Abstract
Treatment-resistant depression (TRD) is a common occurrence in clinical practice. Up to 30% of patients with major depression do not respond to conventional antidepressant treatment, while a significantly greater number of patients experience only partial symptom reduction. Numerous strategies may be applied by the practicing clinician to overcome limitations in the effectiveness of antidepressant monotherapy, including combining drug treatment with evidence-supported psychotherapies, combining antidepressants (combination pharmacotherapy), and combining antidepressants with other non-antidepressant psychotropic medications (augmentation treatment). One such augmentation strategy, the combination of the selective serotonin reuptake inhibitor, fluoxetine (FLX), with the atypical antipsychotic drug, olanzapine (OLZ), is supported by the results of four randomized, double-blind, acute phase studies of patients who had responded inadequately to antidepressant monotherapy. In each study, the FLX/OLZ combination caused rapid reduction in Montgomery-Asberg Depression Rating scale scores, with two of the four studies showing significantly greater improvement than antidepressant monotherapy at study endpoint. Effects of the FLX/OLZ combination were strongest in cases where failure to respond to two antidepressants prior to randomization was established during the current depressive episode. The FLX/OLZ combination was well-tolerated; however, body weight gain and increases in prolactin were greater than that of the antidepressant monotherapy groups, and were comparable to that of OLZ monotherapy. While effective during acute-phase treatment, questions remain regarding the long-term efficacy and safety of FLX/OLZ relative to antidepressant monotherapy and other combination strategies. Efforts aimed at determining the placement of FLX/OLZ among the available options for addressing TRD are limited by lack of comparison and sequential treatment studies. Important aspects of study design and directions for future research are discussed.
Introduction
In the last two decades, there has been a marked increase in the number of antidepressant medications available for the treatment of major depressive disorder (MDD). In spite of this, it has become increasingly evident that the effectiveness of antidepressant monotherapy for MDD is much more modest than what was once believed. For example, a recent meta-analysis of all published double-blind placebo controlled antidepressant trials reported an antidepressant response rate of 53%.Citation1 Although this rate of response was significantly greater than the placebo response rate (36%, P < 0.05), these results have challenged the widely accepted anticipated response rates of 60% to 70% reported in prior reviews of controlled studies.Citation2
Rates of acute and sustained symptom remission on antidepressant monotherapy are even lower, both under ideal treatment conditions that characterize randomized controlled trials, and in actual clinical treatment settings.Citation3–Citation5 For instance, only about 30% of patients achieved clinical remission in the first phase of the landmark Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study after 12 weeks of citalopram monotherapy.Citation6 Only about 70% of patients achieved remission after multiple rounds of medications or cognitive therapy, and the majority of patients relapsed within the first year following remission.Citation7 The importance of this point rests in the fact that the persistence of residual depressive symptoms is associated with substantial disability and increased risk of a full depressive relapse, even among patients who achieve a positive antidepressant response and remain in active treatment.Citation8,Citation9 As such, the majority of antidepressant-treated patients with major depression either fail to improve meaningfully or experience positive but only partial improvement. Clearly, there is an urgent need to develop safe and more effective treatments for major depressive disorder (MDD).
Until such treatments become available, numerous strategies may be applied by the practicing clinician to overcome limitations in the effectiveness of antidepressant monotherapy. In broad terms, these include combining drug treatment with evidence-supported psychotherapies,Citation10 combining antidepressants with different pharmacological profiles (combination pharmacotherapy), and combining antidepressants with psychotropic medications that are not antidepressants (augmentation treatment).Citation11,Citation12 One such augmentation treatment approach is the combination of antidepressants with atypical antipsychotic drugs. There is now controlled evidence supporting the short-term effectiveness of atypical antipsychotic augmentation of antidepressants, including cases of difficult to treat MDD with and without psychotic features.Citation13–Citation15 In addition, one atypical antipsychotic drug, aripiprazole, has a US Food and Drug Administration (FDA) indication as an adjunctive therapy for suboptimal antidepressant response in patients with MDD,Citation16 and one selective serotonin reuptake inhibitor (SSRI)/atypical antipsychotic combination (fluoxetine + olanzapine) is FDA approved for acute bipolar depression.Citation17 Enthusiasm for this approach is tempered by a lack of long-term effectiveness data, as well as long-term metabolic safety and tolerability concerns for many of these agents. This review is focused on the therapeutic rationale for and clinical evidence supporting the combination of fluoxetine and olanzapine for treatment-resistant MDD (TRD). Characteristics and implications of key design issues in each of the reviewed studies will be emphasized.
Mechanisms of action
Neurotransmitter dysfunction in major depression
A substantial body of evidence indicates that dysfunction in serotonin (5-hydroxytryptamine, 5-HT), norepinephrine (NE) and, to a lesser degree, dopamine (DA) neurotransmission are involved in the pathophysiology of major depression,Citation18–Citation20 and that intact 5-HT and NE neurotransmitter systems are needed in order to maintain positive clinical response to antidepressant medications. By now, it is also apparent that early theories that sought to explain the signs and symptoms of major depression solely on the basis of 5-HT and NE depletion have not provided a unifying neurobiological theory explaining why major depression occurs or how antidepressants exert their therapeutic effects. Nevertheless, the monoaminergic neurotransmitter systems have served as the most important pharmacological targets from antidepressant drug development over the last four decades.Citation21 Nearly all currently available antidepressant drugs act as potentiators of monoamine neurotransmission, either by inhibition of enzymes needed for monoamine degradation, or by blocking monoamine reuptake sites.
In recent years, a more sophisticated understanding of the pathophysiology of major depression has emerged that focuses on dysregulation of 5-HT and NE transmission rather than monoamine depletion, per se. Abnormalities in the functional activity of numerous brain regions have been identified in depressed patients using functional neuroimaging, including cortical and limbic structures that are critical for regulation of mood, emotional processing, cognitive and psychomotor functioning, and motivation,Citation22–Citation24 all common symptoms of depression. Importantly, each of these regions receive serotonergic, noradrenergic and/or dopaminergic projections,Citation25 and abnormalities in several of these regions identified on neuroimaging have been shown to resolve following treatment with antidepressants that potentiate 5-HT and/or NE neurotransmission.Citation26–Citation29
Limitations of fluoxetine monotherapy
Monoamine neurotransmission in these key brain regions is tightly regulated, in part, by functional activity of several critical postsynaptic neuroreceptors that are not acted upon directly by SSRIs and other first-line antidepressants. Fluoxetine (FLX) and other SSRIs occupy 5-HT reuptake pumps with relatively high affinity and specificity; however, their pharmacological activity is limited, for all practical purposes, to reuptake pump blockade.Citation30,Citation31 This poses three potential limitations that may result in a lack of meaningful clinical response to monotherapy with FLX for some patients. First, FLX does not act as a pharmacological agonist and cannot directly activate postsynaptic receptors that are important for the control of monoamine functioning in the central nervous system (CNS). Instead, FLX is dependent almost entirely on intact presynaptic serotonergic functioning.Citation32 Second, FLX has only negligible effects on NE and DA reuptake, and does not directly enhance noradrenergic or dopaminergic neurotransmission.Citation30 Furthermore, continuous SSRI treatment is associated with suppression of locus coeruleus firing,Citation33–Citation35 resulting in decreased NE transmission. This is believed to occur as a consequence of enhanced activation of excitatory postsynaptic 5-HT2A receptors located on inhibitory GABAergic interneurons that synapse with NE neurons.Citation36 Third, SSRIs have been shown to suppress ventral tegmental area activity, and therefore reduce DA neurotransmission,Citation37,Citation38 possibly by a 5-HT2C dependent mechanism.Citation39–Citation41 Thus, FLX and other SSRIs may not be able to fully optimize central monoaminergic functioning as stand-alone therapies. These limitations cannot be overcome by increases in medication dosage alone.
Augmentation with atypical antipsychotics: focus on olanzapine
The atypical antipsychotics are a pharmacologically heterogenous group of drugs. Although each is associated with a unique profile of neuroreceptor binding activity, all share the properties of high-potency postsynaptic 5-HT2A antagonism with relatively lower potency dopamine D2 receptor antagonism (or D2 partial agonist effects, in the case of aripiprazole). Importantly, the atypical antipsychotic drug, olanzapine (OLZ), is pharmacologically active at other neuroreceptors that are important for optimizing central monoamine functioning (). As such, adjunctive therapy with OLZ may be a practical means of overcoming many of the mechanistic limitations of FLX monotherapy, and improving clinical response to FLX in patients with treatment resistant major depression.
Table 1 Selected serotonergic neuroreceptor targets of olanzapine with potential relevance for antidepressant augmentation in treatment-resistant depressionCitation13,Citation42,Citation45–Citation47
For example, 5-HT2A blockade results in numerous effects that are relevant to the activity of FLX. In the presence of 5-HT reuptake inhibition, 5-HT2A receptor antagonism has been shown to enhance 5-HT and NE release in rodents.Citation42 In preclinical models, chronic administration of FLX alone suppressed locus coeruleus neuronal activity while acutely administered OLZ significantly increased locus coeruleus firing.Citation43 The combination of FLX + OLZ, however, resulted in enhanced locus coeruleus firing during both acute and chronic administration.Citation43 Increased NE release may be predicted by antagonism of the aforementioned 5-HT2A dependent reduction in NE neuronal firing, while increased NE release may then enhance 5-HT neuronal firing via activation of postsynaptic α-1 adrenergic receptors in the raphe nuclei.Citation42 In addition, OLZ-associated postsynaptic 5-HT2C antagonism and 5-HT1A activation have also been shown to increase NE release in the prefrontal cortex.Citation44 Thus, the combination of FLX + OLZ may result in a greater degree of serotonergic activity than would be expected with FLX alone, and may also reverse FLX-associated NE suppression.
Interactions at serotonin receptors also have important effects on DA neurotransmission. Blockade of 5-HT2A and 5-HT6 receptors, combined with weak D2 receptor antagonism, have both been shown to enhance DA release to a greater degree than blockade of either receptor alone.Citation45,Citation46 In addition to increasing NE efflux, OLZ effects on 5-HT2C and 5-HT1A receptors have been shown to increase DA release in the frontal cortex and nucleus accumbens,Citation44,Citation47 brain regions that are tied to cognitive functioning and motivational drive.Citation48 Clinically, the 5-HT2A antagonists, trazodone and nefazodone, and the 5-HT1A partial agonist, gepirone, have all shown antidepressant effects in clinical trials.Citation49–Citation51 Augmentation of FLX with OLZ may therefore provide a means of enhancing DA activity in brain regions implicated in the pathophysiology of major depression that would not be possible with FLX alone, an effect that may be strong enough to overcome FLX-associated suppression of central DA activity and improve antidepressant response.
Finally, there has been considerable interest in the role of brain derived neurotrophic factor (BDNF), one of a number of neurotrophic factors involved in neuronal maintenance and survival,Citation52 in the both pathogenesis of major depression and the clinical response to antidepressants.Citation53 BDNF has been shown to play an important role in the susceptibility of depression-prone patients to the negative effects of stress.Citation54 In post-mortem brains of depressed patients, low levels of hippocampal and prefrontal BDNF have been documented.Citation53 Abnormally low levels of serum BDNF have also been found in patients with major depression.Citation55 It is noteworthy that long-term exposure to FLX and other antidepressants has been shown to increase BDNF production,Citation56 and that stress-induced reduction in levels of BDNF and Bcl-2, another neurotrophic factor, may be blocked with long-term treatment with OLZ.Citation57 These results suggest that the combination of FLX + OLZ may have synergistic effects on neurotrophin activity, thereby providing an alternative mechanism by which OLZ may enhance the clinical antidepressive effects of FLX.
Efficacy in clinical trials
Results of acute phase studies in TRD
Five randomized, controlled trials have investigated the effect of OLZ augmentation of FLX in acute phase TRD, two of which were publised in a single paper by Thase et al.Citation64 All studies were generally similar in design (). Patients with non-psychotic major depression and a prior history of antidepressant failure (variously defined, as discussed below) received an open-label trial of antidepressant monotherapy during a pre-randomization (lead-in) phase. Individuals who failed to achieve a pre-specified threshold of symptom response during this open-label treatment phase were then eligible for randomization to double-blind acute phase treatment with OLZ + FLX or one of several active control conditions, including continuation treatment with the lead-in phase antidepressant. The design features and key results of the pre-randomization and randomized acute treatment phases of each study are summarized in and , respectively.
Figure 1 Basic design features of acute phase studies of combined fluoxetine and olanzapine (FLX/OLZ) in treatment resistant depression. Citation58,Citation62–Citation64
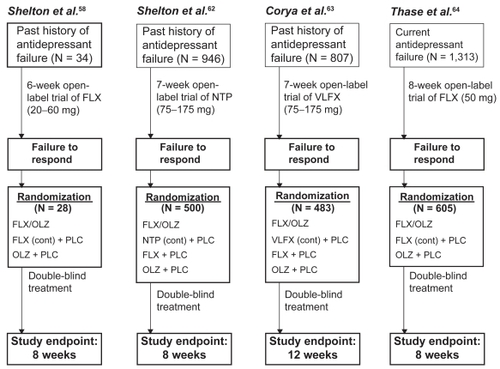
Table 2 Definition of treatment-resistant depression and characteristics of lead-in phase
Table 3 Characteristics of studies of combined fluoxetine and olanzapine in treatment-resistant major depression
The first randomized, controlled study of atypical antipsychotic drug augmentation of antidepressants for TRD compared the effects of FLX + OLZ, FLX monotherapy (FLX + placebo) and OLZ monotherapy (OLZ + placebo).Citation58 There is a paucity of long-term efficacy data for the use of FLX/OLZ combination therapy for TRD. In one single-arm study,Citation67 a mixed sample of 560 patients with TRD (N =145, defined as having history of past failure to respond to at least two trials of antidepressant treatment using agents of different pharmacological classes) and non-treatment refractory major depression (non-TRD, N =407) received an open-label trial. Treatment resistance was confirmed by failure to achieve a partial response to open-label FLX (up to 60 mg/day) during the pre-randomization lead-in phase. Twenty-eight non-responders were then randomly assigned in a double-blind fashion to one of the three acute phase treatment conditions (see for mean doses). Continuation of FLX monotherapy yielded no further improvement in depressive symptoms as measured by the Montgomery-Asberg Depression Rating Scale (MADRS),Citation59 the Hamilton Depression Rating Scale (HAM-D)Citation60 and the Clinical Global Impressions-Depression subscale (CGI-D),Citation61 while OLZ monotherapy achieved a modest benefit over FLX alone. The FLX/OLZ combination resulted in significantly greater improvement in depressive symptoms than FLX or OLZ alone (). The proportion of patients who achieved positive clinical response (≥50% improvement in MADRS scores at endpoint) was greater for the FLX/OLZ group (60%) compared with OLZ (0%) and FLX (10%) monotherapy groups. Pairwise comparisons of the proportion of those achieving positive clinical response were significant only for FLX/OLZ vs OLZ monotherapy, favoring combination therapy.
Four larger-scale randomized, double-blind multi-center studies investigating the effects of FLX/OLZ combination therapy were then completed.Citation62–Citation64 In the first study,Citation62 500 non-psychotically depressed patients with a prior history of failure to respond to a ≥4 week trial of SSRI treatment and prospective failure of open-label NTP () were randomized to one of four treatment groups for the 8-week acute phase study: FLX + OLZ, FLX monotherapy (FLX + placebo), OLZ monotherapy (OLZ + placebo), or continuation treatment with NTP (+ placebo) at the same dosage used during the pre-randomization lead-in phase (). The OLZ/FLX combination produced rapid antidepressant effect and statistically superior improvement in MADRS scores compared with all monotherapy groups through the first four weeks of treatment; however, there were no significant differences between these groups at study endpoint. There were also no significant differences between the FLX + OLZ, FLX monotherapy, OLZ monotherapy, or NTP monotherapy groups in Hamilton Anxiety Scale (HAM-A)Citation65 or Clinical Global Impressions-Illness Severity subscale scores,Citation61 or rates of categorical treatment response (27.5%, 28.9%, 19.3%, and 30.3%, respectively) or remission (16.9%, 13.3%, 12.9%, and 18.2%, respectively), at study endpoint.
The second large-scale study compared the clinical effects of OLZ/FLX combination,Citation63 FLX monotherapy, OLZ monotherapy, and VLFX monotherapy over 12 weeks in a cohort of non-psychotically depressed patients (N = 483) with a past history of failure to respond to a ≥6 week therapeutic SSRI trial and prospective failure of an open-label lead-in trial of VLFX (). Again, there was significant early improvement associated with OLZ/FLX treatment, and significantly greater improvement in MADRS scores for OLZ/FLX over VLFX monotherapy (first 6 weeks only) and FLX monotherapy (first 11 weeks only). At study endpoint, there was no evidence of a FLX/OLZ advantage over any antidepressant monotherapy group (). This included no significant differences between FLX + OLZ, FLX monotherapy, OLZ monotherapy, and VLFX monotherapy groups in rates of categorical treatment response (43.3%, 33.9%, 25.4%, and 50.0%, respectively) or remission (29.9%, 17.9%, 13.6%, and 22.4%, respectively) at study endpoint.
The final two studies were of identical design and were run concurrently (Study 1 and Study 2). Results were reported separately and as pooled data.Citation64 Both studies were randomized, double-blind 8-week comparisons of FLX/OLZ combination therapy, FLX monotherapy (FLX + placebo) and OLZ monotherapy (OLZ + placebo) in a cohort of patients with non-psychotic major depression (N = 605, pooled data set). Eligibility was based on a past history of SSRI failure during the current depressive episode only, followed by prospective failure of an 8-week open-label FLX (50 mg/day) lead-in trial. In Study 1, there was no evidence of a superior antidepressive effect for FLX/OLZ combination treatment over either monotherapy group. However, in Study 2, the FLX/OLZ combination resulted in significantly greater improvement in MADRS scores, and in categorical response and remission rates, compared with both monotherapy groups at study endpoint. The pooled analysis of both projects revealed significantly greater improvement in MADRS scores, higher rates of categorical response (FLX + OLZ, 40.4%; FLX, 29.6%; OLZ, 25.9%) and remission (FLX + OLZ, 27.3%; FLX 16.7%; OLZ 14.7%), and shorter time required for 25% of patients to achieve therapeutic responder status (FLX + OLZ, 30 days; FLX, 55 days; OLZ, 53 days), compared with both monotherapy groups (). There were also advantages for FLX/OLZ over both antidepressant monotherapies in family and leisure functioning as measured by the Sheehan Disability Scale.Citation66 There was no statistically significant advantage observed for work functioning.
Results of long-term studies
There is a paucity of long-term efficacy data for the use of FLX/OLZ combination therapy for TRD. In one single-arm study,Citation67 a mixed sample of 560 patients with TRD (N =145, defined as having history of past failure to respond to at least two trials of antidepressant treatment using agents of different pharmacological classes) and non-treatment refractory major depression (non-TRD, N =407) received an open-label trial FLX/OLZ (mean modal dose = 46.1 ± 20.7 mg/day of FLX/7.5 ± 3.5 mg/day of OLZ) for up to 76 weeks. There was a significant reduction in MADRS and CGI-S subscale scores as early as 0.5 weeks, followed by strong and continuous reductions in these measures througout the remainder of the study in both TRD and non-TRD patients. At study endpoint, 61.6% of patients met categorical response criteria (≥50% reduction in MADRS scores), and 56.3% of patients achieved remission (MADRS scores of ≤8 on two consecutive visits. Higher remission rates (60.7% vs 44.1%) and shorter time to remission were observed in the non-TRD compared with TRD sample. These results were tempered by relatively high droput rates. Only 177 (31.6%) of patients completed 52 weeks of treatment, and only 143 (25.5%) of patients completed the 76-week trial. In addition, of the patients who achieved remission, 12.1% of non-TRD and 25.0% of TRD patients relapsed (defined as a MADRS score of ≥16 at any two visits following remission). More long-term studies are needed.
Tolerability and safety
Safety and tolerability results across each of the acute-phase studies are summarized in . Rates of discontinuation due to treatment-emergent adverse effects (TEAEs) were greatest for FLX/OLZ- and OLZ monotherapy-treated patients. Because those who did not tolerate lead-in antidepressant medication were dropped prior to randomization, discontinuation rates tended to be lowest for drugs that were utilized during the lead-in phase and were continued during the double-blind treatment period.
Table 4 Treatment-emergent adverse effects
Increases in body weight, total non-fasting cholesterol and serum prolactin (PRL) levels were greatest for the FLX/OLZ and OLZ groups. There was little change from baseline in any of these measures for the antidepressant monotherapy groups during double-blind treatment. The magnitude of increases in these measures between FLX/ OLZ and OLZ groups was generally similar. However, increases in total cholesterol were significantly greater for FLX + OLZ vs OLZ monotherapy in a pooled analysis of data from the 5 acute phase studies reviewed above.Citation76 There were no significant differences for change in triglyceride levels between these two groups in the Thase et alCitation64 study. There were no significant between groups differences in baseline to endpoint change in non-fasting glucose in the studies by Shelton et alCitation62 and Thase et al;Citation64 however, in both studies, the numerical increases in non-fasting glucose levels were greatest in the FLX/OLZ and OLZ groups relative to the antidepressant monotherapy groups. Similar increases in weight (+ 5.6 ± 6.6 kg) and non-fasting glucose levels were reported in the long-term open-label study by Corya et alCitation67
Extrapyramidal side effects (EPS) were measured using the Simpson-Angus Scale (SAS),Citation68 Barnes Akathisia Scale (BARS),Citation69 and the Abnormal Involuntary Movement Scale (AIMS)Citation70 in each of the reviewed studies. There were no significant between groups differences in EPS measures in the Shelton et al Citation62 study. There were no significant changes in these measures from baseline in any of the treatment groups in the Corya et alCitation63 study, or in the FLX/OLZ group in the Thase et alCitation64 study. EPS measures for the FLX/OLZ group were reported as being similar to that of the OLZ group. In the long-term open-label study by Corya et alCitation67 there were no significant increases in SAS, BARS or AIMS scores between baseline and the 76-week study endpoint. In the only study that assessed PRL response, FLX/OLZ was associated with significantly greater increases in PRL concentration than FLX, OLZ or NTP monotherapy.Citation62
Discussion
Clinical issues
TRD is a common occurrence in clinical practice, and poses numerous challenges for the practicing clinician. While atypical antipsychotic augmentation of antidepressant medication was shown to be an effective strategy for patients with TRD in a recent meta-analysis of 10 randomized, double-blind, placebo controlled trials,Citation14 their exact place within the mélange of therapeutic options for managing TRD has not been precisely defined. For this to be determined, data from sequential treatment studies and/or head-to-head comparisons would be needed. The largest sequential investigation of therapeutic options in TRD to date, the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study, did not investigate the effects of atypical antipsychotic augmentation. Data from head-to-head comparison trials between FLX/OLZ and other interventions for TRD are not yet available. Nevertheless, based on clinical trial results that are currently available, the strongest clinical evidence supporting a role for atypical antipsychotic drugs as augmenters of antidepressants in TRD exists for olanazapine, quetiapine and aripiprazole, at least for short-term, acute phase treatment.Citation13,Citation14,Citation71–Citation74 Of these, only aripiprazole is currently approved for this indication.
Our review of the literature, which focused on the effects of OLZ augmentation of FLX in TRD, also supports the short-term efficacy of this strategy, is consistent with a prior narrative review of FLZ + OLZ in TRD.Citation75 Effectiveness of this combination appears to be particularly strong for those with an inadequate SSRI response and treatment resistance in the current depressive episode. Focusing on this sub-population of patients has at least two advantages. First, the decision to augment antidepressant therapy with OLZ in actual clinical practice would be based on poor treatment response to antidepressant monotherapy in the current depressive episode, rather than having a history of poor antidepressant response in prior depressive episodes. Next, the consideration of only those subjects who met treatment failure criteria during the current depressive episode would guard against the inclusion of less treatment resistant cases in studies that focus specifically on intervention effects in TRD. This would result in a sample that is more representative of the TRD population encountered in clinical practice. Patients with TRD, defined in this more stringent manner, would also have a greater likelihood of achieving a more favorable clinical response to antidepressant augmentation compared with monotherapy. This position is supported by results of Shelton et al,Citation58 Thase et alCitation64 and the pooled analysis by Trivedi et alCitation76 which focused on patients with TRD based on the current depressive episode only. All showed stronger effects of FLX/OLZ augmentation relative to antidepressant monotherapy than did individual large-scale studies that used a less stringent definition of TRD.Citation62,Citation63
Available evidence of effectiveness FLX + OLZ in TRD is similar to the published data describing the combination’s effectiveness for the short-term acute-phase treatment of depression in patients with bipolar I disorder.Citation77,Citation78 In two randomized, controlled studies, FLX + OLZ resulted in greater 8-week improvement in depressive symptomatology compared with placebo and OLZ monotherapy,Citation79 and greater 7-week improvement than lamotrigine.Citation78 In addition, combination therapy with FLX + OLZ was associated with higher remission rates than placebo or OLZ monotherapy,Citation77 and a similar remission rate as lamotrigine.Citation78 In one small placebo-controlled study comparing the effects of FLX + OLZ, OLZ monotherapy, FLX monotherapy and placebo in depressed patients with bipolar I or II disorder, there were significant reductions in depressive symptoms for the entire cohort; however, the study was under-powered to detect differences between treatment groups.Citation80 In published studies of bipolar depressed patients, FLX + OLZ was generally well tolerated, with limited safety data suggesting that FLX + OLZ combination therapy was associated with weight gain and lipid and prolactin level elevation similar to that of OLZ monotherapy. As is the case with TRD, long-term safety and effectiveness data in bipolar patients are generally lacking. FLX + OLZ combination therapy was associated with significantly greater improvement in depressive symptoms than lamotrigine in a 25-week double blind extension of the 7-week acute phase study by Brown et al.Citation78,Citation79 However, FLZ + OLZ was associated with more frequent weight gain and significantly higher incidence of clinically significant hypercholesterolemia (≥240 mg/dL) and weight gain (≥7% increase in weight from baseline) at the 25 week endpoint.Citation79 Overall, the state of the available evidence for FLX + OLZ therapy in bipolar depression is similar to that of the combination in TRD, with encouraging results shown in short-term acute phase studies, but limited longer-term data.
Enthusiasm is thus tempered by several unanswered questions regarding the use of FLX/OLZ augmentation in TRD. The optimal augmenting dose of OLZ has not been established, though anecdotal reports and uncontrolled evidence suggest that the target dose may be lower (2.5 to 10 mg) than those used in the treatment of schizophrenia or bipolar disorder.Citation81,Citation82 The long-term efficacy and safety profile of FLX/OLZ in TRD has also not been established. There have been no controlled investigations longer than 12 weeks to date. As such, there are also no studies focused on the question of how long OLZ augmentation of FLX is required among patients who respond acutely to the combination.
The paucity of long-term studies is also of vital clinical interest because of concerns about body weight gain and dysmetabolic effects that are associated with OLZ. Of the antipsychotic drugs, OLZ is among the most orexigenic and, with the exception of clozapine, is associated with the greatest short-term weight gain and longer-term risk of hyperglycemia, diabetes, and atherogenic dyslipidemia.Citation83–Citation85 These metabolic risks must be carefully considered when deciding among the varied options available for augmentation of antidepressants in TRD. Apart from the obvious long-term health concerns, individuals with MDD are in physically poorer health than the general populationCitation86 and therefore comprise a patient sub-population that is already at risk for profound cardiovascular morbidity and early mortality. Indeed, major depression has been identified as an independent risk factor for coronary artery disease and premature death due to cardiovascular causes.Citation87–Citation89 As is the case with schizophrenia and bipolar disorder, psychiatric illnesses that are associated with considerable physical health burden and early mortality due to cardiovascular disease,Citation90,Citation91 use of OLZ for patients with major depression requires close metabolic monitoring and follow-up. The stringency of such monitoring should generally be the same that used for OLZ treatment of bipolar disorder and schizophrenia, even if lower doses of OLZ are shown to be effective for antidepressant augmentation.
Characteristics and implications of study design and execution
The definition of TRD is a matter of ongoing debate, and numerous and varying criteria have been used to defined TRD samples in clinical trials.Citation92 To improve uniformity, several definitional models have been proposed, a detailed review of which will not be provided here. The interested reader is referred to several excellent reviews.Citation93–Citation95 For purposes of the current discussion, some design features and issues in study execution, many of which focus on sample selection and study definitions of TRD, may have led to the inclusion of less-treatment resistant depressed patients. This could have introduced bias toward null findings in some of the reviewed studies.
For example, two of the reviewed studies based their definition of TRD, in part, on a history of failure not confined to the current depressive episode.Citation62,Citation63 Only the studies by Shelton et alCitation58 and Thase et alCitation64 randomized patients that had both a retrospective and prospective lead-in antidepressant failure in the current depressive episode. Only the Thase et alCitation64 group randomized patients that had both a retrospective and prospective lead-in antidepressant failure in the current depressive episode only. Planned sub-group analyses of subjects in the Shelton et alCitation62 and Corya et alCitation63 studies who met both retrospective and prospective TRD inclusion criteria for the current depressive episode revealed a more marked treatment effect for FLX/OLZ. In the Corya et alCitation63 study, improvement in MADRS at study endpoint was significantly greater for FLX/OLZ compared with FLX alone in this patient subgroup. A pooled analysis of data from all of the above reviewed acute-phase studies was performed by Trivedi et alCitation76 which focused on only those subjects with a history of antidepressant non-response in the current depressive episode (N = 1146). FLX/OLZ was associated with significantly greater 8-week improvement in MADRS scores (–13.0 vs –8.6 vs –8.2) and higher rates of remission (25.5% vs 17.3% vs 14.0%) compared with FLX or OLZ monotherapy.Citation76
Second, although a 4- to 6-week time interval for lead-in phase treatment could be considered generally adequate, many patients may require longer than this to adequately respond to antidepressant monotherapy,Citation96 a situation that may be even more problematic for drugs that require slower or more prolonged titration to therapeutic doses. These factors may have been particularly relevant for the Shelton et al Citation62 and Corya et alCitation63 studies, both of which utilized antidepressant medications during the lead-in phase that required titration to therapeutic doses (NTP and VLFX, respectively). Assuming that dosage adjustments were made during the first week even after achieving initial target doses, this would have left approximately 6 weeks of lead-in antidepressant treatment at adequate dosage. This time interval would not be sufficient to exclude late responders who, by definition, are not treatment-resistant but would have still been included in the double-blind acute treatment phase of these studies.
Third, investigators in the Shelton et al Citation62 and Corya et al Citation63 studies were not blinded to the double-blind acute phase entry criteria (reduction of ≥30% in MADRS scores) which, as pointed out by the study authors, may have led to unconscious bias toward continued treatment and randomization via an under-rating of MADRS scores at the end of the lead-in phase. The lower than expected rates of partial treatment response at the end of the lead-in phase of both of these studies (18% and 26%, respectively) support this hypothesis; however, response rates for individuals with treatment resistant depression would also be expected to be low. In the study by Thase et alCitation64 investigators were blinded to the double-blind acute phase study entry criteria. As such, the potential limitations posed by lack of blinding to acute phase study criteria do not apply to this study.
Fourth, in each of the reviewed studies, some patients who were eligible for double-blind acute phase treatment were re-randomized to the same antidepressant medication that they received during the lead-in period. These individuals would have received up to 15 weeks of NTP treatment in the Shelton et alCitation62 study, up to 19 weeks of VLFX treatment in the Corya et alCitation63 study, and up to 16 weeks of FLX treatment in the Thase et alCitation64 study, thus causing an imbalance in study design and possible bias in favor of NTP, VLFX, or FLX, respectively. Finally, none of the reviewed studies included a placebo, as it was deemed unethical to deny active treatment to patients with refractory depression. Lack of a placebo group, however, makes it difficult to rule out non-pharmacological causes of clinical improvement across groups.
Summary and conclusions
TRD represents a clinical challenge for which more effective treatments are urgently needed. The combination of FLX and OLZ has shown considerable promise in short-term, acute phase studies; however, little is known about the relative effectiveness of FLX/OLZ augmentation compared with other augmenting or combination strategies for TRD. Several questions remain concerning the optimal dosing and required duration of OLZ in combination with FLX for patients with TRD, and little is known about the long-term efficacy, tolerability and safety of this combination. This includes lack of data regarding long-term effects of FLX/OLZ on surrogate markers of cardiovascular risk (weight gain, dysglycemia, atherogenic dyslipidemia) and important clinical endpoints (eg, development of diabetes and serious cardiovascular disease). Future studies of comparative effectiveness of FLX/OLZ augmentation and other forms of combination therapy for TRD are needed in order to best determine its place among existing treatment options, either in the form of head-to-head comparisons with other TRD treatment options or as a therapeutic option in sequential treatment studies of patients with major depression.
Information sources
PubMed, PsycINFO, Ovid MEDLINE, clinicaltrials.gov.
Disclosures
Dr Bobo has received grant/research support from Cephalon and has served on the speaker’s bureau in the past for Pfizer and Janssen Pharmaceutica.
Dr Shelton has received grant/research support from Eli Lilly and Company, GlaxoSmithKline, Janssen Pharmaceutica, Pfizer, Inc., Sanofi-Aventis, Wyeth, Inc., AstraZeneca Pharmaceuticals, Forest Pharmaceuticals, Otsuka America, Inc., Pamlab, Inc., and Abbott Laboratories; has served as a paid consultant for Pfizer, Inc., Janssen Pharmaceutica, Eli Lilly and Co., Forest Pharmaceuticals, Otsuka America, Inc., Pamlab Inc., and Sierra Neuropharmaceuticals; and has served on the speaker’s bureau in the past for Bristol-Myers Squibb, Eli Lilly and Company, Janssen Pharmaceutica, Pfizer, Inc., GlaxoSmithKline, Wyeth, Inc., and Pamlab, Inc.
References
- PapakostasGIFavaMDoes the probability of receiving placebo influence clinical trial outcome? A meta-regression of double-blind, randomized clinical trials in MDDEur Neuropsychopharmacol2009191344018823760
- SheltonRCTreatment options for refractory depressionJ Clin Psychiatry199960Suppl 4576110086483
- PetersenTPapakostasGIPosternakMAEmpirical testing of two models for staging antidepressant treatment resistanceJ Clin Psychopharmacol2005825433634116012276
- MachadoMIskedjianMRuizIEinarsonTRRemission, dropouts, and adverse drug reaction rates in major depressive disorder: a meta-analysis of head-to-head trialsCurr Med Res Opin20062291825183716968586
- MollerHJOutcomes in major depressive disorder: the evolving concept of remission and its implications for treatmentWorld J Biol Psychiatry20089210211418428079
- TrivediMHRushAJWisniewskiSREvaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practiceAm J Psychiatry20061631284016390886
- RushAJTrivediMHWisniewskiSRAcute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D reportAm J Psychiatry2006163111905191717074942
- GredenJFThe burden of disease for treatment-resistant depressionJ Clin Psychiatry200162Suppl 16263111480881
- KellerMBBolandRJImplications of failing to achieve successful long-term maintenance treatment of recurrent unipolar major depressionBiol Psychiatry19984453483609755357
- ThaseMEFriedmanESBiggsMMCognitive therapy versus medication in augmentation and switch strategies as second-step treatments: a STAR*D reportAm J Psychiatry2007164573975217475733
- ThaseMERushAJWhen at first you don’t succeed: sequential strategies for antidepressant nonrespondersJ Clin Psychiatry199758Suppl 1323299402916
- TrivediMHFavaMWisniewskiSRMedication augmentation after the failure of SSRIs for depressionN Engl J Med2006354121243125216554526
- SheltonRCPapakostasGIAugmentation of antidepressants with atypical antipsychotics for treatment-resistant major depressive disorderActa Psychiatr Scand2008117425325918190674
- PapakostasGISheltonRCSmithJFavaMAugmentation of antidepressants with atypical antipsychotic medications for treatment-resistant major depressive disorder: a meta-analysisJ Clin Psychiatry200768682683117592905
- TyrkaARPriceLHMelloMFMelloAFCarpenterLLPsychotic major depression: a benefit-risk assessment of treatment optionsDrug Saf200629649150816752932
- NelsonJCPikalovABermanRMAugmentation treatment in major depressive disorder: focus on aripiprazoleNeuropsychiatr Dis Treat20084593794819183784
- DeeksEDKeatingGMOlanzapine/fluoxetine: a review of its use in the treatment of acute bipolar depressionDrugs20086881115113718484802
- JansLARiedelWJMarkusCRBloklandASerotonergic vulnerability and depression: assumptions, experimental evidence and implicationsMol Psychiatry200712652254317160067
- SchidkrautJJThe catecholamine hypothesis of affective disorders: a review of supporting evidenceAm J Psychiatry19651225095225319766
- ElhwuegiASCentral monoamines and their role in major depressionProg Neuropsychopharmacol Biol Psychiatry200428343545115093950
- RichelsonEInteractions of antidepressants with neurotransmitter transporters and receptors and their clinical relevanceJ Clin Psychiatry200364Suppl 1351214552650
- LiottiMMaybergHSMcGinnisSBrannanSLJerabekPUnmasking disease-specific cerebral blood flow abnormalities: mood challenge in patients with remitted unipolar depressionAm J Psychiatry2002159111830184012411216
- MaybergHSLiottiMBrannanSKReciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadnessAm J Psychiatry1999156567568210327898
- DrevetsWCBogersWRaichleMEFunctional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolismEur Neuropsychopharmacol20021265274412468016
- MarekGDumanRSKaplanGBHammerRPNeural circuitry and signaling in depressionBrain Circuitry and Signaling in PsychiatryWashington, DCAmerican Psychiatric Publishing, Inc2002153178
- WalshNDWilliamsSCBrammerMJA longitudinal functional magnetic resonance imaging study of verbal working memory in depression after antidepressant therapyBiol Psychiatry20071;62111236124317601497
- IshizakiJYamamotoHTakahashiTTakedaMYanoMMimuraMChanges in regional cerebral blood flow following antidepressant treatment in late-life depressionInt J Geriatr Psychiatry200823880581118214999
- VlassenkoAShelineYIFischerKMintunMACerebral perfusion response to successful treatment of depression with different serotoninergic agentsJ Neuropsychiatry Clin Neurosci200416336036315377745
- DaviesJLloydKRJonesIKBarnesAPilowskyLSChanges in regional cerebral blood flow with venlafaxine in the treatment of major depressionAm J Psychiatry2003160237437612562589
- TatsumiMGroshanKBlakelyRDRichelsonEPharmacological profile of antidepressants and related compounds at human monoamine transportersEur J Pharmacol19973402–32492589537821
- CusackBNelsonARichelsonEBinding of antidepressants to human brain receptors: focus on newer generation compoundsPsychopharmacology (Berl)199411445595657855217
- RosenbaumJFTollefsonGDSchatzbertAFNemeroffCBFluoxetineTextbook of PsychopharmacologyThird Edition edArlington, VAAmerican Psychiatric Publishing, Inc2004231246
- SzaboSTdeMCBlierPModulation of noradrenergic neuronal firing by selective serotonin reuptake blockersBr J Pharmacol1999126356857110188964
- SoderoAPValdomeroACuadraGRRamirezOAOrsingherOALocus coeruleus activity in perinatally protein-deprived rats: effects of fluoxetine administrationEur J Pharmacol20045031–3354215496293
- WestCHRitchieJCBoss-WilliamsKAWeissJMAntidepressant drugs with differing pharmacological actions decrease activity of locus coeruleus neuronsInt J Neuropsychopharmacol200912562764118950545
- SzaboSTBlierPFunctional and pharmacological characterization of the modulatory role of serotonin on the firing activity of locus coeruleus norepinephrine neuronsBrain Res2001922192011730697
- PriscoSEspositoEDifferential effects of acute and chronic fluoxetine administration on the spontaneous activity of dopaminergic neurones in the ventral tegmental areaBr J Pharmacol19951162192319318528581
- DiMascioMDiGiovanniGDiMatteoVPriscoSEspositoESelective serotonin reuptake inhibitors reduce the spontaneous activity of dopaminergic neurons in the ventral tegmental areaBrain Res Bull19984665475549744293
- DiGiovanniGDeDeurwaerderePDiMascioMDiMatteoVEspositoESpampinatoUSelective blockade of serotonin-2C/2B receptors enhances mesolimbic and mesostriatal dopaminergic function: a combined in vivo electrophysiological and microdialysis studyNeuroscience199991258759710366016
- DiMatteoVDiGiovanniGDiMascioMEspositoESB 242084, a selective serotonin2C receptor antagonist, increases dopaminergic transmission in the mesolimbic systemNeuropharmacology19993881195120510462132
- DiMatteoVDiGiovanniGDiMascioMEspositoESelective blockade of serotonin2C/2B receptors enhances dopamine release in the rat nucleus accumbensNeuropharmacology19983722652729680252
- BlierPSzaboSTPotential mechanisms of action of atypical antipsychotic medications in treatment-resistant depression and anxietyJ Clin Psychiatry200566Suppl 8304016336034
- SeagerMAHuffKDBarthVNPhebusLARasmussenKFluoxetine administration potentiates the effect of olanzapine on locus coeruleus neuronal activityBiol Psychiatry200455111103110915158430
- GobertARivetJMLejeuneFSerotonin(2C) receptors tonically suppress the activity of mesocortical dopaminergic and adrenergic, but not serotonergic, pathways: a combined dialysis and electrophysiological analysis in the ratSynapse200036320522110819900
- IchikawaJIshiiHBonaccorsoSFowlerWLO’LaughlinIAMeltzerHY5-HT(2A) and D(2) receptor blockade increases cortical DA release via 5-HT(1A) receptor activation: a possible mechanism of atypical antipsychotic-induced cortical dopamine releaseJ Neurochem20017651521153111238736
- LiegeoisJFIchikawaJMeltzerHY5-HT(2A) receptor antagonism potentiates haloperidol-induced dopamine release in rat medial prefrontal cortex and inhibits that in the nucleus accumbens in a dose-dependent mannerBrain Res2002947215716512176156
- IchikawaJMeltzerHYR(+)-8-OH-DPAT, a serotonin (1A) receptor agonist, potentiated S(-)-sulpiride-induced dopamine release in rat medial prefrontal cortex and nucleus accumbens but not striatumJ Pharmacol Exp Ther199929131227123210565846
- JentschJDRothRHTaylorJRRole for dopamine in the behavioral functions of the prefrontal corticostriatal system: implications for mental disorders and psychotropic drug actionProg Brain Res200012643345311105661
- WeisstaubNVZhouMLiraACortical 5-HT2A receptor signaling modulates anxiety-like behaviors in miceScience2006313578653654016873667
- FeigerADHeiserJFShrivastavaRKGepirone extended-release: new evidence for efficacy in the treatment of major depressive disorderJ Clin Psychiatry200364324324912716264
- McGrathPJStewartJWQuitkinFMGepirone treatment of atypical depression: preliminary evidence of serotonergic involvementJ Clin Psychopharmacol19941453473527806692
- BardeYANeurotrophins: a family of proteins supporting the survival of neuronsProg Clin Biol Res199439045567724649
- MartinowichKManjiHLuBNew insights into BDNF function in depression and anxietyNat Neurosci20071091089109317726474
- DumanRSMonteggiaLMA neurotrophic model for stress-related mood disordersBiol Psychiatry200659121116112716631126
- SenSDumanRSanacoraGSerum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implicationsBiol Psychiatry200864652753218571629
- CastrenEVoikarVRantamakiTRole of neurotrophic factors in depressionCurr Opin Pharmacol200771182117049922
- LuoCXuHLiXMPost-stress changes in BDNF and Bcl-2 immunoreactivities in hippocampal neurons: effect of chronic administration of olanzapineBrain Res200410251–219420215464760
- SheltonRCTollefsonGDTohenMA novel augmentation strategy for treating resistant major depressionAm J Psychiatry2001158113113411136647
- MontgomerySAAsbergMA new depression scale designed to be sensitive to changeBr J Psychiatry1979134382389444788
- HamiltonMA rating scale for depressionJ Neurol Neurosurg Psychiatry196023566214399272
- Clinical Global ImpressionsGuyWECDEU Assessment Manual for Psychopharmacology1976Rockville, MDU.S. Department of Health, Education, and Welfare DHEW Publication No. (ADM) 76–338
- SheltonRCWilliamsonDJCoryaSAOlanzapine/fluoxetine combination for treatment-resistant depression: a controlled study of SSRI and nortriptyline resistanceJ Clin Psychiatry200566101289129716259543
- CoryaSAWilliamsonDSangerTMBriggsSDCaseMTollefsonGA randomized, double-blind comparison of olanzapine/ fluoxetine combination, olanzapine, fluoxetine, and venlafaxine in treatment-resistant depressionDepress Anxiety200623636437216710853
- ThaseMECoryaSAOsuntokunOA randomized, double-blind comparison of olanzapine/fluoxetine combination, olanzapine, and fluoxetine in treatment-resistant major depressive disorderJ Clin Psychiatry200768222423617335320
- HamiltonMGuyWHamiton Anxiety ScaleECDEU Assessment Manual for Psychopharmacology1931981976Rockville, MDU.S. Department of Health, Education, and Welfare
- SheehanDVHarnett-SheehanKRajBAThe measurement of disabilityInt Clin Psychopharmacol199611Suppl 389958923116
- CoryaSAAndersenSWDetkeHCLong-term antidepressant efficacy and safety of olanzapine/fluoxetine combination: a 76-week open-label studyJ Clin Psychiatry200364111349135614658950
- SimpsonGMAngusJWA rating scale for extrapyramidal side effectsActa Psychiatr Scand Suppl197021211194917967
- BarnesTRA rating scale for drug-induced akathisiaBr J Psychiatry19891546726762574607
- GuyWAbnormal Involuntary Movement Scale (AIMS)ECDEU Assessment Manual for Psychopharmacology5345371976Rockville, MDNational Institute for Mental Health
- BermanRMMarcusRNSwaninkRThe efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a multicenter, randomized, double-blind, placebo-controlled studyJ Clin Psychiatry200768684385317592907
- MarcusRNMcQuadeRDCarsonWHThe efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a second multicenter, randomized, double-blind, placebo-controlled studyJ Clin Psychopharmacol200828215616518344725
- ThaseMEQuetiapine monotherapy for bipolar depressionNeuropsychiatr Dis Treat200841112118728771
- DalyEJTrivediMHA review of quetiapine in combination with antidepressant therapy in patients with depressionNeuropsychiatr Dis Treat20073685586719300621
- DoddSBerkMOlanzapine/fluoxetine combination for treatment-resistant depression: efficacy and clinical utilityExpert Rev Neurother2008891299130618759541
- TrivediMHThaseMEOsuntokunOAn integrated analysis of olanzapine/fluoxetine combination in clinical trials of treatment-resistant depressionJ Clin Psychiatry200970338739619284928
- TohenMVietaECalabreseJEfficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depressionArch Gen Psychiatry200360111079108814609883
- BrownEBMcElroySLKeckPEJrA 7-week, randomized, double-blind trial of olanzapine/fluoxetine combination versus lamotrigine in the treatment of bipolar I depressionJ Clin Psychiatry20066771025103316889444
- BrownEDunnerDLMcElroySLOlanzapine/fluoxetine combination vs lamotrigine in the 6-month treatment of bipolar I depressionInt J Neuropsychopharmacol20081211110 [Epub ahead of print]
- AmsterdamJDShultsJComparison of fluoxetine, olanzapine, and combined fluoxetine plus olanzapine initial therapy of bipolar type I and type II major depression – lack of manic inductionJ Affect Disord200587112113015923042
- TakahashiHKamataMYoshidaKHiguchiHIshigookaJAugmentation with olanzapine in TCA-refractory depression with melancholic features: a consecutive case seriesHum Psychopharmacol200823321722018172909
- RasmussenKCreating more effective antidepressants: clues from the clinicDrug Discov Today20061113–1462363116793531
- AllisonDBMentoreJLHeoMAntipsychotic-induced weight gain: a comprehensive research synthesisAm J Psychiatry1999156111686169610553730
- American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of ObesityConsensus development conference on antipsychotic drugs and obesity and diabetesDiabetes Care20042759660114747245
- NewcomerJWSecond-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature reviewCNS Drugs200519Suppl 119315998156
- IosifescuDVTreating depression in the medically illPsychiatr Clin North Am2007301779017362805
- LettHSBlumenthalJABabyakMADepression as a risk factor for coronary artery disease: evidence, mechanisms, and treatmentPsychosom Med200466330531515184688
- DavidsonKWKupferDJBiggerJTAssessment and treatment of depression in patients with cardiovascular disease: National Heart, Lung, and Blood Institute Working Group ReportPsychosom Med200668564565017012516
- Frasure-SmithNLesperanceFDepression--a cardiac risk factor in search of a treatmentJAMA2003289233171317312813125
- HennekensCHPrevention of premature mortality among patients with schizophrenia: the need for primary prevention efforts in cardiovascular diseaseCNS Spectr2008136 Suppl 1091018567981
- Roshanaei-MoghaddamBKatonWPremature mortality from general medical illnesses among persons with bipolar disorder: a reviewPsychiatr Serv200960214715619176408
- BerlimMTTureckiGWhat is the meaning of treatment resistant/ refractory major depression (TRD)? A systematic review of current randomized trialsEur Neuropsychopharmacol2007171169670717521891
- KellerMBIssues in treatment-resistant depressionJ Clin Psychiatry200566Suppl 851216336031
- RushAJThaseMEDubeSResearch issues in the study of difficult-to-treat depressionBiol Psychiatry200353874375312706958
- SackeimHAThe definition and meaning of treatment-resistant depressionJ Clin Psychiatry200162Suppl 16101711480879
- TrivediMHMorrisDWGrannemannBDMahadiSSymptom clusters as predictors of late response to antidepressant treatmentJ Clin Psychiatry20056681064107016086624