presented from a phase II clinical study of placebo, 1200, 2400 or 3600 mg/day of gabapentin enacarbil dosed twice a day for treatment of post-herpetic neuralgia. All doses demonstrated statistically significant improvements over placebo on the change from baseline to the end of maintenance treatment in the 24- hour average pain intensity score. This 14-week, double-blind, placebo-controlled study enrolled 376 subjects experiencing pain for at least 3 months following healing of herpes zoster skin rash. Gabapentin enacarbil was generally well tolerated at all doses, and the most common adverse events were dizziness and somnolence, and most were mild or moderate.
Conclusions
RLS is a sleep-related movement disorder which involves an undesirable sensation to move the legs, which improves with activity. It is typically worse in the evenings and nights. Dopaminergic agents are currently the most commonly prescribed therapy. Long-term use of dopaminergic drugs may cause augmentation or other side effects such as leg edema, dizziness, and nausea. Gabapentin, an analog of GABA, is an anticonvulsant/analgesic agent. Gabapentin is only mildly effective in relieving RLS symptoms, perhaps because of poor absorption from the gastrointestinal tract. Gabapentin enacarbil, a prodrug of gabapentin, is designed to enhance gastrointestinal absorption and hence enhance bioavailability of gabapentin. Clinical trials have shown gabapentin enacarbil to be a safe and effective drug for RLS. An application for approval of gabapentin enacarbil for treatment of RLS is currently pending with FDA for approval, and additional clinical indications are being pursued, including neuropathic pain. The addition of one more therapeutic option to the RLS drug armamentarium would provide more options to physicians dealing with RLS patients with refractory symptoms.
Disclosures
The authors disclose no conflicts of interest.
References
- NicholsDAAllenRPGraukeJHRestless legs syndrome symptoms in primary care: a prevalence studyArch Intern Med20031632323232914581252
- AllenRPWaltersASMontplaisirJRestless legs syndrome prevalence and impact: REST general population studyArch Intern Med20051651286129215956009
- HöglBKiechlSWilleitJRestless legs syndrome: a community-based study of prevalence, severity, and risk factorsNeurology2005641920192415955944
- AllenRPicchierttiDHeningWRestless leg syndrome: diagnostic criteria, special considerations, and epidemiology A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institute of HealthSleep Med2003410111914592341
- YeeBKillickRRestless legs SyndromeAustralian Family Physician20093829630019458798
- TrenkwalderCHeninigWAMontagnaPTreatment of restless legs syndrome: An evidence-based review and implications for clinical practiceMov Disord2008232267230218925578
- Pfizer IncNeurontin Prescribing Information20071 http://www.pfizer.com/files/products/uspi_neurontin.pdfAccessed February 16, 2010
- McLeanMJGabapentin in the management of convulsive disordersEpilepsia199940Suppl 6S3950 discussion S73–7410530682
- AnhutHAshmanPFeuersteinTJGabapentin (Neurontin) as add-on therapy in patients with partial seizures:a double-blind, placebo-controlled study. The International Gabapentin Study GroupEpilepsia1994357958018082624
- IVAX launches gabapentin tablets. IVAX Corp Press Release . August 18, 2004.
- ThorpMLMorrisCDBagbySPA crossover study of gabapentin in treatment of restless legs syndrome among hemodialysis patientsAm J Kidney Dis20013810410811431189
- MicozkadiogluHOzdemirFNKutASezerSSaatciUHaberalMGabapentin versus levodopa for the treatment of Restless Legs Syndrome in hemodialysis patients: an open-label studyRen Fail20042639339715462107
- Garcia-BorregueroDLarrosaOde la LlaveYVergerKMasramonXHernandezGTreatment of restless legs syndrome with gabapentin: a double-blind, cross-over studyNeurology2002591573157912451200
- HappeSKloschGSaletuBZeitlhoferJTreatment of idiopathic restless legs syndrome (RLS) with gabapentinNeurology2001571717171911706121
- HappeSSauterCKloschGSaletuBZeitlhoferJGabapentin versus ropinirole in the treatment of idiopathic restless legs syndromeNeuropsychobiology200348828614504416
- MellickGAMellickLBManagement of restless legs syndrome with gabapentin (Neurontin)Sleep1996192242268723380
- MellickLBMellickGASuccessful treatment of reflex sympathetic dystrophy with gabapentinAm J Emerg Med199513967832967
- BackonjaMBeydounAEdwardsKRGabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trialJAMA1998280183118369846777
- PollackMHMatthewsJScottELGabapentin as a potential treatment for anxiety disordersAm J Psychiatry19981559929939659873
- BockbraderHNBreslinEMUnderwoodBAMultiple-dose, dose-proportionality study of neurontin (gabapentin) in healthy volunteersEpilepsia199637Suppl 5159
- GidalBEDeCerceJBockbraderHNGabapentin bioavailability: effect of dose and frequency of administration in adult patients with epilepsyEpilepsy Res19983191999714500
- GidalBERadulovicLLKrugerSInter- and intra-subject variability in gabapentin absorption and absolute bioavailabilityEpilepsy Res20004012312710863139
- BoydRATurckDAbelRBEffects of age and gender on single-dose pharmacokinetics of gabapentinEpilepsia19994047447910219274
- Neurontin (gabapentin) capsule [package insert]. April 2009.
- StewartBHKuglerARThompsonPRA saturable transport mechanism in the intestinal absorption of gabapentin is the underlying cause of the lack of proportionality between increasing dose and drug levels in plasmaPharm Res1993102762818456077
- UchinoHKanaiYKim doKTransport of amino acid-related compounds mediated by L-type amino acid transporter 1 (LAT1): insights into the mechanisms of substrate recognitionMol Pharmacol20026172973711901210
- RiceASMatonSPostherpetic Neuralgia Study GroupGabapentin in postherpetic neuralgia: a randomised, double blind, placebo controlled studyPain20019421522411690735
- CundyKCBranchRChernov-RoganTXP13512 [(±)-1-([(α-Isobutanoyloxyethoxy)carbonyl] aminomethyl)-1-cyclohexane acetic acid], a novel gabapentin prodrug: I. Design, synthesis, enzymatic conversion to gabapentin, and transport by intestinal solute transportersJ Pharmacol Exp Ther200431131532315146028
- RichterAAntonSEKochPThe impact of reducing dose frequency on health outcomesClin Ther2003252307233514512137
- RiceASMatonSPostherpetic Neuralgia Study GroupGabapentin in postherpetic neuralgia: a randomised, double blind, placebo controlled studyPain20019421522411690735
- EnersonBEDrewesLRMolecular features, regulation and function of monocarboxylate transporters: implications for drug deliveryJ Pharm Sci2003921531154412884241
- WangHHuangWFeiYJHuman placental Na+-dependent multivitamin transporter: cloning, functional expression, gene structure and chromosomal localizationJ Biol Chem1999274148751488310329687
- CundyKCSastrySLuoWClinical pharmacokinetics of XP13512, a novel transported prodrug of gabapentinJ Clin Pharmacol2008481378138818827074
- MerlinoGSerafiniAYoungJJGabapentin enacarbil, a gabapentin prodrug for the treatment of the neurological symptoms associated with disorders such as restless legs syndromeCurr Opin Investig Drugs20091091102
- CundyKCAnnamalaiTBuLXP13512 [(±)-1-([(α-Isobutanoyloxyethoxy) carbonyl] aminomethyl)-1-cyclohexane Acetic Acid], A Novel Gabapentin Prodrug: II. Improved Oral Bioavailability, Dose Proportionality, and Colonic Absorption Compared with Gabapentin in Rats and MonkeysJ Pharmacol Exp Ther200431132433315146029
- BaillieJKPowerIThe mechanism of action of gabapentin in neuropathic painCurr Opin Investig Drugs2006713339
- TaylorCPGeeNSSuTZA summary of mechanistic hypotheses of gabapentin pharmacologyEpilepsy Res1998292332499551785
- Suman-ChauhanNWebdaleLHillDRWoodruffGNCharacterisation of [3H]gabapentin binding to a novel site in rat brain: Homogenate binding studiesEur J Pharmacol19932442933018384570
- JensenAAMosbacherJElgSThe anticonvulsant gabapentin (Neurontin) does not act through γ-aminobutyric acid-B receptorsMol Pharmacol2002611377138412021399
- LanneauCGreenAHirstWDGabapentin is not a GABAB receptor agonistNeuropharmacology20014196597511747901
- NgGYBertrandSSullivanRγ-Aminobutyric acid type B receptors with specific heterodimer composition and postsynaptic actions in hippocampal neurons are targets of anticonvulsant gabapentin actionMol Pharmacol20015914415211125035
- BertrandSNgGYPurisaiMGThe anticonvulsant, antihyperalgesic agent gabapentin is an agonist at brain γ-aminobutyric acid type B receptors negatively coupled to voltage-dependent calcium channelsJ Pharmacol Exp Ther2001298152411408520
- BertrandSNouelDMorinFGabapentin actions on Kir3 currents and N-type Ca2+ channels via GABAB receptors in hippocampal pyramidal cellsSynapse2003509510912923812
- Mixcoatl-ZecuatlTMedina-SantillanRReyes-GarciaGEffect of K+ channel modulators on the antiallodynic effect of gabapentinEur J Pharmacol200448420120814744604
- LeeC-HTsaiT-SLiouH-HGabapentin activates ROMK1 channels by a protein kinase A (PKA)-dependent mechanismBr J Pharmacol200815421622518311184
- SinghLFieldMJFerrisPThe antiepileptic agent gabapentin (Neurontin) possesses anxiolytic-like and antinociceptive actions that are reversed by D-serinePsychopharmacology (Berl)1996127198880937
- DissanayakeVUGeeNSBrownJPWoodruffGNSpermine modulation of specific [3H]-gabapentin binding to the detergent solubilized porcine cerebral cortex α2δ calcium channel subunitBr J Pharmacol19971208338409138689
- FinkKDooleyDJMederWPInhibition of neuronal Ca2+ influx by gabapentin and pregabalin in the human neocortexNeuropharmacology20024222923611804619
- ChenSREisenachJCMcCaslinPPPanHLSynergistic effect between intrathecal non-NMDA antagonist and gabapentin on allodynia induced by spinal nerve ligation in ratsAnesthesiology20009250050610691238
- HayashidaKObataHNakajimaKEisenbachJCGabapentin acts within the locus coeruleus to alleviate neuropathic painAnesthesiology20081091077108419034104
- TaylorCPLevyRHMattsonRHMeldrumBSPeruccaEGabapentin. Mechanism of actionAntiepileptic Drugs5th edPhiladelphiaLippincott Williams & Wilkins2002321334
- GeeNSBrownJPDissanayakeVUThe novel anticonvulsant drug, gabapentin (Neurontin), binds to the α2δ subunit of a calcium channelJ Biol Chem1996271576857768621444
- HendrichJVan MinhATHeblichFPharmacological disruption of calcium channel trafficking by the α2δ ligand gabapentinPNAS20081053628363318299583
- LalRSukbuntherngJLuoWPharmacokinetics and tolerability of single escalating doses of gabapentin enacarbil: a randomized-sequence, double-blind, placebo-controlled crossover study in healthy volunteersClin Ther2009311776178619808136
- CanafaxDMMoorsTLCundyKCSingle- and multi-dose phase I studies of XP13512, a transported prodrug of gabapentin, demonstrate safety, tolerability and dose-proportional gabapentin pharmacokineticsInt Conf Mech Treatment Neuropathic Pain20041146
- CundyKSastryCKLuoWClinical pharmacokinetics of XP13512: A novel transported prodrug of gabapentinNeurology200870Suppl 1A293
- CundyKSastryCKLuoWClinical pharmacokinetics of XP13512: A summary of four healthy volunteer studiesSleep200831Suppl A268Abs 818
- FennerLJCanafaxDMMoorsTLA phase 1 randomized, cross over, single dose study of the safety, tolerability, and pharmacokinetics of XP13512 sustained released tablets vs Neurontin in healthy adult subjectsInt Conf Mech Treatment Neuropathic Pain20041146
- LalRSukbuntherngJLuoWClinical pharmacokinetic drug-interaction studies of XP13512, a novel transported prodrug of gabapentin, with naproxen and cimetidine [abstract]Neurology20087011Suppl 1A295
- LalRSukbuntherngJLuoWClinical pharmacokinetics of gabapentin after oral administration of XP13512/GSK1838262 tablets and naproxen in healthy adultsJ Clin Pharmacol20084891124
- KushidaCBeckerPPerkinsTXP13512 improves symptoms and sleep disturbance in RLS patients: Results of a 2-week, randomized, double blind, placebo controlled cross-over polysomnographic trialSleep200629Suppl SA278
- KushidaCAWaltersASBeckerPA randomized, double-blind, placebo-controlled, crossover study of XP13512/GSK1838262 in the treatment of patients with primary restless legs syndromeSleep20093215916819238802
- EllenbogenALKushidaCABeckerPMXP13512/GSK1838262 1200 mg provides symptomatic relief in restless legs syndrome patients: A randomized, double-blind, placebo-controlled studyMov Disord2008231 Suppl S364Abs 1113
- BeckerPKushidaCEllenbogenAXP13512 reduces restless legs syndrome symptoms and associated sleep impairment: Results of double blind, randomized, placebo-controlled studySleep200831Suppl A268Abs 817
- Xenoport and GlaxoSmithKline report positive top-line results of second phase III restless legs syndrome trial for XP13512/GSK1838262. XenoPort Inc. Press Release, Jan 15, 2008.
- XenoPort and GlaxoSmithKline report positive top-line results of final pivotal trail of XP13512/GSK1838262 for restless legs syndrome. XenoPort Inc. Press Release, Feb 28, 2008
- www.xenoport.com.