Abstract
Objective
To summarize and review the utility of physical interventions in the treatment of psychiatric disorders.
Methods
A systematic review of the literature pertaining to novel physical interventions, namely, transcranial magnetic stimulation, deep brain stimulation, vagus nerve stimulation, and neurosurgery, was conducted using MEDLINE, EMBASE, and PSYCHLIT. Bibliographies of papers were scrutinized for further relevant references along with literature known to the authors.
Results
Currently available physical interventions worldwide are reviewed with respect to efficacy, applications, and putative indications. Physical interventions have experienced a resurgence of interest for both the investigation of brain function and the treatment of neuropsychiatric disorders. The widespread availability of neuroimaging technology has advanced our understanding of brain function and allowed closer examination of the effects of physical treatments. Clinically, transcranial magnetic stimulation seems likely to have a role in the management of depression, and its use in other neuropsychiatric disorders appears promising. Following on from its success in the management of intractable epilepsy, vagus nerve stimulation is undergoing evaluation in the treatment of depression with some success in refractory cases. Deep brain stimulation has improved mood in patients with Parkinson’s disease and may also relieve symptoms of obsessive-compulsive disorder. Neurosurgery has re-invented itself by way of increased technical sophistication, and although further assessment of its efficacy and clinical utility is still needed, its widespread practice reflects its increasing acceptance as a viable treatment of last resort.
Conclusion
It is clear that physical treatments are here to stay and “getting physical” offers a useful addition to the neuropsychiatrist’s therapeutic armamentarium. However, like all new treatments these interventions need to remain under rigorous scientific scrutiny to determine accurately their immediate and long-term effects.
Introduction
The development of psychopharmacological treatments for psychiatric disorders has made physical interventions less popular, evidenced by the marked decline in neurosurgery since its hey-day in the 1960s. Many psychiatric patients are resistant to medications or are unable to tolerate their side-effects, and therefore novel treatments for neuropsychiatric disorders are necessary. In the last decade, several new physical treatments have been introduced that hold the potential to join the mainstream of psychiatric therapy. In this paper we review the efficacy of transcranial magnetic stimulation (TMS), vagus nerve stimulation (VNS), and deep brain stimulation (DBS) and the current status of neurosurgery which, although not new, continues to be novel among neuropsychiatric treatments. Light therapy was excluded from this review as much of the literature pertaining to it is linked with seasonal affective disorder and it does not generally come under the rubric of “physical treatments” in neuropsychiatry.
Method
The literature was reviewed selectively by entering the search terms “transcranial magnetic stimulation”, “vagus nerve stimulation”, “deep brain stimulation”, “psychosurgery”, and “neurosurgery” into MEDLINE, EMBASE, and PSYCHLIT. To identify more-specific articles the names of some of the surgical procedures, eg, “stereotactic subcaudate tractotomy”, “capsulotomy”, “limbic leucotomy”, and “cingulotomy”, were entered. As few randomized controlled trials have been possible or indeed attempted using VNS, DBS, and neurosurgery, the use of strict criteria such as inclusion of placebo or blinding status was not possible and studies were hence included based on their clinical salience. We also included industry-sponsored trials as although these include an interest on the part of the sponsor, they have withstood quality control and audit by bodies such as the FDA and often have attracted the largest numbers of participants.
Transcranial magnetic stimulation (TMS)
TMS is performed by placing an electromagnetic coil on the scalp through which large currents are pulsed to generate rapidly fluctuating magnetic fields. These cross the scalp unimpeded and generate eddy currents in the underlying brain cortex that depolarise neurons and produce an associated effect (CitationRoth et al 1991) (see ). The neural response is contingent upon a number of variables, such as the site of application, the stimulation parameters, and the use of single or multiple stimuli (CitationCohen et al 1990). The application of repeated TMS pulses to a particular site is termed repetitive TMS (rTMS) and the application at frequencies above 1Hz is referred to as fast-frequency repetitive TMS (FF-rTMS). The latter has been most investigated in neuropsychiatric treatment studies.
Figure 1 Illustration of brain regions affected by the physical treatments transcranial magnetic stimulation (a), vagus nerve stimulation (b), deep brain stimulation (c), and neurosurgery (d).
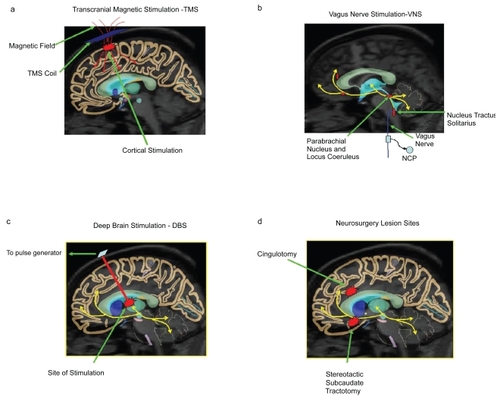
TMS in neuropsychiatric disorders
The rapid and widespread implementation of TMS over the last 20 years has generated considerable debate about the effects and implications of using this noninvasive method of brain stimulation.
Depression
In preclinical studies with rats TMS shows an effect similar to that produced by antidepressants and electroconvulsive shock (CitationBen-Shachar et al 1997; CitationLisanby and Belmaker 2000; CitationKeck et al 2001; CitationLevkovitz and Ng 2001). There have also been findings that support it having an antidepressant action in humans, including reports that it delays the onset of rapid eye movement (REM) sleep in healthy subjects (CitationCohrs et al 1998) and normalizes the dexamethasone suppression test in depressed patients (CitationPridmore 1999).
Guided by neuroimaging studies, the prefrontal cortex was proposed as a suitable target for antidepressant rTMS (CitationGeorge et al 1995), and initial studies of focal rTMS of the left prefrontal cortex (PFC) seemed encouraging (CitationPascual-Leone et al 1996). Comparison across studies has been difficult because of differences in patient populations, study design, duration, and rTMS parameters. A summary of sham-controlled treatment trials of rTMS is shown in .
Table 1 TMS/sham controlled studies
There have been several meta-analyses of these placebo-controlled trials of rTMS for major depression. The Cochrane Collaboration (CitationMartin et al 2003) concluded that two weeks of high-frequency rTMS to the left dorsolateral prefrontal cortex led to significant improvement on the Hamilton Depression Rating Scale (HDRS) (CitationHamilton 1960), but not on a self-rating scale such as Beck (CitationBeck 1961). This improvement was not sustained at 2-week follow-up. The authors concluded that the published evidence for an antidepressant effect of rTMS was weak. CitationBurt et al (2002) conducted an independent meta-analysis of treatment studies, which included controlled and uncontrolled, blind, and open study designs. Analysis of effects in uncontrolled studies found a consistent but modest treatment effect (37% mean reduction in HDRS) and for controlled studies the magnitude of change was even smaller (27% mean reduction in HDRS for TMS group, compared with 7% reduction for sham treatment). In summary, meta-analyses show that statistical evidence for the efficacy of rTMS is fairly robust but that clinical outcomes are modest. An important caveat, though, is that most of the trials to date have only compared rTMS to a sham control over a 2-week period, whereas evidence from trials allowing longer treatment periods, either in an open extension (eg, CitationLoo et al 1999) or within the controlled phase (CitationRumi et al 2005), suggests further improvement occurs with increasing duration of rTMS.
A number of studies randomized depressed subjects to receive electroconvulsive therapy (ECT) or 4 weeks of rTMS. These reported a clear advantage (CitationGrunhaus et al 2000) or emerging trends (CitationPridmore et al 2000; CitationJanicak et al 2002) in favour of ECT, though one study found no difference (CitationGrunhaus et al 2003). Of interest, the results of CitationGrunhaus et al (2000) suggested that rTMS may be equally effective as ECT for treating nonpsychotic depression, but had little efficacy in psychotic depression.
Investigators in the field concur that there still remains a need for large multi-center trials with longer sham-controlled periods and greater scientific rigor including appropriate and documented randomization and assessment as well as increased monitoring of both the patient and the therapeutic characteristics that modulate treatment outcome (CitationHoltzheimer et al 2004).
Another experimental application of TMS has been magnetic seizure therapy (MST), the use of rTMS at high stimulus frequency and intensity to deliberately induce a generalized seizure under anesthesia for the treatment of depression (CitationMorales et al 2004). MST stimulation is more focal than that of ECT, thus leading to the expectation of fewer cognitive side effects. So far, preliminary trials in nonhuman primates and human subjects have confirmed this expectation, while case reports have found that MST led to significant improvement in two medication-resistant depressed subjects (CitationMorales et al 2004).
Bipolar disorder
There have been a number of case reports of the induction of mania in depressed subjects receiving rTMS treatment (CitationGarcia-Toro 1999; CitationDolberg et al 2001; CitationSakkas et al 2003; CitationSu 2005;CitationHausmann et al 2004b). CitationGrisaru et al (1998a) published the first trial of rTMS for the treatment of acute mania, randomizing patients to receive high-frequency rTMS to the left or right prefrontal cortices. Right prefrontal stimulation appeared to have an antimanic effect with 71% mean improvement on the Young Mania Rating Scale (CitationYoung et al 1978), whereas left prefrontal stimulation was associated with 29% mean improvement. Taken together with the results of depression trials demonstrating efficacy for high-frequency left prefrontal rTMS, these findings suggest that rTMS to the left and right prefrontal cortices respectively may have opposing effects. In further open studies, CitationSaba et al (2002) and CitationMichael and Erfurth (2004) also reported that rTMS may be a useful add-on treatment to medication in acute mania. However, the only sham-controlled study of rTMS in mania, which used the exact same rTMS parameters as in the study by CitationGrisaru et al (1998a), failed to find any advantage for right prefrontal rTMS over sham (CitationKapstan et al 2003).
Schizophrenia
Left prefrontal high-frequency rTMS and right prefrontal low-frequency rTMS have also been trialled as treatments in patients with schizophrenia (CitationCohen et al 1999; CitationKlein et al 1999; CitationRollnik et al 2000; CitationHajak et al 2004; CitationHoli et al 2004). Results have shown variable degrees of improvement in positive and negative symptoms and in mood. The inconsistent findings of the sham-controlled studies (CitationKlein et al 1999; CitationRollnik et al 2000; CitationHajak et al 2004; CitationHoli et al 2004) can perhaps be partly explained by the effect of interaction in that daily attendance required by the rTMS treatment protocol may have accounted for some of the changes observed, particularly any reduction in negative symptoms.
Some sham-controlled studies have reported that slow TMS over the left auditory cortex can reduce auditory hallucinations (CitationHoffman et al 2000, Citation2003; CitationPoulet et al 2005). Others have failed to find any significant effects (CitationMcIntosh et al 2004; CitationSchonfeldt-Lecuona et al 2004) despite the use of individual fMRI to specifically identify and target the cortical sites for inner speech production, thought to be involved in the production of hallucinations. Some of the inconsistencies in findings may also be explained by a lack of consensus for rating of auditory hallucinations.
Other psychiatric disorders
There have been preliminary reports examining the use of rTMS in the treatment of other psychiatric disorders such as obsessive-compulsive disorder (OCD) (CitationSachdev et al 2001a; CitationAlonso et al 2001), post traumatic stress disorder (CitationGrisaru et al 1998; CitationMcCann et al 1998a; CitationCohen et al 2004), and Tourette’s syndrome (CitationChae et al 2004). There have also been reports of high-frequency rTMS exacerbating anxiety and panic (CitationGreenberg et al 1997) and low-frequency rTMS alleviating panic disorder (CitationZwanzger et al 2002). The role of rTMS in these disorders is at present unclear.
Neurological disorders
TMS has been investigated particularly in patients with Parkinson’s disease and epilepsy. In Parkinson’s disease, rTMS has been administered in an attempt to treat bradykinesia and improve motor control. During movement, simultaneous stimulation of the motor cortex has been reported to be of no benefit (CitationGhabra et al 1999) and stimulation of the supplementary motor area has been shown to worsen fine movements (CitationCunnington et al 1996). Other studies stimulating the PFC have noted modest benefits (CitationSommer et al 1998; CitationShinamoto et al 1999; CitationSiebner et al 1999), some researchers suggesting that rTMS can diminish bradykinesia and enhance motor speed (CitationSommer et al 1998; CitationSiebner et al 1999).
Interestingly, the application of TMS is associated with a measurable risk of seizure induction and yet, paradoxically, it has been found to be relatively safe in patients with epilepsy (CitationTassinari et al 1990). Indeed, it may in fact be an effective means of reducing seizure activity (Tegaru et al 1999; CitationMenkes and Gruenthal 2000; CitationWerhahn et al 2000), although further research is needed.
Investigational applications of TMS
In cognitive neuroscience TMS has proven to be a versatile and valuable investigational tool, and has been used to examine cortical excitability and various aspects of brain cognition. It has been applied to the motor cortex in humans to examine motor evoked potentials (MEP) and motor threshold (MT). MT is the stimulus intensity required to elicit MEP and in any given individual is relatively constant with interhemispheric differences, but is subject to modulation by drugs and disease.
Paired pulse TMS (ppTMS)
Paired pulse TMS involves the application of a pair of stimuli separated by a variable inter-stimulus interval. The first stimulus is subthreshold whereas the second is above the threshold. Varying the inter-stimulus interval allows modulation of the overall response to the paired stimuli such that response can be facilitated or inhibited. Facilitation can usually be achieved with an inter-stimulus interval of 10–20 msec, and on either side of this (shorter or longer), inhibition is more likely. Neurochemically, inhibition and facilitation are mediated by gamma-amino butyric acid (GABA) and glutamate respectively.
When a TMS pulse elicits a MEP against a background of voluntary muscle contraction, the background activity is suppressed for a period after the MEP. This “silent period” is considered another measure of inhibitory cortical activity (CitationPascual-Leone et al 2002a).
Psychiatric disorders
The above TMS testing paradigms have been used to investigate abnormalities in cortical inhibitory and facilitatory processes in the motor cortex of patients with psychiatric disorders (CitationMaeda and Pascual-Leone 2003). In schizophrenia, abnormalities have been reported in the MEP response to single pulse TMS (CitationAbarbanel et al 1996; CitationPuri et al 1996), and in silent period measurements and response to paired pulse stimulation (CitationDaskalakis et al 2001; Fitzgerald et al 2001), suggesting a reduction in cortical inhibitory processes. However, these investigations were mostly done in medicated patients, and alterations in motor cortical functioning in the presence of antipsychotic medication have been demonstrated elsewhere (CitationZiemann et al 1997; CitationPascual-Leone et al 2002b).
Several studies have reported increased cortical inhibition (CitationSteele et al 2000) or reduced post-exercise cortical facilitation in depressed subjects (CitationSamii et al 1996; CitationShajahan et al 1999), though others have reported that the latter findings appeared to be nonspecific, occurring also in patients with mania and schizophrenia (CitationChroni et al 2002). There are also preliminary reports of abnormalities in motor cortical functioning in OCD (CitationGreenberg et al 2000), and Tourette’s syndrome (CitationZiemann et al 1997).
Cognition
TMS is increasingly being used in neuropsychological investigations (eg, CitationLi et al 2004). During TMS or rTMS, functioning of the stimulated cortical area can be temporarily disrupted, creating in effect a “virtual lesion”. This technique can then be applied to examine the cortical sites involved in a particular function and the critical time periods of their involvement (CitationPascual-Leone et al 1999). For example, rTMS can be used to noninvasively identify the lateralization of verbal functions (Epstein 1998). Studies of the frontal and prefrontal cortex have examined aspects of memory and word generation (CitationGrafman et al 1994; CitationJahanshahi et al 1998). For example, CitationDevlin et al (2005) have recently demonstrated that TMS-induced interference in the left inferior prefrontal cortex has an effect on semantic but not perceptual processing. TMS can also enhance neuropsychological functioning when administered to specific cortical areas with precise timing, eg, picture naming (CitationTopper et al 1998).
Clearly, this innovative field of research has expanded tremendously with the introduction of TMS as an investigative tool and is an area of research that is likely to yield many new insights into the functioning of the normal and diseased brain.
Overall, TMS remains a useful tool for the investigation of abnormal physiological processes in psychiatric disorders. Further research in larger samples of unmedicated subjects is needed before definitive conclusions can be made.
Adverse effects
TMS has few adverse effects as it is relatively noninvasive. The risks that are associated with its use are determined largely by the number, intensity, and frequency of stimuli applied. Occasionally, rTMS results in a headache that may last several hours. Some subjects complain of scalp discomfort during TMS, attributable to the associated stimulation of muscles and nerves near the coil. Low frequency TMS and ppTMS are unlikely to produce seizures, or have any lasting effects on cognition, but rTMS, by increasing cortical excitability, can precipitate seizures even in healthy subjects, though the risk is very low (CitationWassermann 1998).
It is important to note that TMS can cause a temporary shift in auditory threshold and to protect patients from this during stimulation a set of precautionary earplugs should be worn (CitationWassermann 1998).
Vagus nerve stimulation (VNS)
The vagus nerve or the Xth cranial nerve is a mixed nerve, composed predominantly of sensory afferents carrying information from the thorax, abdomen, head, and neck to the brain. Cell bodies of these vagus-sensory afferents lie predominantly in the nodose ganglion and project information primarily to the nucleus tractus solitarius (NTS) as well as the area postrema, the spinal trigeminal nucleus, the medullary reticular formation, the dorsal nucleus of the vagus, and the nucleus ambiguous (CitationHenry 2002). Information is conveyed via these direct projections and by an autonomic feedback loop to the rest of the brain and ascending projections to the forebrain which travel via the parabrachial nucleus and the locus coeruleus. These structures have direct connections with the forebrain, thalamus, hypothalamus, amygdala, and stria terminalis – regions that are important in the modulation of mood (CitationGeorge et al 1997b; CitationVan Bockstade et al 1999). Vagus nerve projections attend brain regions that are thought to be involved in several neuropsychiatric disorders presenting the potential for VNS to have several important clinical roles in addition to its use in treating epilepsy and depression.
In humans, VNS involves stimulation of the left cervical vagus nerve using a commercial device – the Neuro-Cybernetic Prosthesis (NCP) System (CitationSchacter and Saper 1998). This device comprises a bipolar pulse generator, electrodes, and a programming wand. The generator is implanted in the left chest wall below the clavicle and delivers electrical signals via a bipolar lead to an electrode that is wrapped around the vagus nerve in the neck (see ). Implantation usually takes less than an hour and can be conducted under general or local anesthesia (CitationAmar et al 1998). On-line modification and data retrieval are possible as a proprietary instrument is used to program the pulse generator. The latter can be stopped briefly using a hand held magnet but stimulus interruption does not interfere with preprogrammed stimulation which automatically resumes when the magnet is displaced (CitationGeorge et al 2000b).
VNS reduces the excitability of neurons involved in the propagation of seizure activity (CitationZagon and Kemeny 2000); however, its exact mechanisms of action remain unknown though it is thought to produce slow hyperpolarization. EEG and neuroimaging data, using single photon emission computed tomography, implicate the thalamus (CitationRing et al 2000; CitationVonck et al 2000), inhibition of which may prevent the onset or propagation of seizures (CitationVan Laere et al 2000).
Efficacy
To date, the main use of VNS has been to reduce seizure frequency in both adults (CitationBen-Menachem et al 1994; CitationHandforth et al 1998; CitationMorrow et al 2000; CitationWakai and Kotagal 2001) and children (CitationPatwardhan et al 2000; CitationWakai and Katagal 2001) with treatment-resistant epilepsy. Improvements gained appear to be sustained and may continue with time (CitationSalinsky et al 1995; CitationDeGiorgio et al 2000).
Two studies have shown an improvement in mood in epileptic patients receiving VNS compared with controls (CitationHarden et al 1999; CitationHoppe et al 2001) and such observations have led researchers to hypothesize that it may be effective in treating mood disorders (CitationHarden et al 1999; CitationElger et al 2000). The fact that it increases central noradrenergic and serotonergic neurotransmission would be in keeping with such a hypothesis (CitationKrahl et al 1998; CitationJobe et al 1999), and in common with other effective antidepressant therapies it alters limbic system blood flow, involving in particular the cingulate (CitationHenry et al 1999).
Open studies to examine the effects of VNS on mood have been conducted in treatment-resistant depressed patients (CitationRush et al 2000; Sackeim et al 2001). In a multi-center study, 30 depressed patients (n = 21; nonpsychotic treatment-resistant major depression, n = 4; bipolar, and n = 5 bipolar II) underwent 10 weeks of VNS after which 40% of patients reported having a significant reduction in mood-scale scores (Ham-D and MADRS). These findings were sustained during long-term follow-up (CitationRush et al 2000). Further preliminary studies (CitationKosel and Schlaepfer 2003) also suggest that VNS has antidepressant properties; however, there is a need for larger controlled trials and at least one is currently underway (CitationSchlaepfer and Kosel 2004). Several recently published studies provide preliminary support for VNS having an antidepressant role. Firstly, in a multicenter trial CitationRush et al (2005a) compared the effects of 10 weeks’ active as opposed to sham VNS in 222 participants with treatment-resistant depression. They found VNS was linked with greater symptom reduction across measures but the finding did not reach significance and hence could not be considered definitive evidence of its efficacy. This group subsequently published further results of a one-year open trial involving patients who had completed the initial acute phase (CitationRush et al 2005b). The results from 205 participants in this study revealed statistically significant reduction in depressive symptoms and despite the lack of a control group and nonmasking of ratings, these data seem to support further investigation of VNS as an antidepressant. Finally, CitationGeorge et al (2005) compared the results from the one-year open trial with the results from a comparable treatment-as-usual (TAU) group. The primary analysis in this study yielded a significant difference between the groups favoring VNS plus TAU over TAU alone. The addition of VNS to TAU resulting in improved response supports its role as an antidepressant, although this still requires further investigation.
VNS was initially approved in the US by the FDA for the treatment of epilepsy in 1997 and just recently (July 2005) the VNS therapy system has been approved to treat depressed patients of 18 years and over who have not had a response to four or more trials of an antidepressant (CitationCyberonics website 2005). It has also been approved for use in the treatment of depression in the EU and Canada. It would be useful to extend VNS trials to include depressed patients other than those who are treatment resistant, in order to examine its differential efficacy across depressive subtypes and also its effects on cognition (CitationSchacter 2004).
Adverse effects
The adverse effects of VNS are, firstly, those associated with the procedure of implantation and, secondly, those that occur as a consequence of stimulation. Surgical adverse effects include pain, coughing, left-vocal cord paralysis, hoarseness, nausea, and very occasionally infection. Most patients describe these as a moderate inconvenience and the effects are usually transient (CitationSchachter and Saper 1998). Of note, during implantation, there have been no deaths and no reports of serious adverse events such as the alteration of cardiac or pulmonary function. However, transient asystole has occurred in a small number of patients when the stimulator is first activated during in-theatre testing. The most significant stimulation-related adverse effects are those of dyspnea and voice-alteration (CitationCharous et al 2001), which can be reversed by application of the hand-held magnet and prevented by lowering the stimulation current (CitationSchachter and Sapel 1998).
Deep brain stimulation (DBS)
In the management of Parkinson’s disease (PD) and other movement disorders DBS is an important treatment that appears to have an additional antidepressant effect without causing any global cognitive deterioration (CitationFunkiewiez et al 2004). In PD, neural degeneration and transmitter deficiencies lead to neural dysfunction and abnormal activity in motor system relays such as the thalamus, the internal segment of the globus pallidus (GPi) (CitationMiller et al 1987), and the subthalamic nucleus (STN) (CitationWichmann et al 1994), with the last emerging as the most popular target for treatment interventions (CitationBreit et al 2004).
Using implanted quadripolar electrodes connected to a battery-powered pulse-generating device (see ), DBS delivers an electrical current, the strength of which can be adjusted by varying electrode selection and polarity and by altering frequency, amplitude and pulse-width. In the treatment of PD, parameters that are typically used include a voltage of more than 3 V with a pulsewidth of 60–90 μsec and a frequency of 150–185 Hz (CitationLozano 2001).
The mechanism of action of DBS, like other physical treatments, is unknown but both clinical and experimental evidence indicate that the frequency of stimulation affects clinical outcome. CitationBreit et al (2004) outline the main hypotheses that account for the benficial effects of high frequency stimulation as follows: the effect is due to depolarization blocking neuronal transmission through inactivation of voltage-dependent ion channels; the jamming of information imposes an efferent stimulation-driven high frequency pattern; stimulation of inhibitory afferents results in synaptic inhibition to the target nucleus; and stimulation induces neurotransmitter depletion, and hence causes synaptic depression. This explanation is corroborated to some extent by animal models of PD in which there is increased basal spontaneous activity in the STN that drives GPi nucleus inhibitory outflow. The outflow in turn inhibits motor systems within the thalamus, brainstem, and cortex and results in the akinesia and bradykinesia of PD (CitationDeLong and Wichmann 2001). Hence the rationale for surgical intervention is to interrupt the excessive inhibition from these nuclei (CitationLang et al 1999). Electrical stimulation achieves this by producing neuronal inactivation either by direct disruption of neuronal activity or by increasing GABA-mediated inhibitory neurotransmission. Interestingly, the afferents that impinge upon neurones in the GPi nucleus and STN are rich in GABA (see ).
Indications for DBS in PD include advanced idiopathic illness with motor complications, tremor, and related disability. With GPi or STN DBS the symptoms of PD can be improved by up to 80% and in many cases the use of concurrent medications can be significantly reduced if not stopped altogether (CitationLimousin et al 1998; CitationVolkmann et al 1998). Vim thalamus DBS has a greater specificity of action and is effective in alleviating the tremor of PD in up to 80% of patients; however, unlike DBS applied to the GPi and STN, it fails to produce significant functional improvement and has few additional benefits (CitationSchuurman et al 2000) such as alleviating motor fluctuations, bradykinesia, gait disturbances, and drug-induced dyskinesias. Not surprisingly, GPi and STN DBS are being increasingly favored and there is now almost no indication for Vim thalamus DBS as these treatments are far superior in effect. The disadvantage of this trend is that speech and cognition are less responsive and some of the problems that patients face can in fact be exacerbated by surgery (CitationLimousin et al 1998).
Efficacy
As a treatment DBS has the advantage of being precise and reversible (CitationGreenberg and Rezai 2003), a clear advance in comparison to neurosurgery for intractable psychiatric disorders (CitationRees Cosgrove 2004; CitationKopell et al 2004). Furthermore, the strength of stimulation can be controlled allowing treatment to be adjusted according to individual needs, which has made it an increasingly popular choice for the treatment of refractory PD.
DBS has also been found to be beneficial in two of three cases of treatment refractory OCD described by CitationGabriels et al (2003). CitationAouizerate et al (2004) describe DBS of the ventral caudate nucleus as effective in improving functioning and achieving remission in a patient with intractable severe OCD and concomitant major depression. CitationLonzano and Hamani (2004) report an increase in the number of applications for DBS, indicating recognition of its potential for treating neuropsychiatric disorders as well as its continued importance in the treatment of movement disorders.
Adverse effects
Predictably, the adverse effects associated with DBS are largely the consequence of surgery as opposed to stimulation. One of the most common side-effects is that of transient confusion; More serious side-effects can also occur although the likelihood is less than 2%. Stimulation is also associated with speech disturbance, paraesthesiae, eye movement difficulties, and motor contractions. Complications can also arise because of device failure; however, this is easily remedied by replacement of the necessary components.
Neurosurgery
Psychosurgery has been “practised” since antiquity involving trephination thought to release “evil spirits”. At the beginning of the twentieth century neurosurgery experienced a resurgence of interest; however, the development of psychotropic medications largely eliminated the need for standard prefrontal leucotomy (CitationFreeman and Watts 1942), which was used to “treat” schizophrenia and affective psychoses (CitationTooth and Newton 1961). However, neurosurgery for mental disorders has survived in modified form and in many parts of the world remains a treatment option for neuropsychiatric illnesses where all other options have been exhausted.
Modern-day neurosurgical procedures, although more refined and sophisticated, continue to target the limbic system and its connections, particularly frontal lobe circuits that involve striatal structures such as the thalamus and caudate (CitationCummings 1993). Currently, four neurosurgical procedures are performed worldwide namely: cingulotomy, stereotactic subcaudate tractotomy (SST), anterior capsulotomy (AC), and limbic leucotomy (LL) (CitationMalhi et al 1997), with the last in essence combining the lesions of the first two (CitationKelly et al 1973) (see ). The indications, ablative techniques, lesion sites, and targets of these procedures are summarized in .
Table 2 Neurosurgical procedures used in the treatment of neuropsychiatric disorders
It is noteworthy that all three neurosurgical lesions interrupt the interconnecting pathways of the limbic system and the prefrontal cortex, in particular the amygdalofugal pathways and those of the limbic loop, explaining perhaps the overlapping effects of these procedures.
Efficacy
Psychosurgery for psychiatric disorders is almost always considered a treatment of last resort and as a consequence psychiatric patients that undergo surgical procedures are by definition treatment refractory. Nevertheless, in countries where these procedures are available, strict regulations govern the selection and consent of patients to ensure that all reasonable alternatives have been adequately explained (CitationClinical resource audit group 1996).
In the treatment of OCD, psychosurgery has been reasonably effective with significant improvement in 40%–60% of cases undergoing anterior capsulotomy (CitationMindus and Nyman 1991; CitationMindus and Jenike 1992; CitationRasmussen et al 2000). In comparison, SST has generally been less helpful in OCD, with improvement occurring in only a third of patients (CitationHodgkiss et al 1994); however, it is effective in mood disorders, with more than a third of patients achieving a good outcome (CitationHodgkiss et al 1994; CitationMalizia 1994; CitationPoynton et al 1995; CitationMalhi and Bartlett 2000). Recently, the mechanism of action of SST in depression has been investigated (CitationDalgleish et al 2004), with improvement thought to occur by way of an acquired insensitivity to negative information. This interesting but provocative suggestion warrants further investigation.
In addition to its use in OCD, capsulotomy has also been used to treat refractory social phobia, generalized anxiety disorder, and panic disorder. Long term it appears to produce a notable reduction in anxiety, with 67% of subjects showing significant response (CitationRuck et al 2003); however, concerns have been raised as to whether the procedure causes frontal lobe dysfunction, which may be masked by seeming improvement.
In contrast to capsulotomy and SST, cingulotomy introduced by Fulton and refined by Ballantine et al (1997) (CitationMashour et al 2005) has been used almost exclusively to treat OCD with reasonable success in approximately one third of patients (CitationJennike et al 1991), and because of the conservative nature of the procedure many patients benefit from a second operation to extend the original lesion. However, worsening of obsessionality has also been described, with one study reporting pre-operative obsessive traits predisposing epileptic patients to develop OCD (CitationKulaksizoglu et al 2004).
Limbic leucotomy has been less widely used than the other procedures but has been utilized to treat a broad range of symptoms with modest success (treatment response of 36%–50%) in patients with major depression and OCD (CitationMontoya et al 2002).
Adverse effects
All surgery confers some degree of risk and this is particularly true of neurosurgery in as the operation involves structural reorganization of cortical matter. The most common complaints following SST are confusion, a transient lack of sphincter control, and lethargy, with the latter persisting beyond one week in 12% of patients (CitationMalhi et al 1997). These are similar to the adverse effects associated with AC except that no deaths have been reported and documented seizures have been rare. Similarly, cingulotomy has not resulted in any reported deaths, and reports of seizures are scarce; however, it has caused two cases of hemiplegia (CitationBallantine et al 1987; CitationMarino Junior and Cosgrove 1997).
Despite the relative safety of these procedures, concerns remain about the effects of neurosurgical procedures on personality and behaviour (CitationHappe et al 2001) and there is some evidence to suggest that frontal lobe deficits such as disinihibition, apathy, and cognitive inflexibility have occurred following cingulotomy (CitationIrle et al 1998; CitationRuck et al 2003). CitationCumming et al (1995) also found no differences between post-surgical patients and controls on tests of memory and global ability; however, there were deficits present in formation and shifting of set. CitationBejerot (2003) makes the point that many patients are asked to self-report on symptoms post-operatively and that the validity of information gathered in this context is questionable, especially if patients have compromised frontal lobe function.
Discussion
Limited knowledge of the neuropsychiatric disorders themselves, as well as the mechanisms underpinning physical treatments, continues to hamper growth in this field that is further constrained by a tendency for polarization of opinions in relation to its evidence base and ethics. Thus far, TMS is the only physical treatment that has acquired a firm footing, with studies demonstrating its efficacy in the treatment of depression. Equally valuable is its application into research where it has been used to create “virtual lesions”. Its relative noninvasiveness and acceptance by patients have made it a popular therapy.
More invasive treatments such as DBS and VNS offer greater control than neurosurgery by allowing stimulation to be titrated to achieve an optimal response. In some cases the specificity of these interventions makes them preferable to pharmacotherapy which, although more widely acceptable, is relatively blunt as regards site of action and timing of effect. With respect to the latter, DBS produces effects over a matter of milliseconds, in tune with the electrophysiology of the brain. It is therefore potentially capable of mimicking normal physiological function and its use in the management of PD has paved the way for wider application. Initial studies of its efficacy in the treatment of refractory psychiatric illnesses are encouraging. Similarly, the application of VNS has expanded beyond its role as an “anticonvulsant” to studies examining its antidepressant properties. A recent study indicated 15.5% of patients with treatment-resistant depression achieved sustained remission compared with 4.6% of the participants receiving treatment as usual (CitationGeorge et al 2005). Recently, it has been approved as an antidepressant treatment in the EU and Canada, and this opens up the possibility of its application in a range of psychiatric illnesses including pain syndromes, addictions, and eating disorders.
Somewhat surprisingly neurosurgery has regained popularity as an effective treatment for refractory psychiatric disorders, even though it is still considered only following the failure of traditional methods. Part of the reason for this is its apparent success in a patient population that has a very low rate of placebo response. However, psychosurgical research has been plagued by the lack of standardized nosology and the inherent referral bias, as patients undergoing such procedures are clearly not representative of the respective phenotypes to which they belong. Clearly, the fact that few procedures are performed and they are necessarily invasive limits the prospects of sham-controlled prospective studies. Improvements in technology and surgical techniques with increasing sophistication of instrumentation will likely make such studies possible in the future. However, in the interim research along the lines of CitationDagliesh et al (2004) has shed light on the specific cognitive processes altered by such procedures and is likely to inspire greater clinical confidence.
Conclusion
In the past decade physical interventions have once again captured the imagination of neuroscientists and clinicians alike. However, in comparison with pharmacological treatments they remain under-researched due to a range of political, economic, and sociological factors. It is also difficult to compare efficacy rates of pharmacologically treated patients with those who receive physical interventions as the characteristics of the two groups vary substantially in terms of resistance, duration of illness, and prior treatment. Despite this, these treatments provide novel insights into the neurobiology of these neuropsychiatric disorders and interventions such as TMS are beginning to establish themselves as viable therapeutic options. However, if history is not to repeat itself, the field has to be cautious in its predictions and claims. Key advances in neuroimaging, for instance, have ensured much better localization and monitoring of the effects of these interventions but the longer-term effects have yet to be determined. In reality, each of the physical treatments described requires much further clinical investigation. Fortunately, this is at last a possibility and many researchers have taken up the challenge. It is hoped that in the coming decade some of these interventions in their modified forms will become more widely accepted as mainstream treatments and benefit the many patients with intractable neuropsychiatric disorders.
References
- AbarbanelJMLembergTYaroslavskiU1996Electrophysiological responses to transcranial magnetic stimulation in depression and schizophreniaBiol Psychol4014850
- AlexanderGEDeLongMRStrickPL1986Parallel organization of functionally segregated circuits linking basal ganglia and cortexAnnu Rev Neurosci935781
- AlexanderGECrutcherMDDeLongMR1990Basal gangliathalamocortical circuits; parallel substrates for motor, occulomotor, ‘prefrontal’ and ‘limbic’ functionsProg Brain Res85119462094891
- AlonsoPPujolJCardonerN2001Right prefrontal repetitive transcranial magnetic stimulation in obsessive-compulsive disorder: a double-blind, placebo-controlled studyAm J Psychiatry1581143511431238
- AmarAPHeckCNLevyML1998An institutional experience with cervical vagus nerve trunk stimulation for medically refractory epilepsy: Rationale, technique, and outcomeNeurosurgery431265809848840
- AouizerateBCunyEMartin-GuehlC2004Deep brain stimulation of the ventral caudate nucleus in the treatment of OCD and major depressionJ Neurosurg101682615481726
- BallantineHTBouckomsAJThomasEK1987Treatment of psychiatric illness by sterotactic cingulotomyBiol Psychiatry22807193300791
- BartlettJRBridgesPKSweetWH1977Neurosurgical treatment in psychiatry, pain and epilepsyBaltimoreUniversity Park Press
- BeckATWardCHMendelsonM1961An inventory for measuring depressionArch Gen Psychiatry45617113688369
- Ben-MenachemEManon-EspaillatRRistanovicR1994Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizuresEpilepsia35616268026408
- Ben-ShacharDBelmakerRHGrisaruN1997Transcranial magnetic stimulation induces alterations in brain monoaminesJ Neural Transm10419179203081
- BerjerotS2003Psychosurgery for obsessive compulsive disorder-concerns reaminActa Psychiatr Scand107241312662245
- BermanRNarasimhanMSanacoraG2000A randomized clinical trial of repetitive transcranial magnetic stimulation in the treatment of major depressionBiol Psychiatry47332710686268
- BingleyTLeksellTMeyersonBALaitenenLVLivingstoneKE1973Stereotactic anterior capsulotomy in anxiety and compulsive statesSurgical approaches in psychiatryLancasterMedical and Technical Publishing Co15964
- BreitSSchulzJBBenabidAL2004Deep brain stimulationCell Tissue Res3182758815322914
- BurtTLisanbySHSackeimHA2002Neuropsychiatric applications of transcranial magnetic stimulation: a meta analysisInt J Neuropsychopharmacol57310312057034
- ChaeJHNahasZWassermannE2004A pilot safety study of repetitive transcranial magnetic stimulation (rTMS) in Tourette’s syndromeCogn Behav Neurol171091715453520
- CharousSJKempsterGMandersE2001The effect of vagal nerve stimulation on voiceLaryngoscope11120283111801991
- ChroniELekkaNPTsoussisI2002Effect of exercise on motor evoked potentials elicited by transcranial magnetic stimulation in psychiatric patientsJ Clin Neurophysiol19240412226569
- Clinical resource audit group (CRAG)1996Neurosurgery for mental disordersScotlandHMSO
- CohrsSTergauFRiechS1998High-frequency repetitive transcranial magnetic stimulation delays rapid eye movement sleepNeuroreport93439
- CohenEBernardoMMasanaJ1999Repetitive transcranial magnetic stimulation in the treatment of chroic negative schizophrenia: a pilot studyJ Neurol Neurosurg Pyschiatry6712930
- CohenHKaplanZKotlerM2004Repetitive transcranial magnetic stimulation of the right dorsolateral prefrontal cortex in post-traumatic stress disorder: A double blind, placebo controlled studyAm J Psychol16151524
- CohenLGRothBJNilssonJ1990Effects of coil design on delivery of focal magnetic stimulation. Technical considerationsElectroencephalogr Clin Neurophysiol7535071691084
- CummingSHayPLeeT1995Neuropsychological outcome from psychosurgery for obsessive –compulsive disorderAust N Z J Psychiatry2929387487794
- CummingsJL1993Frontal-subcortical circuits and human beahviourArch Neurol50873808352676
- CunningtonRIansekRThickbroomGW1996Effects of magnetic stimulation over supplementary motor area on movement in Parkinson’s diseaseBrain119815228673493
- Cyberonics website2005Accessed 9 September 2005 URL: http://www.cyberonics.com
- DalgleishTYiendJBramhamJ2004Neuropsychological processing associated with recovery from depression after stereo tactic subcaudte tractotomyAm J Psychiatry1611913615465992
- DaskalakisZJKapurSChristensenBK2001Direct demonstration of cortical inhibition in schizophrenia using TMSSchizophr Res49s2001
- DegiorgioCMSchachterSCHandforthA2000Prospective long-term study of vagus nerve stimulation for the treatment of refractory seizuresEpilepsia41119520010999559
- DeLongMRWichmannT2001Deep brain stimulation for Parkinson’s diseaseAnn Neurol49142311220732
- DevinskyOLucianoDVogtBA1993Neurobiology of cingulate cortex and limbic thalamusBostonBirkhauser
- DevlinJTRushworthMFMatthewsPM2005Category-related activation for written words in the posterior fusiform is task specificNeuropsychologica436974
- DolbergOTDannonPNSchreiberS2002Magnetic motor threshold and response to TMS in major depressive disorderActa Psychiatr Scand106220312197860
- DolbergOSchreibergSGrunhausL2001Transcranial magnetic stimulation-induced switch into mania: a report of two casesBiol Psychiatry494687011274660
- EbertDEbmeierKP1996The role of the cingulated gyrus in depression: from functional anatomy to neurochemistryBiol Psychiatry391044508780840
- ElgerGHoppeCFalkaiP2000Vagus nerve stimulation is associated with mood improvements in epilepsy patientsEpilepsy Res422031011074193
- EschweilerGWegererCSchlotterW2000Left prefrontal activation predicts therapeutic effects of repetitive transcranial magnetic stimulation (rTMS) in major depressionPsychiatry Res991617211068197
- FitzgeraldPBBrownTLDaskalakisZJ2002A transcranial magnetic stimulation study of inhibitory deficits in the motor cortex in patients with schizophreniaPsychiatry Res114112211864806
- FitzgeraldPBBrownTLMarstonNA2003Transcranial magnetic stimulation in the treatment of depression: a double-blind, placebo-controlled trialArch Gen Psychiatry601002814557145
- FreemanWWattsJW1942PsychosurgerySpringfieldCharles C Thomas
- FunkiewiezAArdouinCCaputoE2004Long term effects of bilateral subthalamic nucleus stimulation on cognitive function, mood, and behaviour in Parkinson’s diseaseJ Neurol Neurosurg Psychiatry75834915145995
- GabrielsLCosynsPNuttlinB2003Deep brain stimulation for treatment refractory obsessive compulsive disorder: psychopathological and neuropsychological outcome in three casesActa Psychiatr Scand1072758212662250
- Garcia-ToroM1999Acute manic symptomatology during repetitive transcranial magnetic stimulation in a patient with bipolar depressionBr J Psychiatry17549110789285
- Garcia-ToroMMayolAArnillasH2001Modest adjunctive benefit with transcranial magnetic stimulation in medication-resistant depressionJ Affect Disord64271511313095
- GeorgeMSWassermannEMWilliamsW1995Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depressionNeuroreport6185368547583
- GeorgeMSWassermannEMWilliamsW1997Mood improvements following daily left prefrontal repetitive transcranial magnetic stimulation in patients with depression. A placebo-controlled crossover trialAm J Psychol15417521756
- GeorgeMNahasZMolloyMA2000acontrolled trial of daily left prefrontal cortex TMS for treating depressionBiol Psychiatry489627011082469
- GeorgeMSNahasZBohningDE2000bVagus nerve stimulation: A new form of therapeutic brain stimulationCNS Spectr5435218188148
- GeorgeMSRushAJMarangellLB2005A one-year comparison of vagus nerve stimulation with treatment as usual for treatment-resistant depressionBiol Psychiatry583647316139582
- GhabraMBHallettMWassermannEM1999Simultaneous repetitive transcranial magnetic stimulation does not speed fine movement in PDNeurology527687010078725
- GoktepeEOYoungLBBridgesPK1975A further review of the results of stereoactic subcaudate tractotomyBr J Psychiatry1262708047773
- GrafmanJPasual-LeoneAAlwayD1994Induction of a recall deficit by rapid-rate transcranial magnetic stimulationNeuroreport52517207696593
- GreenbergBDGeorgeMSMartinJD1997Effect of prefrontal repetitive transcranial magnetic stimulation in obsessive-compulsive disorder: a preliminary studyAm J Psychiatry15486799167520
- GreenbergBDRezaiAR2003Mechanisms and the current state of deep brain stimulation in neuropsychiatryCNS Spectr8522612894033
- GreenbergBDZiemannUCora-LocatelliG2000Altered cortical excitability in obsessive compulsive disorderNeurology54142710636140
- GrisaruNAmirMCohenH1998aEffect of transcranial magetic stimulation in posttraumatic stress disorder: a preliminary studyBiol Psychiatry445259646883
- GrisaruNChudakovBYaroslavskyY1998bTMS in mania. A controlled studyAm J Psychiatry1551608109812128
- GrunhausLDannonPSchreiberS2000Repetitive transcranial magnetic stimulation is as effective as electroconvulsive therapy in the treatment of nondelusional major depressive disorder: an open studyBiol Psychiatry473142410686266
- GrunhausLShcriberSDolbergO2003A randomised controlled comparison of electroconvulsive therapy and repetitive transcranial magnetic stimulation in severe and resistant nonpsychotic major depressionBiol Psychiatry533243112586451
- HajakGMarienhagenJLangguthB2004High-frequency repetitive transcranial magnetic stimulation in schizophrenia: a combined treatment and neuroimaging studyPsychol Med3411576315697042
- HamiltonM1960A rating scale for depressionJ Neurol Neurosurg Psychiatry23566214399272
- HandforthADeGiorgioCMSchachterSC1998Vagus nerve stimulation theapy for partial-onset seizures: A randomized active control trialNeurology5148559674777
- HappeFMalhiGSCheckleyS2001Acquire mind-blindness following frontal lobe surgery? A single case study of impaired ‘theory of mind’Neuropsychologia39839011115657
- HardenCLPulverMCNikolovB1999Effect of vagus nerve stimulation on mood in adult epilepsy patientsNeurology52Suppl 2A238P03122
- HausmannAKemmlerGWalpothM2004bNo benefit derived from repetitive transcranial magnetic stimulation in depression: a prospective, single centre, randomised, double blind, sham controlled “add on” trialJ Neurol Neurosurg Psychiatry75320214742619
- HausmannAKramer-ReinstadlerKLechner-SchonerT2004bCan bilateral prefrontal repetitive transcranial magnetic stimulation (rTMS) induce mania? A case reportJ Clin Psychiatry651575615554775
- HenryTRVotawJRPennellPB1999Acute blood flow changes and efficacy of vagus nerve stimulation in partial epilepsyNeurology5211667310214738
- HenryTR2002Therapeutic mechanisms of vagus nerve stimulationNeurology59Suppl 4S31412270962
- HodgkissADMaliziaALBartlettJRBridgesPK1994Outcome after the physiological operation of stereotactic subcaudate tractotomyJ Neuropsychiatr72304
- HoffmanREBoutrosNNHuS2000Transcranial magnetic stimulation and auditory hallucinations in schizophreniaLancet3551073510744097
- HoffmanREHawkinsKAGueorguievaR2003Transcranial magnetic stimulation of left temporoparietal cortex and medication-resistant auditory hallucinationsArch Gen Psychiatry60495612511172
- HoliMMEronenMToivonenK2004Left prefrontal repetitive transcranial magnetic stimulation in schizophreniaSchizophr Bull304293415279057
- HoltzheimerPEAveryDSchlaepferTE2004Antidepressant effects of repetitive transcranial magnetic stimulationBr J Psychiatry184541215172952
- HoppeCHelmstaedterCScherrmannJ2001Self reported mood changes following 6 months of vagus nerve stimulation in epilepsy pateitnsEpilepsy Behav23354212609210
- HoppnerJSchulzMIrmischG2003Antidepressant efficacy of two different rTMS procedures. High frequency over left versus low frequency over right prefrontal cortex compared with sham stimulationEur Arch Psychiatry Clin Neurosci253103912799750
- IrleEExnerCThielenK1998Obsessive-compulsive disorder and ventromedial frontal lesions: clinical and neuropsychological findingsAm J Psychiatry155255639464207
- JahanshahiMProficePBrownRG1998The effects of transcranial magnetic stimulation over the dorsolateral prefrontal cortex on suppression of habitual counting during random number generationBrain1211533449712014
- JanicakPGDowdSMMartisB2002Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: preliminary results of a randomized trialBiol Psychiatry516596711955466
- JennikeMABaerLBallantineHT1991Cingulatomy for refractory obsessive-compulsive disorder. A long-term follow-up of 33 patientsArch Gen Psychiatry48548522039338
- JobePCDaileyJWWernickeJF1999A noradrenergic and serotonergic hypothesis of the linkage between epilepsy and affective disordersCrit Rev Neurobiol133175611028680
- JorgeRERobinsonRGTatenoA2004Repetitive transcranial magnetic stimulation as treatment of poststroke depression: a preliminary studyBiol Psychiatry5539840514960293
- KaptsanAYaroslavskyYApplebaumJ2003Right prefrontal TMS versus sham treatment of mania: a controlled studyBipolar Disord536912656936
- KauffmannCDCheemaMAMillerBE2004Slow right prefrontal transcranial magnetic stimulation as a treatment for medication-resistant depression: a double-blind, placebo-controlled studyDepress Anxiety19596214978787
- KeckMWeltTPostA2001Neuroendocrine and behavioural effects of repetitive transcranial magnetic stimulation in a psychopathological animal model are suggestive of antidepressant-like effectsNeuropsychopharmacology243374911182529
- KellyDRichardsonAMitchell-HeggsNA1973Sterotactic limbic tractotomy: neuropsychological aspects and operative techniqueBr J Psychiatry123133404582234
- KleinEKreininIChistyakovA1999Therapeutic efficiency of right prefrontal slow repetitive transcranial magnetic stimulation in major depression: a double blind controlled trialArch Gen Pyschiatry5631520
- KnightG1965Sterotactic tractotomy in the surgical treatment of mental illnessJ Neurol Neurosurg Psychiatry283041014338119
- KoerselmanFLamanDMvan DuijnH2004A 3-month, follow-up, randomized, placebo-controlled study of repetitive transcranial magnetic stimulation in depressionJ Clin Psychiatry651323815491234
- KopellBHGreenbergBRezaiAR2004Deep brain stimulation for psychiatric disordersJ Clin Neurophysiol21516715097294
- KoselMSchapeflerTE2003Beyond the treatment of epilepsy: New applications of vagus nerve stimulation in PsychiatryCNS Spect8515521
- KrahlSEClarkKBSmithDC1998Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulationEpilepsia39709149670898
- KulaksizogluIBBebekNBaykanB2004Obsessive compulsive disorder after epilepsy surgeryEpilepsy Behav51131814751216
- LangAELozanoAMMontgomeryE1997Posteroventral medial pallidotomy in advanced Parkinson’s diseaseN Engl J Med3371036429321531
- LangAEDuffJSaint-CyrJATrepanierL1999Posteroventral medial pallidotomy in Parkinson’s diseaseJ NeurolS246II284110526000
- LevkovitzYNgM2001Transcranial magnetic stimulation and anti-depressive drugs share similar cellular effects in rat hippocampusNeuropsychopharmacology246081611331140
- LiXNahasZPerry AndersonRN2004Can left frontal rTMS be used as a maintenance treatment for bipolar depression?Depress Anxiety209810015390210
- LimousinPKrackPPollakP1998Electrical stimulation of the sub-thalamic nucleus in advance Parkinson’s diseaseN Engl J Med3391105119770557
- LisanbySBelmakerR2000Animal models of the mechanisms of action of repetitive transcranial magnetic simulation (RTMS): comparisons with electroconvulsive shock (ECS)Depress Anxiety121788711126193
- LisanbyHPascual-LeoneASampsonS2001Augmentation of sertraline antidepressant treatment with transcranial magnetic stimulationBiol Psychiatry4981S
- LooCMitchellPCrokerV2003Double-blind controlled investigation of bilateral prefrontal transcranial magnetic stimulation for the treatment of resistant major depressionPsychol Med33334012537034
- LooCMitchellPSachdevP1999Double-blind controlled investigation of transcranial magnetic stimulation for the treatment of resistant major depressionAm J Psychiatry156946810360138
- LozanoAM2001Deep brain stimulation for Parkinson’s diseaseParkinsonism Relat Disord719920311331187
- LozanoAMHamaniC2004The future of deep brain stimulationJ Clin Neurophysiol2168915097295
- McCannUDKimbrellTAMorganCM1998Repetitive transcranial magentic stimulation for posttraumatic stress disorderArch Gen Psychiatry5527689510224
- McIntoshAMSempleDTaskerK2004Transcranial magnetic stimulation for auditory hallucinations in schizophreniaPsychiatry Res12791715261700
- MaedaFPascual-LeoneA2003Transcranial magnetic stimulation: studying motor neurophysiology of psychiatric disordersPsychopharmacology1683597612830365
- MalhiGSBartlettJR1998A new lesion for the psychosurgical operation of stereotactic subcaudate tractotomy (SST)Br J Neurosurg1233510070426
- MalhiGSBartlettJR2000Depression: A role for neurosurgery?Br J Neurosurg144152311198762
- MalhiGSBridgesPKMaliziaAL1997Neurosurgery for mental disorders (NMD): a clinical worldwide perspective: past, present and futureInt J Psychiatr Clin Pract111929
- MaliziaALSenskyT1994Stereotactic subcaudate tractotomy in the management of severe psychiatric disordersPsychiatry in Europe, directions and developmentsLondonGaskell8594
- ManesFJorgeRMorcuendeM2001A controlled study of repetitive transcranial magnetic stimulation as a treatment of depression in the elderlyInt Psychogeriatr132253111495396
- Marino JuniorRCosgroveGR1997Neurosurgical treatment of neuropsychiatric illnessPsychiatr Clin N Am2093343
- MartinJLRBarabanojMJScalaepflerTE2003Repetitive transcranial magnetic stimulation for the treatment of depression: Systematic review and meta-analysisBr J Psychiatry1824809112777338
- MashourGAWalkerEEMartuzaRL2005Psychosurgery: past, present and futureBrain Res Brain Res Rev484091915914249
- MenkesDLGruenthalM2000Slow-frequency repetitive transcranial magnetic stimulation in a patient with focal cortical dysplasiaEpilepsia41240210691123
- MeyersonBAMindusPLundsfordLD1988The role of the anterior interior capsulotomy in psychiatric surgeryModern stereotactic neurosurgeryBostonMartinus
- MichaelNErfurthA2004Treatment of bipolar mania with right prefrontal rapid transcranial magnetic stimulationJ Affect Disord78253715013251
- MillerR1987The time course of neuroleptic therapy for psychosis: role of learning processes and implications for concepts of psychotic illnessPsychopharmacol9240515
- MindusP1993Present-day indications for capsulotomyActa Neurochir58Suppl2933
- MindusPJenikeMA1992Neurosurgical treatment of malignant obsessive compulsive disorderPsychiatr Clin North Am15921381461805
- MindusPNymanH1991Normalization of personality characteristics in patients with incapacitating anxiety disorders after capsulotomyActa Psychiatr Scand83283912028804
- MontgomerySAAsbergM1979A new depression scale designed to be sensitive to changeBr J Psychiatry1343829444788
- MontoyaAWeissAPPriceBH2002MRI guided stereotactic limbic leucotomy for treatment of intractable psychiatric diseaseNeurosurgery501043911950407
- MoralesOSackeimHBermanR2004Magnetic seizure therapy: development of a novel intervention for treatment resistant depressionClinical Neuroscience Research45970
- MorrowJIBinghamECraigJJ2000Vagal nerve stimulation in patients with refractory epilepsy. Effect on seizure frequency, severity and quality of lifeSeizure9442510986004
- MosimannUPSchmittWGreenbergBD2004Repetitive transcranial magnetic stimulation: a putative add-on treatment for major depression in elderly patientsPsychiatry Res1261233315123391
- NahasZKozelFALiX2003Left prefrontal transcranial magnetic stimulation (TMS) treatment of depression in bipolar affective disorder: a pilot study of acute safety and efficacyBipolar Disord540712656937
- NewcombeR1975The lesion in stereotactic subcaudate tractotomyBr J Psychiatry126478811092399
- OsviewFFrimDM1997Neurosurgery for psychiatric disordersJ Neurol Neurosurg Psychiatry6370159416800
- PadbergFZwanzgerPKeckME2002Repetitive transcranial magnetic stimulation (rTMS) in major depression: relation between efficacy and stimulation intensityNeuropsychopharmacology276384512377400
- PadbergFZwanzgerPThomaH1999Repetitive transcranial magnetic stimulation (rTMS) in pharmacotherapy-refractory major depression: comparative study of fast, slow and sham rTMSPsychiatry Res881637110622338
- Pascual-LeoneADaveyNJRothwellJ2002aHandbook of transcranial magnetic stimulationLondonArnold
- Pascual-LeoneAManoachDSBirnbaumR2002bMotor cortical excitability in schizophreniaBiol Psychiatry52243112079727
- Pascual-LeoneARubioBPallardoF1996Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depressionThe Lancet3482337
- Pascual-LeoneABartres-FazDKeenanJP1999Transcranial magnetic stimulation: studying the brain-behaviour relationship by induction of ‘virtual lesions’Philos Trans R Soc Lond B Biol Sci35412293810466148
- PatwardhanRVStongBBebinEM2000Efficacy of vagal nerve stimulation in children with medically refractory epilepsyNeurosurgery471353811126906
- PouletEBrunelinJBediouB2005Slow transcranial magnetic stimulation can rapidly reduce resistant auditory hallucinations in schizophreniaBiol Psychiatry571889115652879
- PoyntonAMKartsounisLDBridgesPK1995A prospective clinical study of stereotactic subcaudate tractotomyPsychol Med25763707480453
- PridmoreS1999Rapid transcranial magnetic stimulation (rTMS) and normalization of the dexamethasone suppression test (DST)Psychiatry Clin Neurosci5333710201281
- PridmoreSBrunoRTurnier-SheaY2000Comparison of unlimited numbers of rapid transcranial magnetic stimulation (rTMS) and ECT treatment sessions in major depressive episodeInt J Neuropsychopharmacol31293411343589
- PuriBKDaveyNJEllawayPH1996An investigation of motor function in schizophrenia using transcranial magnetic stimulation of the motor cortexBr J Psychiatry16969058968625
- RasmussenSGreenbergBMindusP2000Neurosurgical approaches to intractable obsessive-compulsive disorderCNS Spect52334
- Rees CosgroveG2004Deep brain stimulation and psychosurgeryJ Neurosurg101574615481708
- RingHAWhiteSCostaDC2000A SPECT study of the effect of vagal nerve stimulation on thalamic activity in patients with epilepsySeizure9380410985992
- RothBJCohenLGHallettMLecyWJCraccoRQBarkerAT1991The electrical field induced during magnetic stimulation, magnetic motor stimulationBasic principles and clinical experience (EEG Suppl 43)AmsterdamElsevier26878
- RollnikJDHuberTJMogkH2000High frequency repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex in schizophrenic patientsNeuroreport1140131511192620
- RuckCAndreewitchSFlycktK2003Capsulotomy for treatment refractory anxiety disorders: long term follow up of 26 patientsAm J Psychiatry1605132112611833
- RumiDOGattazWFRigonattiSP2005Transcranial magnetic stimulation accelerates the antidepressant effect of amitriptyline in severe depression: a double-blind placebo-controlled studyBiol Psychiatry57162615652875
- RushAJGeorgeMSSackeimHA2000Vagus nerve stimulation (VNS) for treatment-resistant depressions: a multicenter studyBiol Psychiatry472768610686262
- RushAJMarangellLBSackeimHA2005aVagus nerve stimulation for treatment-resistant depression: a randomized, controlled acute phase trialBiol Psychiatry583475416139580
- RushAJSackeimHAMarangellLB2005bEffects of 12 months of vagus nerve stimulation in treatment-resistant depression: a naturalistic studyBiol Psychiatry583556316139581
- SabaGRocamoraJFKalalouK2002Catatonia and transcranial magnetic stimulationAm J Psychiatry159179412359696
- SachdevPMcBrideRLooC2001aRight vs. left prefrontal transcranial magnetic stimulation for obsessive-compulsive disorder: a preliminary investigationJ Clin Psychiatry62981411780880
- SakkasPMihalopoulouPMourtzouhouP2003Induction of mania by rTMS: report of two casesEur Psychiatry18196812814856
- SalinskyMCGeorgeRSonnenA1995A randomized controlled trial of chronic vagus nerve stimulation for treatment of medically intractable seizuresNeurology45224307854516
- SamiiAWassermannEMIkomaK1996Decreased postexercise faciliatation of motor evoked potentials in patients with chronic fatigue syndrome or depressionNeurology471410148960719
- SchachterSCSaperCB1998Vagus nerve stimulationEpilepsia39677869670894
- SchachterSC2004Vagus nerve stimulation:mood and cognitive effectsEpilepsy Behav5S56914725847
- SchlaepferTKoselM2004Novel physical treatments for major depression, transcranial magenetic stimulation and magnetic seizure therapyCurr Opin Psychiatry171520
- SchuurmanPRBoschDABossuytPMM2000A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremorN Engl J Med342461810675426
- Schonfeldt-LecuonaCGronGWalterH2004Stereotaxic rTMS for the treatment of auditory hallucinations in schizophreniaNeuroreport1516697315232304
- ShajahanPMGlabusMFGoodingPA1999Reduced cortical excitability in depression. Impaired postexercise motor facilitation with transcranial magnetic stimulationBr J Psychiatry1744495410616615
- ShimamotoHMorimitsuHSugitaS1999Therapeutic effect of repetitive transcranial megnatic stimulation in Parkinson’s diseaseRinsho Shinkeigaku391264710791094
- SiebnerHRMentschelCAuerC1999Repetitive transcranial megnetic stimulation has a benficial effect on bradykinesia in Parkinson’s diseaseNeuroreport105899410208595
- SommerMKammTTergauF1998Beneficial effects of repetitive transcranial megnetic stimulation (rTMS) on fine motor control in Parkinson’s diseaseMov Disord13Suppl 2298
- Strom-OlsenRCarlisleS1971Bifrontal sterotactic tractotomy: a follow-up of its effects on 210 patientsBr J Psychiatry118141544930549
- SteeleJDGlabusMFShajahanPM2000Increased cortical inhibition in depression: a prolonged silent period with transcranial magnetic stimulation (TMS)Psychol Med305657010883712
- SuTPHuangCCWeiIH2005Add-on rTMS for medication-resistant depression: a randomized, double-blind, sham-controlled trial in Chinese patientsJ Clin Psychiatry66930716013911
- TassinariCAMichelucciRFortiA1990Transcranial magnetic stimulation in epileptic patients: usefulness and safetyNeurology40113232113205
- ToothGCNewtonMP1961Leucotomy in England and Wales 1942–1954Reports on Public Health and Medical SubjectsLondonHMSO
- TopperRMottaghyFMBrugmannM1998Facilitation of picture naming by focal transcranial magnetic stimulation of Wernicke’s areaExp Brain Res12137189746143
- Van BockstaeleEJPeoplesJValentinoRJ1999Anatomic basis for differential regulation of the rostrolateral perilocus coeruleus region by limbic afferentsBiol Psychiatry4613526310578450
- Van LaereKVonckKBoonP2000Vagus nerve stimulation in refractory epilepsy: SPECT activation studyJ Nucl Med4111455410914903
- VolkmannJSturmVFreundH-J1998High-frequency deep brain stimulation for the treatment of movement disordersAktuelle Neurologie2528896
- VonckKBoonPVan LaereK2000Acute single photon emission computed tomographic study of vagus nerve stimulation in refractory epilepsyEpilepsia41601910802767
- WakaiSKotagalP2001Vagus nerve stimulation for children and adolescents with intractable epilepsiesPediatr Int4361511208002
- WassermanEM1998Risk and safety of repetitive transcranial magnetic stimulation. Report and suggested guidelines from the International Workshop in the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7Electroencephofr Clin Neurophysiol108116
- WerhahnKJLieberJClassenJ2000Motor excitability in patients with focal epilepsyEpilepsy Res411798910940619
- WichmannTBergmanHDeLongMR1994The primate subthalamic nucleus. III. Changes in motor behaviour and neuronal activity in the internal pallidum induced by subthalamic inactivation in the MPTP model of parkinsonismJ Neurophysiol72521307983516
- YoungRCBiggsJTZieglerVE1978A rating scale for mania: reliability, validity and sensitivityBr J Psychiatry13342935728692
- ZagonAKemenyAA2000Slow hyperpolarization in cortical neurons: A possible mechanism behind vagus nerve simulation therapy for refractory epilepsyEpilepsia411382911077451
- ZaldDHKimSW1996Anatomy and function of the orbital frontal cortex, II: function and relevance to obsessive-compulsive disorderJ Neuropsychiatry Clin Neurosci824961
- ZiemannUWinterMReimersCD1997Impaired motor cortex inhibition in patients with amyotrophic lateral sclerosis. Evidence from paired transcranial magnetic stimulationNeurology49129289371911
- ZwanzgerPMinovCEllaR2002Transcranial magnetic stimulation for panicAm J Psychiatry159315611823280