Abstract
Fibromyalgia (FM) is a complex syndrome characterized by chronic widespread musculoskeletal pain which is often accompanied by multiple other symptoms, including fatigue, sleep disturbances, decreased physical functioning, and dyscognition. Due to these multiple symptoms, as well as high rates of comorbidity with other related disorders, patients with FM often report a reduced quality of life. Although the pathophysiology of FM is not completely understood, patients with FM experience pain differently from the general population, most likely due to dysfunctional pain processing in the central nervous system leading to both hyperalgesia and allodynia. In many patients with FM, this aberrant pain processing, or central sensitization, appears to involve decreased pain inhibition within the spinal tract, which is mediated by descending pathways that utilize serotonin, norepinephrine, and other neurotransmitters. The reduced serotonin and norepinephrine levels observed in patients with FM suggest that medications which increase the levels of these neurotransmitters, such as serotonin and norepinephrine reuptake inhibitors (SNRIs), may have clinically beneficial effects in FM and other chronic pain conditions. Milnacipran is an SNRI that has been approved for the management of FM. In clinical trials, treatment with milnacipran for up to 1 year has been found to improve the pain and other symptoms of FM. Because FM is characterized by multiple symptoms that all contribute to the decreased quality of life and ability to function, the milnacipran pivotal trials implemented responder analyses. These utilized a single composite endpoint to identify the proportion of patients who reported simultaneous and clinically significant improvements in pain, global disease status, and physical function. Other domains assessed during the milnacipran trials include fatigue, multidimensional functioning, mood, sleep quality, and patient-reported dyscognition. This review article provides information intended to help clinicians make informed decisions about the use of milnacipran in the clinical management of patients with FM. It draws primarily on results from 2 of the pivotal clinical trials that formed the basis of approval of milnacipran in the United States by the Food and Drug Administration.
Introduction
Fibromyalgia (FM) is a chronic pain disorder that affects 0.5% to 5% of the general population worldwide, more frequently in women than in men.Citation1–Citation3 The management of this disorder is complicated by the occurrence not only of pain, but of multiple other symptoms including fatigue, stiffness, sleep disturbances, physical dysfunction, and cognitive problems.Citation4–Citation6 It is also complicated by frequent comorbidity with conditions that share clinical and genetic characteristics and possibly a common pathophysiology. These comorbid conditions include low back pain, diabetic peripheral neuropathy, chronic fatigue syndrome, irritable bowel syndrome (IBS), migraine headache, interstitial cystitis, multiple chemical sensitivities, and temporomandibular disorder (TMD).Citation5,Citation7 The common theme to these conditions appears to be heightened sensitivity to discomfort/stimuli in various regions of the body.
Patients with FM often experience a significantly diminished quality of life, and frequently report an inability to work and feelings of isolation due to their withdrawal from social activities.Citation5,Citation8 FM can result in distressing physical, social and psychological consequences. Therefore, the approval of 3 agents over the past few years for the management of FM represents important progress in the ongoing development of evidence-based therapies for FM.
In the late 1990s, Cypress Bioscience, Inc. observed a large, unmet medical need revolving around the satisfactory treatment of chronic pain patients who were being diagnosed with FM. Although the FM diagnosis was somewhat controversial at the time, there was a large group of patients with numerous somatic complaints who were dissatisfied with their current health status. Moreover, physicians who managed FM patients were expressing frustration with the lack of effective treatment options. Various medications were being used off-label to treat these patients, including tricyclic antidepressants (TCAs), selective serotonin reuptake inhibitors (SSRIs), opioids, nonsteroidal anti-inflammatory drugs (NSAIDs), growth hormone, corticosteroids, and sedative hypnotics. While the TCAs have been found to provide some relief from FM symptoms (particularly sleep-related complaints), their use is often limited by safety and tolerability considerations.Citation9,Citation10 The SSRIs offer a better tolerability profile, but results from clinical trials in patients with FM have been disappointing.Citation11,Citation12 Evidence for the other medications such as opioids and NSAIDs is either lacking or weak.Citation11,Citation13
In response to this unmet need in FM, Cypress began searching for a medication with a suitable neurotransmitter reuptake profile that could be developed as a potential first-line treatment option for patients with FM. One factor contributing to the choice of milnacipran for clinical development was the extensive safety data that already existed for this drug. Although milnacipran was not commercially available in the United States before its approval for the management of FM in 2009, it had been widely used in Europe (since 1997) and in Asia (since 2000) for the treatment of major depressive disorder. Its tolerability in depressed patients had been established in a number of clinical trialsCitation14 and was supported by several million patient-months of postmarketing safety data at the time Cypress licensed the rights for US development. Pierre Fabre Médicament, the original developer of milnacipran, was the licensor and still maintains the global milnacipran safety database.
The scientific rationale behind the development of milnacipran for FM was that, as a serotonin and norepinephrine reuptake inhibitor (SNRI), this drug should have clinically significant analgesic effects. In the central nervous system, both serotonin and norepinephrine have been found to play important roles in pain perception via their involvement in descending antinociceptive pathways.Citation15 Dysfunction in these descending pathways is thought to result in the hyperalgesic (heightened sensitivity to pain) and allodynic (painful response to nonpainful stimuli) states experienced by patients with FM and other related central sensitization syndromes such as IBS and TMD.Citation16,Citation17 The potential benefit of milnacipran in the treatment of FM was further supported by analgesic effects of SNRIs in animal models of pain,Citation18 as well as findings showing decreased cerebrospinal fluid levels of serotonin and norepinephrine metabolites in patients with FM.Citation19 Moreover, therapeutic benefits in FM had already been observed with drugs that inhibit the reuptake of both serotonin and norepinephrine, such as the TCA, amitriptyline.Citation20,Citation21 However, it should be noted that amitriptyline has not been tested in recent clinical trials using the same rigorous standards currently required by the US Food and Drug Administration (FDA) for approval of drugs for the management of FM. For example, recent FM clinical trials with now-approved medications such as milnacipran had much larger sample sizes and longer treatment durationsCitation22 than the older studies involving amitriptyline.Citation10 In addition, these more recent studies have consistently implemented multiple efficacy measures, including pain, global improvements, fatigue, mood, and multidimensional functioning in an effort to address the complex, multisymptomatic nature of FM.
Unlike the TCAs, milnacipran has no significant direct action on adrenergic, muscarinic, or histaminergic receptors – pharmacologic actions associated with many of the unpleasant side effects of TCAs.Citation23 Based on this pharmacologic profile, it was postulated that milnacipran would be clinically beneficial to patients with FM, providing improvements in pain with fewer adverse effects. Moreover, it was thought that a dual reuptake inhibitor would have more potential as an analgesic than medications that selectively block the reuptake of serotonin, which have not consistently demonstrated effectiveness in treating FM symptoms.Citation11,Citation24 Interestingly, drugs that selectively target noradrenergic activity alone also appear to have limited efficacy in patients with FM.Citation22 This suggests that both noradrenergic and serotoninergic activity are required to produce clinically significant pain relief.
Milnacipran received its approval for the management of FM based on the safety and efficacy results of 2 pivotal trials conducted in the United States.Citation25,Citation26 In addition to reviewing the data from these trials, this article describes the various outcome measures that were used to establish the efficacy of milnacipran in patients with FM. The aim of this review article is to highlight the results of these clinical trials in order to provide clinicians with a better understanding of milnacipran as a treatment option in FM.
Pharmacology
Milnacipran is highly water soluble, leading to rapid and wide absorption, with maximum concentration observed within 2 to 4 hours after dosing.Citation27,Citation28 The bioavailabilty of milnacipran is high (approximately 85% to 90%), and absorption is not affected by food intake. Milnacipran undergoes minimal first-pass metabolism, with approximately 55% of the drug excreted unchanged in urine.Citation28 Its relatively short half-life (approximately 6 to 8 hours) is compatible with the recommended twice-daily dosing.Citation28 Pharmacokinetic studies indicate that dose adjustment is not necessary based on age, gender, mild-to-moderate renal impairment, Citation29 or mild-to-moderate hepatic impairment.Citation30 However, caution should be exercised in patients with moderate renal impairment or severe hepatic impairment. In patients with severe renal impairment, the maintenance dose of milnacipran should be reduced by approximately 50% to 50 mg/day (25 mg twice daily).
The low extent of hepatic metabolism, low protein binding (13%), and minimal effects on cytochrome P450 enzymesCitation28,Citation31,Citation32 indicate a low potential for pharmacokinetic drug-drug interactions. In healthy volunteers, co-administration of carbamazepine, digoxin, and lorazepam did not have clinically meaningful effects on the pharmacokinetics of milnacipran. Switching from fluoxetine to milnacipran also did not have an effect on milnacipran pharmacokinetics.Citation28 In vitro, milnacipran did not demonstrate any significant affinity for adrenergic, serotonergic, dopaminergic, opiate, histaminergic, muscarinic, benzodiazepine, or gamma-aminobutyric acid receptors.Citation28 These pharmacologic characteristics of milnacipran may be advantageous for the treatment of patients with FM, many of whom routinely take multiple medications or experience chemical sensitivities.
Clinical efficacy of milnacipran in FM trials
After the completion of a phase 2 trial that demonstrated the potential therapeutic benefits of milnacipran for patients with FM,Citation33 Cypress Bioscience partnered with Forest Laboratories, Inc. to continue the clinical development of this drug for the management of FM. To date, 3 randomized, double-blind, placebo-controlled, multicenter phase 3 trials have been completed in the United States,Citation25,Citation26,Citation34 and a phase 3 trial sponsored by Pierre Fabre Médicament has been conducted in Europe.Citation35 This review focuses on data from the first 2 pivotal US trials upon which the FDA approval was based.Citation25,Citation26 Most of these data have been previously published or presented at scientific meetings; some of the information included in this review, however, is from data on file (Forest Laboratories, Inc.).
The first US pivotal trial included 888 patients with FM (based on the 1990 American College of Rheumatology [ACR] criteria for FM)Citation36 and lasted 6 months (Study 1).Citation25 The second included 1196 patients and lasted 3 months (Study 2).Citation26 In both trials, patients were randomized to placebo, milnacipran 100 mg/day, or milnacipran 200 mg/day. In Study 1, twice as many patients were randomized to the 200 mg/day group than were randomized to either the 100 mg/day or placebo groups in order to better assess the long-term effects of this higher dose. Patients enrolled in the milnacipran trials were required to discontinue centrally acting pharmacotherapies and nonpharmacologic therapies commonly used to treat FM symptoms. Provision for limited use of rescue medication with hydrocodone was allowed in these 2 trials (dosage: ≤60 mg/day for ≤5 days). Patients were instructed not to take hydrocodone during the 48-hour period prior to scheduled study visits and the 2-week period prior to endpoint data collection. For patients who used rescue medication during the critical time periods, a prespecified data handling provision caused these patients to be analyzed as non-responders to treatment, regardless of their actual pain scores. The percentage of patients taking hydrocodone was similar in all treatment arms.
Patient characteristics
Baseline patient characteristics were similar between the first 2 milnacipran FM pivotal trials. In both trials, the patients were mostly women (>90%), mostly white (>90%), and had a mean age of approximately 50 years.Citation25,Citation26 Based on the body mass index (BMI) criteria issued by the World Health Organization,Citation37 more than 75% of the patients were either overweight (BMI ≥25 to 30) or obese (BMI ≥30) at baseline.Citation38 Before initiating treatment, the patients were similarly impaired in terms of baseline pain scoresCitation39 and overall disease activity, as measured by the Fibromyalgia Impact Questionnaire (FIQ).Citation40 Mean baseline scores on the Short Form-36 Health Survey (SF-36) Physical Component Summary (PCS) indicated that enrolled FM patients had markedly decreased physical functioning compared to US norms.Citation41 Patients with current major depressive episodes were excluded from the trials, but approximately 35% of randomized patients in both studies reported a history of depression.Citation25,Citation26 Additionally, mean baseline Beck Depression Inventory (BDI) scores in both trials were 14, indicative of mild depressive symptoms.
Composite responder analyses
The milnacipran trials were designed to evaluate the effect of milnacipran on multiple symptom domains of FM, including pain, fatigue, global improvement, sleep, and physical and mental functioning. The outcomes evaluated in these studies are consistent with the key symptom domains proposed by the Outcome Measures in Clinical Rheumatology Trials (OMERACT) fibromyalgia working group.Citation42–Citation44 One type of efficacy endpoint recognized by OMERACT for its usefulness in FM clinical trials is the composite responder index.Citation43,Citation44 This identifies the proportion of individual patients who have simultaneous, clinically meaningful improvements in multiple symptom domains. Composite endpoints have been used in clinical trials for various rheumatologic conditions, including rheumatoid arthritisCitation45 and osteoarthritis,Citation46 and provide information about specific patient responses as opposed to group mean changes. Although composite endpoints have some limitations,Citation47 one strength is that they are inherently conservative estimates of clinical efficacy. Patients who do not meet the criteria for each of the component domains cannot be counted as having responded to treatment, even if they show dramatic improvement for a single domain. In other words, composite endpoints can only be as robust as their weakest constituent component (ie, the one most difficult to change) and require that the therapy produces clinically significant changes in multiple domains in the same patient. As such, they set a high hurdle for success.
At the time the milnacipran studies were designed, it was not clear whether there was an appropriate instrument for the assessment of physical function as part of a composite responder definition. Therefore, 2 definitions of response were evaluated during the pivotal registration trials: a 2-measure composite responder analysis that required simultaneous improvements in pain and global status; and a more stringent 3-measure composite that required simultaneous improvements in pain, global status, and physical function.Citation25,Citation26 To be classified as 2-measure composite responders, individual patients were required to experience ≥30% improvement from baseline in pain, as measured by Visual Analog Scale (VAS) 24-hour recall scores (recorded on a patient experience diary [PED], as described below), and a rating of “Much Improved” or “Very Much Improved” on the Patient Global Impression of Change (PGIC). Patients who experienced a ≥6-point improvement from baseline in their SF-36 PCS score, in addition to meeting the pain and PGIC criteria, were classified as 3-measure composite responders. All of the definitions of improvement were based on changes that met or exceeded established minimal clinically important differences for those measures.Citation48–Citation50
All statistical analyses were conducted on the intent-to-treat (ITT) population. Several imputation methods were used to handle missing data in these registration trials. Baseline observation carried forward (BOCF), a conservative approach requested by the FDA for the approval of milnacipran for the management of FM,Citation51 was the primary imputation method for analyzing the composite responder endpoints. In BOCF, a patient who does not successfully complete the final trial visit – irrespective of the reason – is declared a nonresponder, regardless of the actual data recorded up to that point. Sensitivity analyses were also conducted using last observation carried forward (LOCF) and observed cases (OC). LOCF is a widely employed imputation method that uses the last recorded value for patients with missing data, while OC is a straightforward method that only analyzes recorded observations and does not impute missing data. As an estimate of outcomes among patients who comply with and tolerate treatment,Citation52 OC analyses of the milnacipran data may provide practicing physicians with useful information. OC results are therefore highlighted in this summary.
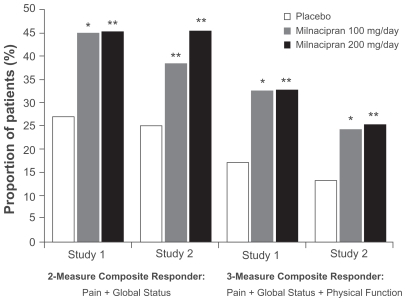
Patients who were treated with milnacipran experienced multidimensional improvements in pain, global status, and physical function. After 3 months of treatment in each of the pivotal studies, a significantly higher proportion of patients in the milnacipran groups met the 2-measure and 3-measure composite responder criteria, as compared with the placebo group (P < 0.01, both doses vs placebo; OC) ().Citation25,Citation26 For the more stringent 3-measure composite analysis, response rates among milnacipran-treated patients were approximately twice the rates found in placebo-treated patients. Results after 6 months of treatment were similar to those found at the 3-month endpoint. At 6 months, response rates for the 2-measure composite responder analysis were 43.8%, 45.2%, and 27.9% for milnacipran 100 mg/day, 200 mg/day, and placebo, respectively (P < 0.05, both doses vs placebo; OC).Citation25
Figure 1 Percentage of patients with fibromyalgia meeting the 2-measure and 3-measure composite responder criteria at 3 months, observed cases. From Study 1Citation25 and Study 2.Citation26
*P < 0.01; **P ≤ 0.001, vs placebo.
Pain
Improvement in pain was included as part of the composite responder analyses because chronic widespread pain is central to the definition of FM and is rated by both patients and physicians as the most important core domain to be assessed in FM clinical trials.Citation42,Citation43 In addition to being included as one component of the primary composite endpoints, pain was evaluated separately in the milnacipran trials using various secondary outcome measures, given the primacy of this symptom in the experience of patients with FM. Pain data was collected on electronic PEDs that prompted patients to record their 24-hour recall pain, weekly recall pain, and current level of pain (“real-time”) by marking VAS scales displayed on these hand-held electronic diaries. The PEDs, which were customized for use in the milnacipran trials, provided patients with a more accurate tool to report on their pain experiences. In post hoc analyses of the milnacipran pivotal trials,Citation53,Citation54 these electronic PEDs were found to be more discriminatory and sensitive than paper-based pain assessments. This was probably due to the minimization of recall bias and the ability to capture data in the patients’ home environment. Use of these electronic diaries also helped to satisfy the FDA’s recent rigorous approach to the use of patient-reported outcomes in registration trials. At the time of application for FDA approval, over 1 million pain data points had been collected from patients enrolled in the milnacipran FM trials. The PED pain data were supplemented by paper VAS pain assessments captured from patients at each study visit.
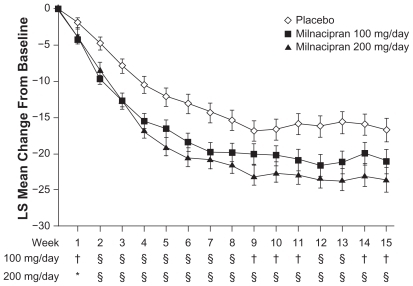
Milnacipran has proven to be effective in reducing FM pain.Citation25,Citation26,Citation33–Citation35 Compared with placebo, milnacipran was associated with significant improvements in PED and paper-based VAS pain measures.Citation25,Citation26 Significant sustained pain reductions were observed as early as 1 week after stable-dose treatment with milnacipran (P < 0.05 vs placebo), and maximal pain relief was reached by 9 weeks of treatment ().Citation25,Citation26 The pain component of the composite responder analysis (ie, ≥30% improvement from baseline PED VAS 24-hour recall pain score) represents a clinically meaningful improvement in FM pain.Citation48,Citation49 A significantly higher proportion of patients experienced ≥30% improvements in pain with milnacipran than with placebo in Study 1 (52.8%, 100 mg/day; 56.2%, 200 mg/day; placebo, 40.2%; P < 0.05, both doses vs placebo; OC)Citation25 and Study 2 (52.3%, 100 mg/day; 54.8%, 200 mg/day; 38.4%, placebo; P < 0.01, both doses vs placebo; OC).Citation26
Figure 2 Least squares (LS) mean change from baseline in weekly average 24-hour morning recall pain scores. From Study 2,Citation26 observed cases.
*P < 0.05; †P < 0.01; and §P < 0.001, vs placebo.
A post hoc analysis of these 2 pivotal trials was conducted to determine the proportion of days in which patients experienced a ≥30% improvement in pain.Citation55 Over a 3-month treatment period, patients treated with milnacipran vs placebo had a significantly higher percentage of days with clinically meaningful pain relief (45%, 100 mg/day; 46%, 200 mg/day; 33%, placebo; P < 0.0001, both doses vs placebo; OC). Significant differences between milnacipran and placebo in the proportion of pain relief days were also detected using a more stringent measure of ≥50% improvement from baseline in PED VAS 24-hour recall pain scores (27%, 100 mg/day; 29%, 200 mg/day; 18%, placebo; P < 0.0001, both doses vs placebo; OC).
Patient global
“Patient global” is one of the core domains identified by OMERACT as being essential for inclusion in FM clinical trial designs.Citation42–Citation44 While most FM patients have symptoms involving multiple domains, not all patients rate all symptoms as being of similar importance. Therefore, a measure that implicitly evaluates those domains of most importance to an individual patient has practical utility and face validity. In the milnacipran studies, patient global was incorporated into the composite responder endpoints using the PGIC, a simple instrument that asks patients to rate their overall improvement by completing a single statement (“Since the start of the study, overall, my fibromyalgia is”) using a Likert scale ranging from 1 (“Very Much Improved”) to 7 (“Very Much Worse”). After 3 months of treatment, approximately one-half of the patients treated with milnacipran had marked global improvements (ie, PGIC score ≤2) compared with approximately one-third of the placebo-treated patients (49.4%, 100 mg/day; 52.8%, 200 mg/day; 33.8%, placebo; OC; pooled data on file).
In analyses conducted to evaluate which symptom domains in the milnacipran trials were associated with global well-being, pain was the strongest independent factor correlating with PGIC scores among patients who reported any global improvements (PGIC score ≤3).Citation56 Vitality, sleep, dyscognition, and physical function were also significantly and independently associated with PGIC improvements. These findings are similar to those recently reported by OMERACT.Citation44 Using the PGIC as a surrogate of overall improvement, OMERACT researchers examined data from 10 clinical trials of FM and found that measures of pain, fatigue, multidimensional function, physical function, and stiffness had the highest correlation with PGIC ratings. These types of correlations suggest that overall changes in patient global well-being reflect changes in multiple FM symptoms, underscoring the need for therapies that have multidimensional clinical benefits.
Functioning
Given the impact of pain on daily functioning, patient-reported quality of life measures have been increasingly utilized in pain clinical trialsCitation57 and are now considered to be an essential area of assessment for the approval of chronic pain medications.Citation4 OMERACT has similarly recognized the importance of using quality of life measures to assess the efficacy of FM therapies.Citation43,Citation44 One such measure is the SF-36, a validated health status scale that has been used in over 70 studies involving FM clinical patients.Citation44,Citation58 The SF-36 includes 36 items assessing 8 domains (physical functioning, bodily pain, role limitations due to physical problems, role limitations due to emotional problems, general health, mental health, energy/vitality, and social functioning) from which the PCS and Mental Component Summary (MCS) scores are derived.Citation41
In a recent review comparing the health status profile of individuals with FM with that of the general population and patients with other health conditions, FM patients were found to have poorer scores on all 8 SF-36 domains than did patients with hypertension, recent acute myocardial infarction, type II diabetes, congestive heart failure, or chronic obstructive pulmonary disease.Citation58 This analysis also found that high levels of bodily pain and decreased energy/vitality scores were characteristic of FM, as compared with other chronic pain conditions (ie, herniated disc, epicondylitis, osteoarthritis, and rheumatoid arthritis). Such findings underscore the importance of prescribing FM medications that not only reduce pain, but also improve multidimensional functioning and increase energy/vitality.
In the pivotal trials, milnacipran treatment improved physical and mental functioning in patients with FM, as demonstrated by significant differences between active drug and placebo in the SF-36 PCS, SF-36 MCS, and each of the individual SF-36 domains (, pooled data on file). Clinically meaningful improvement for the SF-36 PCS was defined in the milnacipran trials as ≥6-point improvement from baseline score, a more conservative criterion than the minimum clinically important differences (SF-36 PCS changes, 2.5 to 5.0 points) that have been established for various rheumatic conditions.Citation50 After 3 months of treatment, a higher proportion of milnacipran-treated patients were found to have clinically meaningful improvements in the SF-36 PCS as compared with placebo-treated patients (44.5%, 100 mg/day; 42.5%, 200 mg/day; 32.7%, placebo; OC; pooled data on file).
Table 1 Additional significant outcomes receiving 3 months of milnacipran treatment
Fatigue
Fatigue is a common complaint among patients with FM, usually described as being physically or emotionally draining.Citation4,Citation6 Like pain, fatigue is a constant presence that fluctuates in intensity throughout the day, affecting a patient’s ability to perform daily tasks, to function in the work force, and to enjoy social or recreational activities.Citation4,Citation6,Citation59 Therefore, medications that reduce fatigue are important to both patients with FM and treating physicians.
To assess the efficacy of milnacipran on fatigue, the pivotal trials utilized the Multidimensional Fatigue Inventory (MFI), a 20-item self-report instrument that measures several dimensions of fatigue: general fatigue, physical fatigue, mental fatigue, reduced motivation, and reduced activity.Citation60 The MFI has been used to assess fatigue levels in various chronic conditions such as rheumatoid arthritis,Citation61 ankylosing spondylitis,Citation62 and Sjögren’s syndrome.Citation63 In the milnacipran pivotal trials, significant improvements in fatigue were observed with milnacipran 200 mg/day compared with placebo, as evidenced by MFI total and all subscale scores ().Citation64 Patients treated with milnacipran 100 mg/day compared with placebo showed significant improvements in MFI total scores and subscale scores of general fatigue, physical fatigue, and reduced motivation.
Cognitive impairment
The cognitive functions most affected in patients with FM are working memory, episodic memory, and semantic memory; other impairments include attentional control, susceptibility to distraction, and loss of vocabulary.Citation65 Patients who have cognitive dysfunction often describe their experiences as feeling disorganized, having difficulty planning, and being unable to remember words.Citation59 Like pain and fatigue, this “fibro-fog” affects daily functioning, particularly with regard to a patient’s ability to work and drive.Citation59
The milnacipran clinical trials assessed self-reported cognitive function in FM patients by using the Multiple Ability Self-Report Questionnaire (MASQ). The MASQ assesses 5 cognitive domains: language, verbal memory, visuo-perceptual ability, visual memory, and attention.Citation66 Significant improvements in the MASQ total score were observed in patients treated with milnacipran 200 mg/day compared with placebo (, pooled data on file). Similarly, significant results with both doses of milnacipran were found for the MASQ verbal memory and attention domains, both of which are reflective of FM cognitive deficits. These results indicate that milnacipran improves cognitive function in patients with FM, particularly in the domains (eg, memory and attention)Citation65 most affected by this disorder.
Long-term studies
Until recently, FM clinical trials have tended to be short in duration, generally lasting 3 months or less. Given the chronic nature of FM, experts in the field have recommended that longer clinical trials be conducted in order to evaluate whether the benefits of FM treatments persist over time.Citation21,Citation67 To this end, 2 randomized, double-blind extension studies of milnacipran have been conducted in the United States: a 28-week extension study to the 6-month pivotal trial (N = 449)Citation68 and an extension study adding up to 39 weeks to the 3-month pivotal trial (N = 384).Citation69 Additionally, a 12-month extension of the 3-month European milnacipran trial has been completed.Citation70 In the US studies, patients who received milnacipran 200 mg/day during the lead-in pivotal trials, were continued on the same dosage in a blinded fashion during the extension studies. Patients who received placebo or milnacipran 100 mg/day were re-randomized to milnacipran 100 mg/day or 200 mg/day.
Results from the extension studies indicate that milnacipran effectively improves the pain and other symptoms of FM for at least 1 year.Citation68,Citation69 The clinical benefits of milnacipran were maintained in patients who received milnacipran in both the lead-in and extension studies (ie, 12 months of continuous milnacipran treatment). In these patients, efficacy outcomes at the end of the extension study (eg, pain VAS scores, FIQ total and physical function scores, PGIC scores) were similar to those observed at the end of the lead-in study. A subgroup of patients (n = 100) re-randomized from placebo to milnacipran 200 mg/day after 6 months of placebo in the double-blind lead-in trial experienced additional improvements in pain, FIQ, and PGIC scores during the extension study period.Citation68 By the end of 12 months, this subgroup of patients had achieved the same relative degree of pain and global improvement reported by those treated continuously with milnacipran for the entire 12 months. Results from another subgroup of patients who were re-randomized from 100 mg/day to 200 mg/day (n = 92) indicate that some patients may benefit from increasing the milnacipran dosage.Citation68 In these patients, additional improvements in pain and other outcomes were observed during the extension study period during which they received the higher dosage. Data from patients receiving milnacipran for up to 3 years in the US trials will be available after the conclusion of a currently ongoing study.
Safety and tolerability
The safety and tolerability information presented here is from patients with FM who were enrolled in the phase 2 clinical trialCitation33 and the 2 pivotal registration trials.Citation25,Citation26 Together, these trials included 2209 patients, 1557 of whom were treated with milnacipran and 652 of whom received placebo.
Discontinuation due to adverse events (AEs) occurred in 23.0% and 26.0% of patients receiving milnacipran 100 mg/day and 200 mg/day, respectively, compared with 12.1% of placebo-treated patients.Citation71 Nausea and palpitations were the only AEs resulting in discontinuation in ≥2% of milnacipran patients and at an incidence greater than placebo. Treatment-emergent AEs (TEAEs) in the milnacipran trials were generally mild to moderate in severity, with nausea being the most common TEAE in all treatment groups. The placebo-corrected rates of nausea for milnacipran 100 mg/day and 200 mg/day were 14.9% and 19.7%, respectively.Citation71 Nausea typically occurred early in treatment and was generally manageable by recommending that medication be taken with food, incorporating gradual dose escalation, and providing patient counseling. While the clinical trials were designed to have relatively inflexible dose escalation phases lasting 2 weeks, recent anecdotal reports from commercial usage suggest that a slower, more flexible approach to dose increases may be beneficial.
Headache was the second most common TEAE, occurring in 18.6%, 17.2%, and 13.7% of all patients receiving milnacipran 100 mg/day, 200 mg/day, and placebo, respectively.Citation71 TEAEs that occurred in ≥5% of all milnacipran-treated patients at an incidence at least twice that of placebo were constipation, hot flush, hyperhidrosis (sweating), palpitations, vomiting, increased heart rate, dry mouth, and hypertension. Most of the aforementioned TEAEs would appear to be directly related to the effect of increased norepinephrine levels in the periphery. No new safety concerns were observed with prolonged exposure to milnacipran in the extension studies.Citation68,Citation69
As with other drugs in this class, cardiovascular effects have been reported with milnacipran. In the FM clinical trials, treatment with milnacipran 100 mg/day was associated with mean increases in blood pressure up to 3.1 mmHg, as well as mean increases in pulse rate of 7 to 8 bpm.Citation28 Potentially clinically significant increases in supine systolic blood pressure (≥180 mmHg with an increase of ≥20 mmHg) occurred rarely (≤0.2%) in patients from all treatment groups.Citation71 Potentially clinically significant increases in supine diastolic blood pressure (≥110 mmHg with an increase of ≥15 mmHg) occurred in 0.9% of patients receiving milnacipran, compared with 0.3% of patients receiving placebo. Potentially clinically significant increases in supine pulse rate (≥120 bpm with an increase of ≥20 bpm) occurred in 0.7% and 0% of patients receiving milnacipran and placebo, respectively (pooled data on file). Results from a recently published study indicate that at supratherapeutic doses, milnacipran does not significantly affect cardiac repolarization or contribute to QTc prolongation.Citation72
Milnacipran was not associated with weight gain, which can occur with other medications used to treat FM.Citation73 During the course of therapy, patients who received milnacipran tended to lose weight. At 3 and 6 months, the proportion of patients with clinically significant weight loss (≥5% of baseline body weight) was significantly higher with milnacipran vs placebo (P < 0.01, both doses vs placebo; both endpoints).Citation38 Similar nausea rates in milnacipran-treated patients who lost or gained weight indicate that this weight loss was unrelated to nausea. The lack of weight gain has been observed for up to 12 months of milnacipran treatment.Citation68 In contrast to the use of SSRIs for FM,Citation74 sexual side effects were reported in <1.0% of milnacipran-treated patients enrolled in the placebo-controlled FM trials (data on file). These findings of lack of sexual side effects were also supported by the lack of a significant difference between milnacipran and placebo groups on the Arizona Sexual Experience Scale.Citation25 In the small population of males with FM (n = 87) included in the placebo-controlled trials, genitourinary AEs were reported in at least 2% of male patients treated with milnacipran and occurred at a rate greater than in placebo-treated male patients.Citation28 This observation is consistent with the mechanism of milnacipran in which the increased peripheral norepinephrine level causes an increase in muscle tone, including in the urethra.
Conclusion: place in therapy
The complexity and heterogeneity of FM limits the utility of single-instrument outcomes in determining therapeutic efficacy. The multifaceted approach used to evaluate efficacy in the milnacipran clinical trials reflects ongoing discussions by groups such as OMERACT regarding the key domains and assessment tools needed to adequately evaluate FM outcomes.Citation42–Citation44 In the milnacipran clinical trials, the implementation of 2 composite responder indices allowed investigators to identify the proportion of individual patients who experienced simultaneous improvements in multiple symptom domains. As the development of pharmacotherapies for FM and other chronic pain syndromes progresses, it is expected that other clinical studies, such as the recently reported trials with sodium oxybate,Citation75,Citation76 will continue to use such outcome measures in order to better address the multisymptomatic nature of these disorders.
Results of the responder analyses in the milnacipran trials indicate that the therapeutic benefits of this medication extend beyond its analgesic effects.Citation25,Citation26,Citation34,Citation35 Based on the OC analyses of patients who were compliant with treatment in the clinical trial program, the rate of response with milnacipran treatment was approximately 50% on the 2-measure composite endpoint (clinically meaningful reduction in pain plus “much improved” or “very much improved” global status). These encouraging results suggest that if patients with FM are counseled on the importance of medication compliance and are adequately informed about the side effects of milnacipran, which are generally mild and transient, approximately half of these patients will achieve clinically significant and meaningful improvement in multiple domains.
There were no statistical differences in composite responder rates between milnacipran 100 mg/day and 200 mg/day, although these trials were not powered to detect such differences. Moreover, with the exception of nausea, the AE profile was similar between the dosages. In clinical settings, however, some patients may benefit from the higher dose, as evidenced by results from the extension studies in which patients who were re-randomized to the higher dose for an additional 6 to 9 months of treatment experienced additional improvements in pain and multidimensional function. Thus, although milnacipran 100 mg/day is the recommended starting dose, some FM patients can be escalated to the higher dose on an as-needed basis and if tolerability allows. Due to the potential for cardiovascular side effects, blood pressure and pulse rate should be measured before starting therapy with milnacipran and periodically monitored in all patients throughout the course of treatment. If baseline hypertension or tachycardia is detected, this should be treated and controlled prior to starting therapy with any drug that impacts noradrenergic activity.
One aim of the milnacipran clinical development program was to differentiate the analgesic effects of milnacipran from possible antidepressant effects. Although milnacipran was found to relieve pain in both depressed and nondepressed FM patients during the phase 2 trial,Citation33 a change was made in the phase 3 trial design to exclude patients with current major depressive episodes, so that the analgesic and other therapeutic effects could be more clearly assessed in the absence of a potentially confounding effect on depression. In the phase 3 trials, improvements in pain and other efficacy outcomes were robust in nondepressed FM populations,Citation25,Citation26,Citation34,Citation35 with an analysis of Study 1 showing that milnacipran resulted in global improvements regardless of baseline depressive symptom severity.Citation77
Standardized guidelines for the management of FM have not been established, although it has been recommended that drugs with strong clinical evidence be used as first-line therapies in patients with moderate to severe pain.Citation67 Strong evidence has been found for all of the FDA-approved medications for FM (milnacipran, duloxetine, pregabalin),Citation12 although no direct comparisons between these medications can be made in the absence of head-to-head clinical trials. Making comparisons based on available clinical trial results is also not possible due to the use of different study designs and primary and secondary outcome measures in the trials.Citation78 However, it has been suggested that SNRIs, such as milnacipran and duloxetine, may be tried in patients with comorbid mood disturbances and/or physical function deficits whereas pregabalin, an alpha-2-delta ligand, may be more appropriate for patients with prominent sleep disturbances or anxiety.Citation24,Citation67 For patients who have a partial response to monotherapies, combination treatment with medications having different mechanisms of action may be beneficial.Citation67,Citation79 The pending results from a multicenter, randomized, controlled pilot study investigating the addition of milnacipran to pregabalin treatment are expected to help clarify the potential benefits of combination therapy.Citation80 Such research may soon be complemented by findings from genetic and pharmacogenetic studies,Citation81 which are being conducted with the goal of allowing more personalized approaches to FM treatment in the future.
Although the approval of milnacipran and other medications represents progress in the treatment of FM, none of these treatments has proven to be completely effective in treating chronic pain conditions. Additional therapeutic approaches including combination and multimodal therapies will likely be needed. Development of personalized approaches, perhaps coupled with agents that modulate other aspects of the CNS pain processing system, will be needed as we strive to improve the treatment of chronic pain conditions.
Acknowledgments
The authors would like to thank Prescott Medical Communications Group (Chicago, IL) for providing medical writing assistance, which was supported by funding from Forest Laboratories, Inc.
Disclosures
Drs Kranzler and Gendreau are officers of and shareholders in Cypress Bioscience, Inc., one of the companies involved in the development of milnacipran for fibromyalgia.
References
- WolfeFRossKAndersonJRussellIJHebertLThe prevalence and characteristics of fibromyalgia in the general populationArthritis Rheum199538119287818567
- WhiteKPHarthMClassification, epidemiology, and natural history of fibromyalgiaCurr Pain Headache Rep20015432032911403735
- BrancoJCBannwarthBFaildeIPrevalence of fibromyalgia: a survey in five European countriesSemin Arthritis Rheum2009
- MeasePFibromyalgia syndrome: review of clinical presentation, pathogenesis, outcome measures, and treatmentJ Rheumatol200532Suppl 7562115630715
- BennettRMJonesJTurkDCRussellIJMatallanaLAn internet survey of 2,596 people with fibromyalgiaBMC Musculoskelet Disord200782717349056
- BennettRMClinical manifestations and diagnosis of fibromyalgiaRheum Dis Clin North Am200935221523219647138
- AaronLABuchwaldDChronic diffuse musculoskeletal pain, fibromyalgia and co-morbid unexplained clinical conditionsBest Pract Res Clin Rheumatol200317456357412849712
- CrooksVAExploring the altered daily geographies and lifeworlds of women living with fibromyalgia syndrome: a mixed-method approachSoc Sci Med200764357758817079063
- ClauwDJFibromyalgia: update on mechanisms and managementJ Clin Rheumatol200713210210917414543
- NishishinyaBUrrutiaGWalittBAmitriptyline in the treatment of fibromyalgia: a systematic review of its efficacyRheumatology (Oxford)200847121741174618697829
- ClauwDJPharmacotherapy for patients with fibromyalgiaJ Clin Psychiatry200869Suppl 2252918537460
- MeasePJChoyEHPharmacotherapy of fibromyalgiaRheum Dis Clin North Am200935235937219647148
- GoldenbergDLBurckhardtCCroffordLManagement of fibromyalgia syndromeJAMA2004292192388239515547167
- NakagawaAWatanabeNOmoriIMMilnacipran versus other antidepressive agents for depressionCochrane Database Syst Rev20093CD00652919588396
- MillanMJDescending control of painProg Neurobiol200266635547412034378
- StahlSMFibromyalgia – pathways and neurotransmittersHum Psychopharmacol200924Suppl 1S11S1719479906
- AblinKClauwDJFrom fibrositis to functional somatic syndromes to a bell-shaped curve of pain and sensory sensitivity: evolution of a clinical constructRheum Dis Clin North Am200935223325119647139
- MochizuckiDSerotonin and noradrenaline reuptake inhibitors in animal models of painHum Psychopharmacol200419Suppl 1S151915378668
- RussellIJVaeroyHJavorsMNybergFCerebrospinal fluid biogenic amine metabolites in fibromyalgia/fibrositis syndrome and rheumatoid arthritisArthritis Rheum19923555505561374252
- ArnoldLMKeckPEJrWelgeJAAntidepressant treatment of fibromyalgia. A meta-analysis and reviewPsychosomatics200041210411310749947
- O’MalleyPGBaldenETomkinsGSantoroJKroenkeKJacksonJLTreatment of fibromyalgia with antidepressants: a meta-analysisJ Gen Intern Med200015965966611029681
- RaoSGCurrent progress in the pharmacological therapy of fibromyalgiaExpert Opin Investig Drugs2009181014791493
- CatesMBoggsAAFeldmanJChisholm-BurnsMAWellsBGSchwinghammerTLMalonePMKolesarJMMajor depressive disorderPharmacotherapy Principles and PracticeNew YorkMcGraw-Hill2008569584
- AbelesMSolitarBMPillingerMHAbelesAMUpdate on fibromyalgia therapyAm J Med2008121755556118589048
- MeasePJClauwDJGendreauRMThe efficacy and safety of milnacipran for treatment of fibromyalgia. a randomized, double-blind, placebo-controlled trial [published correction appears in J Rheumatol 2009;36(3):661]J Rheumatol200936239840919132781
- ClauwDJMeasePPalmerRHGendreauRMWangYMilnacipran for the treatment of fibromyalgia in adults: a 15-week, multicenter, randomized, double-blind, placebo-controlled, multiple-dose clinical trial [published correction appears in Clin Ther 2009;31(2):446]Clin Ther200830111988200419108787
- PuozzoCPanconiEDeprezDPharmacology and pharmacokinetics of milnacipranInt Clin Psychopharmacol200217Suppl 1S253512369608
- Forest Laboratories, IncSavella package insert72009 Available from www.savella.comAccessed December 8, 2009
- PuozzoCPozetNDeprezDBaillePUngHLZechPPharmacokinetics of milnacipran in renal impairmentEur J Drug Metab Pharmacokinet19982322802869725494
- PuozzoCAlbinHVinconGDeprezDRaymondJMAmourettiMPharmacokinetics of milnacipran in liver impairmentEur J Drug Metab Pharmacokinet19982322732799725493
- PuozzoCLensSRehCLack of interaction of milnacipran with the cytochrome p450 isoenzymes frequently involved in the metabolism of antidepressantsClin Pharmacokinet200544997798816122284
- ParisBLOgilvieBWScheinkoenigJANdikum-MofforFGibsonRParkinsonAIn vitro inhibition and induction of human liver cytochrome p450 enzymes by milnacipranDrug Metab Dispos200937102045205419608694
- GendreauRMThornMDGendreauJFEfficacy of milnacipran in patients with fibromyalgiaJ Rheumatol200532101975198516206355
- ArnoldLMGendreauRMSperaAGendreauJWangYMilnacipran 100 mg/day in the treatment of fibromyalgia: a randomized, double-blind, placebo-controlled trial [abstract]Arthritis Rheum200960Suppl 10S527
- BrancoJCZachrissonOPerrotSMainguyYon behalf of The Multinational Coordinator Study GroupA European multicenter randomized double-blind placebo-controlled monotheraphy clincal trial of milnacipran in the treatment of fibromyalgiaJ Rheumatol201037485185920156949
- WolfeFSmytheHAYunusMBThe American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the Multicenter Criteria CommitteeArthritis Rheum19903321601722306288
- World Health OrganizationObesity: Preventing and Managing the Global EpidemicGeneva, SwitzerlandWorld Health Organization2000 Available from http://whqlibdoc.who.int/trs/WHO_TRS_894.pdfAccessed December 8, 2009
- WernerPClauwDJArnoldLMEffect of milnacipran on body weight in patients with fibromyalgia: a pooled analysis of 2 randomized, placebo-controlled trial [poster presentation]Presented at the 24th American Academy of Nurse PractitionersJune 17–21, 2009Nashville, Tennessee
- BennettRMScheinJKosinskiMRHewittDJJordanDMRosenthalNRImpact of fibromyalgia pain on health-related quality of life before and after treatment with tramadol/acetaminophenArthritis Rheum200553451952716082646
- BennettRMBushmakinAGCappelleriJCZlatevaGSadoskyABMinimal clinically important difference in the fibromyalgia impact questionnaireJ Rheumatol20093661304131119369473
- WareJEJrSF-36 health survey updateSpine (Phila Pa 1976)200025243130313911124729
- MeasePJClauwDJArnoldLMFibromyalgia syndromeJ Rheumatol200532112270227716265715
- MeasePArnoldLMBennettRFibromyalgia syndromeJ Rheumatol20073461415142517552068
- MeasePArnoldLMChoyEHFibromyalgia syndrome module at OMERACT 9: domain constructJ Rheumatol200936102318232919820221
- SchwietermanWDIssues in the design of new clinical trials for rheumatoid arthritis therapeuticsNat Clin Pract Rheumatol200841264164819037225
- BinghamCO3rdBirdSRSmugarSSXuXTershakovecAMResponder analysis and correlation of outcome measures: pooled results from two identical studies comparing etoricoxib, celecoxib, and placebo in osteoarthritisOsteoarthritis Cartilage200816111289129318514551
- Ferreira-GonzalezIPermanyer-MiraldaGBusseJWMethodologic discussions for using and interpreting composite endpoints are limited, but still identify major concernsJ Clin Epidemiol200760765165717573977
- DworkinRHTurkDCWyrwichKWInterpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendationsJ Pain20089210512118055266
- FarrarJTYoungJPJrLaMoreauxLWerthJLPooleRMClinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scalePain200194214915811690728
- StrandVSinghJAImproved health-related quality of life with effective disease-modifying antirheumatic drugs: evidence from randomized controlled trialsAm J Manag Care200814423425418415967
- US Food and Drug AdministrationDrug Approval Package, Savella (milnacipran HCl tablets) Available from www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022256s000TOC.cfmAccessed December 8, 2009
- LittleRJARubinDBStatistical Analysis With Missing Data2nd edHoboken, N.JWiley2002
- WilliamsDAGendreauRMClauwDJElectronic diaries have superior discrimination compared to paper-based pain assessment in individuals with fibromyalgia [abstract]Arthritis Rheum200756Suppl 9S607
- WilliamsDAGRMClauwDJA comparison between electronic diaries and paper-based pain assessment in individuals with fibromyalgia [abstract]J Pain200894 Suppl 2P16
- MeasePPalmerRHWangYHuffordMRDay-to-day pain relief in fibromyalgia patients: results from 2 clinical trials [abstract]Ann Rheum Dis200968Suppl 3692693
- GeisserMEClauwDJStrandVGendreauRMPalmerRWilliamsDAContributions of change in clinical status parameters to Patient Global Impression of Change (PGIC) scores among person with fibromyalgia treated with milnacipranPain2010322 [Epub ahead of print]
- GatchelRJTheodoreBREvidence-based outcomes in pain research and clinical practicePain Pract20088645246019000173
- HoffmanDLDukesEMThe health status burden of people with fibromyalgia: a review of studies that assessed health status with the SF-36 or the SF-12Int J Clin Pract200862111512618039330
- ArnoldLMCroffordLJMeasePJPatient perspectives on the impact of fibromyalgiaPatient Educ Couns200873111412018640807
- SmetsEMGarssenBBonkeBDe HaesJCThe Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigueJ Psychosom Res19953933153257636775
- HewlettSHehirMKirwanJRMeasuring fatigue in rheumatoid arthritis: a systematic review of scales in useArthritis Rheum200757342943917394228
- van TubergenACoenenJLandeweRAssessment of fatigue in patients with ankylosing spondylitis: a psychometric analysisArthritis Rheum200247181611932872
- d’EliaHFRehnbergEKvistGEricssonAKonttinenYMannerkorpiKFatigue and blood pressure in primary Sjogren’s syndromeScand J Rheumatol200837428429218612929
- ClauwDJPalmerRHHuffordMRZablockiRWangYMilnacipran improves fatigue in patients with fibromyalgia: results from two randomized, double-blind, placebo-controlled trials [poster presentation]Presented at the 72nd Annual American College of Rheumatology and 43rd Annual Association of Rheumatology Health Professionals MeetingSan Francisco, CA2008
- GlassJMFibromyalgia and cognitionJ Clin Psychiatry200869Suppl 2202418537459
- SeidenbergMHaltinerATaylorMAHermannBBWylerADevelopment and validation of a Multiple Ability Self-Report QuestionnaireJ Clin Exp Neuropsychol1994161931048150893
- ArnoldLMBiology and therapy of fibromyalgia. New therapies in fibromyalgiaArthritis Res Ther20068421216762044
- GoldenbergDLClauwDJPalmerRHMeasePChenWGendreauRMDurability of therapeutic response to milnacipran treatment for fibromyalgia. Results of a randomized, double-blind, monotherapy 6-month extension studyPain Med201011218019420002596
- FerreraRPalmerRChenWGendreauRImprovements in fibromyalgia symptoms are sustained for 1 year with milnacipran treatment: results from 2 double-blind, dose-controlled extension studies [abstract]J Pain2009104 Suppl 1S60
- BrancoJCCherinPSpäthMMainguyYLong-term therapeutic response to milnacipran treatment for fibromyalgia. A European 1-year extension study following a 3-month study [abstract]Arthritis Rheum200960Suppl 10S529
- GendreauJPalmerRHThackerKMilnacipran is safe and well tolerated in the treatment of fibromyalgia [poster presentation]Presented at the 27th Annual Scientific Meeting of the American Pain SocietyMay 8–10, 2008Tampa, Florida
- PericlouAPalmerRHZhengHLindamoodCEffects of milnacipran on cardiac repolarization in healthy participantsJ Clin Pharmacol201050442243320103694
- Pfizer IncLyrica package insert42009 Available from www.lyrica.comAccessed December 8, 2009
- FergusonJMSSRI antidepressant medications: adverse effects and tolerabilityPrim Care Companion J Clin Psychiatry201031222715014625
- RussellIJPerkinsATMichalekJESodium oxybate relieves pain and improves function in fibromyalgia syndrome: a randomized, double-blind, placebo-controlled, multicenter clinical trialArthritis Rheum200960129930919116896
- RussellIJAlvarez-HorineSZhengYGuintaDRHolmanAJSwickTJEffect of sodium oxybate on pain, PGIc, and composite scores in fibromyalgia – results from a phase 3 controlled trial [abstract]Arthritis Rheum200960Suppl 10S528
- GendreauMRClauwDJPalmerRHEfficacy of milnacipran in the treatment of fibromyalgia among patients with varying degrees of depressed mood [poster presentation]Presented at the 161st Annual Meeting of the American Psychiatric AssociationMay 3–8, 2008Washington, DC, USA
- BoomershineCSFirst pregabalin and now duloxetine for fibromyalgia syndrome: closer to a brave new world?Nat Clin Pract Rheumatol200841263663718982001
- MeasePJSeymourKFibromyalgia: should the treatment paradigm be monotherapy or combination pharmacotherapy?Curr Pain Headache Rep200812639940518973731
- US National Institutes of Health, Clinical Trials Registry Available from www.clinicaltrials.govAccessed December 8, 2009
- BuskilaDSarzi-PuttiniPAblinJNThe genetics of fibromyalgia syndromePharmacogenomics200781677417187510