Abstract
This study compared patterns of frontal-lobe dysfunction in alcoholics with Korsakoff’s syndrome (KS: n = 9), non-Korsakoff alcoholics (AL: n = 28), patients with Parkinson’s disease (PD: n = 18), and patients with rupture and repair of the anterior communicating artery (ACoA: n = 4) relative to healthy non-neurological control (NC) participants (n = 70). The tests administered were sensitive to functions of dorsolateral prefrontal and orbito-frontal subsystems. Measures included perseverative errors on the Wisconsin Card Sorting Test (WCST-pe), errors on object alternation (OA), errors on Trails B, number of words generated on the Controlled Oral Word Association Test (COWAT), and number of categories completed on the WCST (WCST-cc). KS patients were as impaired as AL participants on orbitofrontal measures and, on dorsolateral prefrontal measures, were impaired relative to AL participants, whose performance did not differ from controls. Patients with PD also were impaired on tests of orbitofrontal and dorsolateral prefrontal functioning but to a lesser extent than the KS patients. Moreover, most of the PD deficits were driven by the impaired performance of patients whose initial symptoms were on the right side of the body. The ACoA patients were significantly impaired on tests of orbitofrontal but not dorsolateral prefrontal functioning relative to the control group. Together, the results confirm different patterns of frontal-system impairments in patient groups having compromised frontal lobe functioning consequent to varying etiologies.
Introduction
Behavioral manifestations of dysfunctional of human frontal brain systems have been consistently demonstrated in many neurological conditions, including alcoholism with and without Korsakoff’s syndrome (KS), Parkinson’s disease (PD), and rupture and repair of aneurysms of the anterior communicating artery (ACoA) (for reviews see CitationOscar-Berman and Bardenhagen 1998; CitationLichter and Cummings 2001; CitationMoselhy et al 2001). The variability in behavioral abnormalities in these several disorders suggests differential vulnerability of frontal subsystems. Frontal-system features have been only partially defined, but it is generally agreed that prefrontal cortex is host to at least two subsystems: dorsolateral and orbitofrontal (on the ventral surface) (CitationFuster 1997; CitationOscar-Berman and Bardenhagen 1998). Whereas the dorsolateral system contains extensive reciprocal connections with other neocortical sites, its connections with limbic sites are less striking than are those of the orbitofrontal system. The dorsolateral system is important for successful performance on tasks that require intact visuospatial, mnemonic, and attentional functions, for set shifting and rule discovery, and for verbal and spatial working memory (see CitationRoyall et al 2002 for a review). By contrast, functions involved in response inhibition have been linked to the ventral surface of the orbitofrontal system, which is extensively connected with basal forebrain and limbic structures. The orbitofrontal system is especially important for maintaining normal inhibitory influences on behavior, such as inhibiting abnormal perseverative responding (CitationOscar-Berman and Bardenhagen 1998), including disengagement from previously reinforced responses (CitationRolls 2004). Although difficulties with cognitive inhibition, attention, and set shifting reminiscent of frontal dysfunction occur in alcoholics with and without KS, patients with PD, and patients with rupture and repair of the ACoA, the more specific cognitive pictures of the individual disorders are dissimilar.
Alcoholics with and without KS often display deficiencies in behaviors suggestive of compromised frontal-lobe integrity such as planning and monitoring socially appropriate behaviors (CitationBates and Convit 1999; CitationOscar-Berman et al 2004). In neuroimaging studies in which alcoholics demonstrated diminished metabolic activity of frontal areas, this reduction often was associated with neuropsychological impairment of frontal functioning (CitationOscar-Berman and Evert 1997; CitationOscar-Berman 2000; CitationSullivan 2000). Neuropsychological findings indicate that KS patients exhibit signs of frontal-system damage including perseveration, disinhibition, apathy, and personality changes. Structural abnormalities of the frontal lobes have been reported in alcoholics with and without KS (CitationOscar-Berman 2000; CitationSullivan 2000), and CitationMelgaard et al (1990) showed a positive relationship between severity of alcoholism and extent of blood flow reduction in the frontal cortex. Collectively, neurobehavioral, neuropathological, and neuroimaging studies are suggestive of diminished frontal-lobe integrity in alcoholism (CitationPfefferbaum et al 1997; CitationHoaken et al 1998), but it is not clear if one subsystem is disproportionately affected.
Although PD is considered a movement disorder due to the preponderance of motor deficits and damage to the basal ganglia, there are numerous corticostriatothalamic loops connecting basal ganglia structures with the frontal lobes (CitationTaylor et al 1986; CitationMiddleton and Strick 2001; CitationSaint-Cyr 2003). Further, PD patients exhibit a wide range of cognitive deficits including impairments reflective of frontal system dysfunction (CitationLevin et al 1991). Damage to the prefrontal cortex in PD is not direct, but rather may be characterized as deafferentation from the basal ganglia and related structures, as supported by several studies illustrating that the subcortical lesions are sufficient to cause frontal-type impairments (CitationRoyall et al 2002). Cognitive sequelae of PD are thought to arise from disruption of dopaminergic corti-costriatothalamic loops through the dorsolateral and orbital regions of the frontal lobes. CitationMiddleton and Strick (2001) described four separate topographically organized dorsolateral frontostriatal circuits, two lateral orbitofrontostriatal circuits, and three medial orbitofrontostriatal circuits. The substantia nigra, ventral tegmental area, and the substantia nigra pars compacta all project to the head of the caudate nucleus, which receives input from the dorsolateral and orbital prefrontal cortices. CitationFreedman and Oscar-Berman (1986b) found no impairment in the performance of non-demented patients with PD on tasks sensitive to dysfunction of the dorsolateral prefrontal cortex. By contrast, CitationFreedman (1990) used OA, a task sensitive to orbitofrontal function, and reported mean error rates of another group of PD patients that were significantly higher than for a neurologically healthy control group.
Patients with a history of ACoA disease demonstrate several cognitive impairments, but they are especially impaired on tests of frontal-lobe function (CitationDeLuca 1993; CitationJorn and Rybarczyk 1995; CitationDiamond et al 1997). CitationDeLuca (1993) suggested that the amnesia resulting from rupture of an ACoA aneurysm is a result of a basal forebrain infarct. The ACoA connects the anterior cerebral arteries and completes the anterior segment of the vascular Circle of Willis. Rupture of the ACoA typically results in damage to the basal forebrain and anterior portion of the limbic system (CitationCarpenter 1991; CitationVictor and Ropper 2001). The ACoA and its branches perforate the ventral and medial surfaces of the frontal lobes and basal forebrain, as evidenced by imaging scans. Rupture of the ACoA often results in damage to the nucleus basalis, medial septal nuclei, anterior commissure, and columns of the fornix (CitationDunker and Harris 1976). CitationFreedman and Oscar-Berman (1986a) compared the performance patterns of ACoA patients with those of abstinent alcoholic control participants on tasks sensitive to dorsolateral prefrontal dysfunction. They found that ACoA patients did not significantly differ from control participants in performance of those tasks. These results suggest spared dorsolateral prefrontal function.
The purpose of the present study was to characterize the nature of prefrontal dysfunction in groups of patients with frontal system damage: alcoholics with and without KS, patients with PD, and patients with rupture and repair of the ACoA. The tasks selected are sensitive to the dorsolateral and orbitofrontal subsystems within the frontal lobes, and the patient groups chosen have damage in different frontal brain systems. The current study allows comparison of the extents to which the dorsolateral- and orbital- prefrontal subsystems are affected by the neuropathology of each group relative to neurologically intact participants.
The measures selected to assess dorsolateral prefrontal function were: the number of categories completed on the Wisconsin Card Sort Test (WCST-cc; CitationBerg 1948), the Controlled Oral Word Association Test (COWAT; CitationBenton and Hamsher 1989; CitationSpreen and Strauss 1998), and the Trail Making Test, Part B (Reitan and Wolfson 1995; U.S. Army 1944). Together, these three tests draw on verbal and spatial working memory, cognitive skills reliant upon the dorsolateral prefrontal cortex (CitationRoyall et al 2002). Measures selected to assess orbitofrontal function were the number of perseverative errors on the WCST (WCST-pe) and the number of errors committed before reaching a learning criterion on an Object Alteration (OA) task. The OA task has been shown to be a particularly sensitive measure of neuropsychological dysfunction, and reflective of damage to the orbitofrontal system in human and non-human primates alike (CitationOscar-Berman and Bardenhagen 1998). CitationFreedman and colleagues (1998) examined OA performance in patients with bilateral frontal-lobe lesions, and found that these patients were significantly impaired relative to a neurologically healthy control group. Based upon computerized tomography images of the brains of these patients, the investigators concluded that several regions of the orbitofrontal cortex were critical to successful OA performance. The authors further noted that, in monkeys, lesions of cytoarchitectonically homologous frontal regions have been associated with impaired performance on OA, strengthening their argument that the orbitofrontal cortex (eg, Brodmann’s Area 47) is a critical lesion site in humans. CitationFreedman and colleagues (1998) also administered the WCST (CitationHeaton et al 1993), a standard, widely used test that provides information about response strategies. The authors concluded that the number of perseverative errors on the WCST was associated with impaired OA performance, and both measures were indicative of medial and orbitofrontal damage. It should be noted that although the WCST is often conceptualized as a measure of dorsolateral prefrontal function, the literature on this subject is incon sistent, and components of the WCST measure distinct cognitive functions (CitationLezak 1995; CitationMountain and Snow 1993). The perseverative error score reflects a participant’s inability to abandon a previously rewarded task strategy in favor of a strategy that is presently reinforced. WCST-pe has also been directly tied to errors on OA (CitationFreedman 1998), a test known to be reflective of orbitofrontal function. As such, both OA and WCST-pe are thought to directly reflect the patient’s ability to inhibit inappropriate behavioral responses.
Using the various tests of dorsolateral and orbitofrontal functioning, we predicted that the KS group would commit significantly more errors overall than the non-Korsakoff alcoholics (AL), because the former group has more extensive diffuse frontal pathology than the latter (CitationOscar-Berman 2000). However, the AL group was expected to commit significantly more errors than healthy nonneurological control (NC) subjects on these same experimental measures. Additionally, and because PD patients exhibit behavioral deficits consistent with frontal-system dysfunction (presumably the result of interrupted dopaminergic fron-tostriatal circuitry originating in the basal ganglia), these patients were expected to demonstrate significantly impaired performance on all frontal tasks. However, due to the nature of the frontal-system damage in PD, as well as the fact that our patients were in the mild to moderate stages of the disease, we expected that some frontal functions would be less disrupted than others. Finally, because OA is thought to be sensitive to orbitofrontal damage, and the ACoA supports the basal forebrain and ventral and medial portions of the frontal lobes (CitationCarpenter 1991), these patients were expected to show impairment on OA. Their performance on tests of dorsolateral function was not expected to be impaired, however, since the dorsolateral prefrontal cortex is impacted to a much lesser extent than orbitofrontal cortex from rupture of an ACoA aneurysm.
Methods
Participants
A total of 129 individuals comprised the study groups. The neurobehavioral groups consisted of 28 non-Korsakoff alcoholics (21 men, 7 women), 9 alcoholic KS patients (8 men, 1 woman); 4 patients (all women) with ruptured and repaired ACoA aneurysms; and 18 patients with PD (12 men, 6 women). Seventy neurologically intact control participants (NC; 22 men, 48 women) also were included. In order to equate the control participants with the diagnostic groups on demographic variables, NC subgroups were selected for the purposes of statistical comparisons with each of the neurobehavioral groups. Thus, all groups consisted of men and women equated as closely as possible for socioeconomic status, age, and educational level. Patient participation was solicited from the Neurology, Psychology, Psychiatry, General-Medical, Movement Disorder, and Out-patient clinics of the Boston University Medical Center, the Department of Veterans Affairs (VA) Healthcare System, Boston Campus, and its after-care programs in the Boston area. The AL and NC participants were also recruited through advertisements in local newspapers. Informed consent for participation in the research was obtained from each subject prior to testing, and participants were reimbursed for time and travel expenses.
The exclusion criteria for the experimental and control groups included history of epilepsy, stroke, Alzheimer’s disease and other neurodegenerative diseases (with the exception of PD), major psychiatric disease (eg, schizophrenic disorders and current major depression), electro-convulsive therapy, serious head injury resulting in a loss of consciousness of more than 15 minutes, history of radiation to the head, history of polydrug abuse, and clinical evidence of active hepatic disease. Individuals whose ability to comprehend the experimental conditions or respond to the instructions was in doubt were not included; these participants were identified using the Mini-Mental State Examination (MMSE; CitationFolstein et al 1975). Participants scoring one or more standard deviations below the mean (ie, below the 16th percentile) on the MMSE for their age and education were not included in the study; normative data provided by CitationSpreen and Strauss (1998) were consulted to determine participants’ percentile scores. To screen for other exclusion criteria, detailed health questionnaires were administered prior to testing, and hospital records were examined when available.
Alcoholics with and without Korsakoff’s syndrome
All of the alcoholic participants met DSM-IV criteria (CitationAPA 1994) for moderate to severe alcohol abuse and dependence, using a computerized version of the Diagnostic Interview Schedule-Revised (DIS-R; CitationRobins et al 1989) as the screening instrument, and had a drinking history of 21 or more drinks per week for a minimum of 5 years. Additionally, the KS patients were diagnosed by the Psychology and Neurology Services of the VA or affiliated facilities, and had an IQ within normal range. A discrepancy of 10 points or more existed between the Wechsler Adult Intelligence Scale, 3rd edition (WAIS-III; CitationWechsler 1997) Verbal IQ score and the General Memory score of the Wechsler Memory Scale, 3rd edition (WMS-III; CitationWechsler 1997) with IQ being better than General Memory. In order not to confound the long-term effects of alcoholism with those of current drinking habits, only those alcoholics who had reported abstinence for a minimum of 4 weeks were included, as this is important for obtaining stable levels of performance (CitationNIAAA 1993).
The scores of 72 individuals from 3 groups (with equivalent demographic characteristics) were included in this set of analyses: Thirty-five were NC participants (9 men, 26 women), 28 were AL participants (21 men, 7 women), and 9 were KS patients (8 men, 1 woman). summarizes the mean ages, educational levels, Full Scale IQ (FSIQ) scores, General Memory Quotients (GMQ), and MMSE scores of the NC, AL, and KS groups.
Table 1 Means and standard deviations for for age, educational level, Wechsler Adult Intelligence Scale (WAIS-III) Full Scale IQ (FSIQ), Wechsler Memory Scale (WMS-III) General Memory Quotient (GMQ), and Mini Mental State Exam (MMSE) score. The comparison groups consist of healthy Control participants, non-Korsakoff alcoholics, and patients with alcoholic Korsakoff’s syndrome
Patients with PD
Patients with PD (n = 18) were recruited from the Boston Medical Center Movement Disorder Clinic and local support groups. The scores of the PD patients were compared to those of 28 NC subjects (see ) in the analyses. Eight of the PD patients had experienced initial motor symptoms on the left side of the body (LPD; 6 men), and 10 first experienced symptoms on the right (RPD; 6 men). All of the PD patients had a diagnosis of idiopathic PD assigned by their neurologists, and no patients whose symptoms were due to other neurological conditions, or who had undergone neurosurgery, were included in the study. The PD patients had a mean disease duration of 6.8 years (SD = 4.9). All but 2 of the PD patients, who had bilateral motor symptoms and appeared to be in the mild to moderate stages of the disease, were administered the CitationHoehn and Yahr (1967) scale to assess severity of motor dysfunction, and all participants were found to be in the mild to moderate stages. For the PD patients with left-side motor symptom onset, 3 participants had a Hoehn and Yahr score of 3, and 4 had scores of 2. Seven of the RPD patients had scores of 2, and 2 had scores of 3. The RPD and LPD patients were equated for Hoehn and Yahr scores and disease duration.
Table 2 Means and standard deviations for healthy control participants and patients with Parkinson’s disease (PD) for age, educational level, Mini Mental State Exam (MMSE) score, Wechsler Adult Intelligence Scale (WAIS-III) Full Scale IQ (FSIQ), and Wechsler Memory Scale (WMS-III) General Memory Quotient (GMQ)
At the time of testing, all PD participants were taking dopamine agonists, such as pramipexole and pergolide. Additionally, several were taking levodopa-carbidopa, a dopamine precursor; one was taking amantadine, which stimulates dopamine release; another was taking selegiline hydrochloride, a monoamine oxidase inhibitor; one was taking clonazepam, a benzodiazepine derivative; two were taking the selective serotonin reuptake inhibitor fluoxetine for depression. Patients on antidepressants were not excluded due to the high incidence of depression in PD, and they were given the Hamilton Depression Inventory (CitationHamilton 1960). A score of 14 on the Hamilton Depression Inventory is indicative of depression; the PD group was not depressed (Hamilton range of scores, 0–13). All but one were taking multivitamins and some combination of other medications for hypertension, prostate problems, incontinence, or elevated cholesterol levels.
Patients with rupture and repair of the ACoA
Four patients with rupture and repair of the ACoA (all women) were included in this set of analyses; the low incidence of ruptured ACoA aneurysms limited the number of patients available. The ACoA patients had diagnoses assigned by their neurologists. They were compared with seven neurologically healthy NC participants (3 men, 4 women). summarizes the mean ages, educational levels, FSIQ, GMQ, and MMSE scores of the NC and ACoA groups.
Table 3 Healthy non-neurological control participants are compared with patients with rupture and repair of the anterior communicating artery (ACoA). Group means and standard deviations are provided for age, educational level, Mini Mental State Exam (MMSE) score, Wechsler Adult Intelligence Scale (WAIS-III) Full Scale IQ (FSIQ), and Wechsler Memory Scale (WMS-III) General Memory Quotient (GMQ)
Procedures
Tests of orbitofrontal function
Two measures of orbitofrontal function were obtained: Errors to criterion on the OA task, and perseverative errors on the WCST (WCST-pe). The OA task was administered in a Wisconsin General Test Apparatus, modified for human testing (CitationOscar-Berman and Zola-Morgan 1980). The administrator and participant sat opposite each other on either side of a wooden frame. A dark curtain was attached to the frame, which when lowered, hid 2 stimuli (a red and a blue disc covering 2 reinforcement wells) from the participant’s view. On the first trial of the OA task, both wells were baited so that a subject’s first response was always rewarded. From then on, the correct object was alternated. That is, the administrator placed a penny beneath the other disc (previously incorrect). The placement of the penny varied randomly; the administrator alternated the disc under which the penny was located after each correct response made by the participant. The participant’s task was to try to collect as many pennies as possible. Participants were tested until they reached a learning criterion of 12 consecutive correct alternations, or until the failure criterion was met. The failure criterion was 20 consecutive trials without learning the alternation strategy. Errors on the OA task are characterized as “perseverative,” because the participant continues to choose the unbaited object after receiving negative feedback from the experimenter on the previous trial. The following instructions were read to each of the participants:
“This test is a little unusual because I can't tell you very much about how to do it. I am going to place a penny in one of these two wells (administrator points), and cover it with either the red or blue cover. I want you to try to get as many pennies as you can. I will add the amount you get to your total when you’re being paid.”
The WCST was administered in accordance with the standardized method outlined in the test manual (CitationHeaton et al 1993). The WCST requires strategic sorting of cards based on color, shape, or number of items on the face of each card; these sorting strategies are referred to as “categories”. The participant places a card in a pile and is then told whether it is correct or incorrect by the test administrator. Based on this feedback, the participant is to determine the sorting strategy. The participant is not told which strategy to use and must discern this from the administrator’s feedback. After the participant achieves 10 correct answers, the criterion for sorting changes, and the participant again must determine a new strategy based upon the feedback from the test administrator. Perseverative errors on the WCST are committed when a participant continues to sort by a particular strategy that no longer is correct (ie, fails to inhibit an inappropriate response).
Tests of dorsolateral prefrontal cortex function
Data from three measures of dorsolateral prefrontal functioning were obtained: the number of categories completed on the WCST (WCST-cc); the number of words generated on the COWAT; and errors on Trails B.
The procedure for administration of the WCST was described above. If a participant accurately completed the WCST, he or she would have completed 6 categories in 128 trials.
The COWAT is a test of phonemic verbal fluency. Participants were asked to generate as many words as possible that began with the letters F in a 60-second period; they were then asked to do the same thing using the letter A, and finally, using the letter S. Participants were instructed to refrain from providing proper nouns (eg, “Boston” or “Bob”), numbers (eg, “nine” or “ninety”), and 2 forms of the same word (eg, “bus” and “buses”). The number of words generated was summed across all three letters, yielding the variable used in the analyses.
Part B of the Trail Making Test challenged participants to connect 13 numbered and lettered dots, alternating between number and letter (eg, 1-A-2-B-3-C). Participants were instructed to alternate between number and letter and to complete the task as quickly as possible without making any errors or lifting their pencil from the paper. The total number of errors was the variable used in the analyses.
Results
presents the means and standard deviations, by group, for all of the experimental measures, and shows the patterns of group performance across all tasks. Univariate analyses of variance (ANOVA) were conducted to compare the performance of the experimental groups to the control groups. When necessary, analyses of covariance (ANCOVA) were conducted to determine the effect of age and education on the experimental variables. If either age or education or both were not significantly related to the experimental variable, they were eliminated as covariates in a step-wise fashion, with the least significant covariate removed first. Post-hoc comparisons were conducted using the least significant difference method.
Figure 1 Group patterns of dorsolateral prefrontal and orbitofrontal function relative to non-neurological control (NC) participants, except where noted (— indicates that post hoc tests were not carried out, because the group main effect of the ANOVA was not significant). Downward arrows indicate observed deficits, and equal signs indicate absence of deficits. The groups consisted of Korsakoff patients (KS), non-Korsakoff alcoholics (AL), patients with rupture and repair of the anterior communicating artery (ACoA), and patients with Parkinson’s disease (PD), including subgroups of PD patients with right-side motor symptom onset (RPD) or left-side motor symptom onset (LPD).
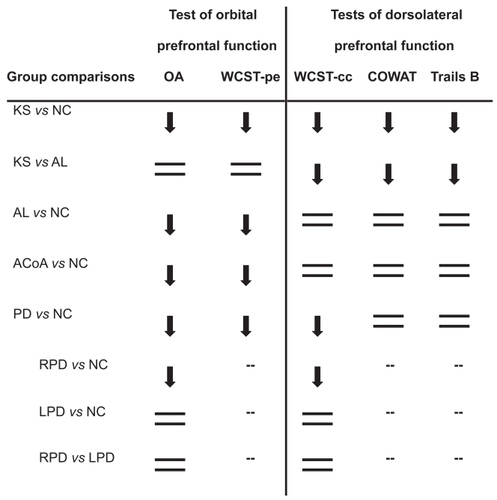
Table 4 Means and standard deviations by group for number of errors on Object Alternation (OA), percentile score on perseverative error measure of the Wisconsin Card Sorting Test (WCST-pe), number of categories completed on the Wisconsin Card Sorting Test (WCST-cc), total number of words generated on the Controlled Oral Word Association Test (COWAT), and total number of errors on Trails B
Tests of orbitofrontal function
Alcoholics with and without Korsakoff’s syndrome
An ANOVA revealed a significant main effect of group (F[2,69] = 4.2, p < 0.02) for total errors committed on OA. Post-hoc analyses revealed that the effect was driven by performance differences between the AL (mean = 38.4, SD = 22.3) and NC groups (mean = 23.8, SD = 19.5; p < 0.01), and between the KS (mean = 37.8, SD = 23.4) and NC groups (p < 0.01); the KS patients did not differ significantly from the AL group (mean difference = 0.5; p = 0.95).
An ANOVA of perseverative errors on WCST (WCST-pe) revealed a significant main effect of group (F[2,68] = 3.4, p < 0.04). Post-hoc analyses revealed significant differences between the NC (mean = 56.4, SD = 28.1) and KS groups (mean = 33.9, SD = 38.9; p < 0.04) and between the NC and AL groups (mean = 38.0, SD = 26.7; p < 0.02), but no significant difference between the KS and AL groups (mean difference = 5.7; p = 0.71).
PD patients
An ANOVA revealed a significant main effect of group (F[1, 46] = 5.8; p < 0.02) for mean number of errors on OA, with the PD patients committing significantly more errors (mean = 35.9, SD = 18.6) than the NC group (mean = 22.9, SD = 17.2).
A subsequent ANOVA was conducted to determine if the effect was related to side of symptom onset in PD patients. The overall ANOVA revealed a significant main effect of side-of-onset subgroup (F[2,43] = 5.1; p < 0.01); post hoc analyses indicated that the effect was driven by PD patients who first experienced motor symptoms on the right side of their bodies (RPD; p < 0.003). The RPD subgroup (mean = 43.6, SD = 18.2) committed significantly more errors on OA than the NC group (mean = 22.9, SD = 17.2), whereas they did not differ from LPD patients (mean = 31.0, SD = 18.9). LPD patients did not differ significantly from NC participants.
An ANOVA of WCST-pe revealed a significant main effect of group (F[1, 17] = 6.8, p < 0.01); the PD group had a significantly lower mean percentile score (mean = 29.7, SD = 20.6) the NC group (mean = 51.1, SD = 26.2). An ANOVA was conducted to determine if the effect was related to side of symptom onset. Although RPD patients had a larger mean number of perseverative errors, no significant effect of PD subgroup was found.
ACoA patients
Preliminary t-tests revealed a significant difference between the ACoA and NC groups on educational level (t = –2.44, p < 0.04); education was, therefore, entered into the analyses as a covariate. Unless otherwise noted, preliminary ANCOVAs revealed no significant relation between education and the experimental variable, and education was, therefore, removed from subsequent analyses.
A one-way ANCOVA with education as the covariate revealed a significant relation between Education and OA performance (F[1,8] = 8.41, p < 0.02); the NC group had a higher mean educational level (mean = 13.57, SD = 1.3) than the ACoA group (mean = 12.25, SD = 0.5). The ANCOVA also revealed a significant main effect of group even when the effect of education on OA performance was accounted for (F[1,8] = 8.66, p < 0.02]; the ACoA group made significantly more errors (mean = 42.5, SD = 5.3) than the NC group (mean = 31.86, SD = 13.4).
An ANOVA revealed a significant main effect of group on WCST-pe (F[1,8] = 6.25; p < 0.03), with the ACoA group achieving a significantly lower mean percentile score (mean = 18.5, SD = 13.4) than the NC group (mean = 62.1, SD = 21.0).
Tests of dorsolateral prefrontal function
Alcoholics with and without Korsakoff’s syndrome
An ANOVA revealed a significant main effect of group (F[2,68] = 4.02, p < 0.01) for number of categories completed on the WCST (WCST-cc). Post-hoc analyses indicated that the effect was driven by the KS group, with the KS patients completing significantly fewer categories (mean = 2.2, SD = 2.2) than the NC (mean = 5.2, SD = 2.0; p < 0.001) and AL groups (mean = 4.6, SD = 1.8; p < 0.001). The number of categories completed did not differ significantly between the AL and NC groups.
An ANOVA of total words generated beginning with the letters F, A, and S revealed a significant main effect of group (F[2,68] = 10.15, p < 0.01). Post-hoc analyses indicated that the effect was driven by the performance of the KS patients, who generated significantly fewer words (mean = 8.8, SD = 8.8) than the NC (mean = 16.7, SD = 5.3; p < 0.001) and the AL groups (mean = 14.8, SD = 4.6; p < 0.001). The AL and NC groups were statistically equivalent in number of words generated.
An ANOVA of number of errors committed on Trails B revealed a significant main effect of group (F[2,69] = 3.4; p < 0.04], with the KS group committing significantly more errors (mean = 1.9, SD = 1.9) than the NC (mean = 0.7, SD = 1.3; p < 0.02) and AL groups (mean = 0.6, SD = 1.2; p < 0.02). The AL and NC groups were statistically equivalent in number of errors committed on Trails B.
PD patients
An ANOVA revealed a significant main effect of group (F[1,17) = 4.41, p < 0.05) for WCST-cc, with the PD patients completing significantly fewer categories (mean = 4.4, SD = 1.8) than the NC group (mean = 5.6, SD = 1.3).
A subsequent ANOVA was conducted to determine if the effect was related to side of symptom onset in PD patients. The overall ANOVA revealed a significant main effect of side-of-onset subgroup (F[2,36] = 3.6; p = 0.4]; post hoc analyses indicated that the effect was driven by RPD patients. The RPD subgroup completed significantly fewer categories (mean = 4.0, SD = 2.5) than the controls (mean difference = 1.7, SD = 1.3; p < 0.01) on the WCST, whereas the LPD subgroup (mean = 5.6, SD = 1.0) did not significantly differ from the controls. There was no significant difference between the RPD and LPD subgroups.
ANOVAs revealed no significant main effects of group on either the COWAT or on Trails B.
ACoA patients
In comparisons between the ACoA and NC groups, individual ANOVAs revealed no significant main effects of group for WCST-cc, mean number of errors on Trails B, nor mean number of words generated on the COWAT.
Summary of findings
All patient groups were impaired on tests of orbitofrontal function relative to healthy control participants. The non-Korsakoff AL group was as impaired as the KS group on those measures. The impairment of PD patients on OA was driven by those patients who first experienced motor symptoms on the right side of the body.
On all tests of dorsolateral prefrontal function, the KS patients were impaired relative to AL and NC participants. The PD group demonstrated impairment on WCST-cc, but not on the COWAT nor on Trails B, relative to neurologically healthy participants. Post-hoc analyses revealed that on the WCST-cc, the effect was again driven by RPD patients. ACoA patients were not impaired on any of the tests of dorsolateral prefrontal function.
Discussion
As was summarized in , the present study describes patterns of prefrontal functioning in alcoholics with and without KS, in patients with PD, and in patients with rupture and repair of the ACoA.
Alcoholics with and without Korsakoff’s syndrome
The KS patients were impaired on all tasks. Of interest, the KS and the AL groups’ performance levels were not significantly different on tests of orbitofrontal function. These data suggest that excessive consumption of alcohol may take a toll on orbitofrontal function whether or not it affects dorsolateral prefrontal functioning or results in the amnesia characteristic of KS. Further support for this interpretation of the data is evident in the performance of the AL group relative to controls. In the present study, the AL group performed similarly to the NC group on tests of dorsolateral prefrontal functioning, despite displaying impaired performance, equal to that of KS patients, on tests of orbitofrontal function.
Our data support other findings regarding the neuropathology and behavioral impairments associated with chronic alcohol abuse. After the acute effects of alcohol abuse have subsided, there often remain several cognitive and affective deficits such as difficulty regulating emotion, impulsivity, and difficulty switching sets (for reviews, see CitationLevin et al 1991; CitationMoselhy et al 2001; CitationRoyall et al 2002; CitationOscar-Berman et al 2004; CitationOscar-Berman and Marinkovic 2003). Given that chronic alcoholism is known to damage limbic structures (CitationRoyall et al 2002), and that the orbitofrontal cortex and the limbic system are extensively interconnected (CitationOscar-Berman and Bardenhagen 1998; CitationMiddleton and Strick 2001), it is possible that the orbitofrontal dysfunction observed in the present study is an indirect effect of compromised limbic integrity rather than a direct reflection of damaged orbitofrontal cortex per se. It is accordingly not surprising that individuals who demonstrate impairments in laboratory measures of orbitofrontal dysfunction experience everyday difficulty in the cognitive and affective domains mentioned above.
PD patients
The PD group demonstrated a different pattern of performance across tasks. As a whole, the PD group performed similarly to the NC group on two out of the three tests of dorsolateral prefrontal function (ie, COWAT and Trails B). On the third measure, WCST-cc, the PD group demonstrated impaired performance relative to controls. Further analyses revealed a significant effect of subgroup between patients who first experienced motor symptoms on the right side of the body (RPD), those who had experienced them initially on the left side of the body (LPD), and the NC group. Specifically, the effect was driven entirely by RPD patients. The same was true of performance on the OA task. As a whole, the PD group was impaired on OA relative to the NC group. Again, analyses revealed that the effect was driven by RPD patients, who committed significantly more errors than either the NC group or LPD subgroup.
These data provide a possible explanation for the results of CitationFreedman (1990) who observed an increased overall PD error rate, but no statistically significant difference between PD and control groups on OA. Freedman’s results may have been influenced by side-of-symptom onset, although that analysis was not conducted. It is possible that LPD patients (greater right hemisphere damage) were able to use a more extensive verbal strategy in these tasks than RPD patients (greater left hemisphere damage). Because RPD patients have primary damage (or more extensive damage) to the left basal ganglia, left dopaminergic corticostriatal circuits are presumably more severely affected, rendering verbal mediation of cognitive tasks more difficult. This inference is supported by several SPECT studies that provided evidence for greater dopamine depletion in the hemisphere contralateral to the side of motor symptom onset (CitationAntonini et al 1995; CitationBooij et al 1997; CitationTissingh et al 1998; CitationMozley et al 2000), by studies demonstrating that asymmetrical dopamine depletion persists after motor symptoms appear bilaterally (CitationLeenders et al 1990; CitationAntonini et al 1995), and by post-mortem studies that found significant neuronal loss in the hemisphere contralateral to the side of the body on which motor symptoms first appeared (CitationKempster et al 1989). Based on the OA results, the same pattern of performance was expected on WCST-pe. Although the PD group as a whole was impaired relative to the NC group, the ANOVA conducted to determine the influence of side of symptom onset showed no significant main effect of subgroup.
ACoA patients
Like the AL group, ACoA patients demonstrated normal levels of performance on tasks of dorsolateral prefrontal function compared to controls. Both the ACoA group and the NC group committed very few errors on Trails B; thus, it is important to note that the non-significant result on this measure of dorsolateral prefrontal function may have been due to a ceiling effect. By contrast, ACoA patients demonstrated impaired performance on OA and WCST-pe. These results are consistent with the neuropathology of rupture and repair of the ACoA. The ACoA and its perforating branches supply blood to the basal forebrain and limbic system, which, through connectivity with the orbitofrontal cortex, mediates response inhibition, a function necessary to avoid perseverative errors on the WCST and successfully complete OA (CitationLevin et al 1991; CitationRoyall et al 2002). Because performance by ACoA patients on tests of dorsolateral prefrontal function was similar to that of the NC group, the findings indicate that orbitofrontal function is more severely affected by the pathology of a ruptured and repaired ACoA aneurysm than are other functions controlled by prefrontal brain subsystems.
As predicted, ACoA patients did not demonstrate impairment on tests of dorsolateral prefrontal function, suggesting a differential effect of an ACoA aneurysm on prefrontal subsystems. One possible explanation for these data is that impairments on tests sensitive to orbitofrontal dysfunction are reflective of sustained damage to the basal forebrain and/or limbic system. While the small number of participants in the ACoA group may have limited the ability to detect a significant difference between the groups, and, therefore, limits the conclusions that can be drawn from these data, it remains a hypothesis suitable for further study.
Overall patterns of performance
Patterns of performance on the measures used varied among the groups. While KS patients were impaired on all experimental measures, AL and ACoA participants were impaired only on orbitofrontal tests. Patients with PD were also impaired on OA and WCST-pe, but they demonstrated impairment on only one test of dorsolateral frontal function, the WCST-cc. Across all groups, performance on OA was never impaired in the absence of impaired performance on WCST-pe; this pattern of performance supports the conceptualization of the two measures as reflective of similar cognitive functions. These data fit well with those of CitationFreedman et al (1998), who also observed a relation between perseverative errors on the WCST and impaired performance on OA. The findings support the view that the orbitofrontal cortex and its neural networks mediate the ability of people to inhibit inappropriate behavioral responses and allow them to switch cognitive sets (CitationBechara 2004; CitationHappaney et al 2004). Although the nature of the deficit in response inhibition is not entirely clear, the coincidental impairment on these tasks suggests that they may rely on a similar cognitive process. One possible explanation may lie in the taxonomy of perseveration proposed by CitationSandson and Albert (1984), who described a “stuck-in-set” type of perseveration.
Stuck-in-set perseveration emphasizes the inappropriate maintenance of a particular response strategy. Errors on OA are committed when the participant fails to learn the task strategy. This failure could occur for several reasons: the individual fails to establish set (ie, he or she never determines that the penny location alternates between objects), the participant recognizes the pattern but is unable to maintain performance consistent with it, or impulsivity draws a response to one object over that of another (ie, disinhibition). Similarly, perseverative errors on the WCST might be committed if the individual chooses a priori a particular sorting strategy and is not able to adjust performance based on the administrator’s feedback (ie, set is never established), or because set is established contingent upon sorting for either color, shape, or number, but when the task calls for a novel sorting strategy, the participant is unable to inhibit previously correct but currently inappropriate behavioral responses (ie, sort by a different characteristic). In their review, CitationSandson and Albert (1984) noted that perseverative responding on tests requiring cognitive flexibility is often observed in non-human primates with orbitofrontal lesions, on the WCST in patients with frontal-lobe damage, and in patients with PD.
All of the patient groups in the present study have been shown to demonstrate impairments suggestive of compromised prefrontal integrity. It seems logical to infer dysfunctional orbitofrontal and/or limbic system activity from these data given the connectivity between limbic and orbitofrontal systems, and the view that the limbic system is important in facilitating cognitive flexibility (CitationRoyall et al 2002). Successful performance on WCST-pe and OA requires behavioral inhibition; as such, the task demands of these measures may provide the ideal means by which to elicit stuck-in-set perseveration. If such is the case, the relation between group performances on these two tasks could be explained by the similar nature of task demands (eg, response inhibition).
Limitations
Although these data provide valuable information regarding the effect of neurological disease on prefrontal subsystems, interpretation of the results is limited by the fact that performance on the experimental measures was not compared directly across clinical groups. This was due primarily to the small sample sizes of the clinical groups, namely the KS and ACoA patient groups, and to a lesser extent the PD patient subgroups. Ideally, patient populations large enough to conduct robust comparisons across clinical groups would allow a more detailed picture of the degree of dysfunction affected by neurological compromise related to disease and/or to substance abuse. However, such a comparison would remain limited by the imprecise etiology of neurological compromise. As emphasized by CitationOscar-Berman and Bardenhagen (1998), research using tasks sensitive to prefrontal damage with comparison groups of patients having discrete brain lesions would be ideal, but difficult to garner for obvious ethical considerations. As such, we are limited in our interpretation of these data by the fact that the specificity of such measures in humans has not yet been definitively established.
Conclusions
The results of the present study illustrate different patterns of frontal-system impairment in alcoholics with and without KS, in patients with PD, and in patients with rupture and repair of the ACoA. These data suggest that differences in performance are related to the specific neuropathology of each patient group, and that compromised orbitofrontal integrity, as assessed by OA, may be related to compromise of the limbic system and/or basal forebrain pathology. This interpretation is consistent with other published data that is extensively reviewed by CitationOscar-Berman and Bardenhagen (1998). There, the authors discussed how results of many studies using OA and other comparative neuropsychological paradigms in both human and nonhuman animal subjects has helped to outline the differential impairment of frontal subsystems in patients with neurological diseases. Specifically, the authors noted that in the absence of discrete lesions restricted to precise areas of the prefrontal cortex, data from neurobehavioral experiments using human patients is limited to suggesting differing degrees of dysfunction and damage.
Our data also clearly demonstrate that damage affected by diverse neurological conditions differs with respect to the relative location within the prefrontal cortex as well as the nature and degree of functional impairment. Furthermore, these data are consistent with models of prefrontal function by CitationFuster (1997), CitationRolls (2004), Farah (CitationFellows and Farah 2005), and Shallice and colleagues (CitationShallice 2002; CitationStuss et al 2005) that divide the prefrontal cortex into distinct functional subregions, each controlling correspondingly distinct functional domains arising from specific cortico-cortical and subcortical connections. Although the various models address diverse underlying cognitive and affective operations of prefrontal functional subsystems (which can be measured separately by sensitive neurobehavioral tests), all of the models agree that orbitofrontal cortex is necessary for one’s ability to alter behavior flexibly. It is not surprising, therefore, that patients with damage to the orbitofrontal cortex and related limbic structures demonstrated poor performance on OA and make many perseverative errors on the WCST, as both measures are reflections of a person’s inability to strategically adapt a previously rewarded response (eg, see CitationRolls 2004). Finally, the data from the PD group support the view that this disease has different cognitive consequences with regard to the lateralization of pathology (CitationHappaney et al 2004). While all of the PD patients demonstrated compromised frontal-lobe integrity, the nature and extent of the deficits were specific to differential impairment of left and right frontal subsystems. In future studies, results of combined neuroimaging and neuropsychological tests with these patients will determine whether damage to right orbitofrontal cortex is associated with deficits in decision-making, emotional processing, and social conduct, as would be predicted by the models of CitationBechara (2004), CitationRolls (2004), and CitationShallice (2002).
Acknowledgments
Support for this work was provided by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) grants F31 AA05557, R37–AA07112, and K05–AA00219, and the Medical Research Service of the U.S. Department of Veterans Affairs.
References
- [APA] American Psychiatric Association1994Diagnostic and statistical manual of mental disordersFourth edition (DSM-IV)Washington, D.CAPA
- AntoniniAVontobelRPsyllaM1995Complementary positron emission tomographic studies of the striatal dopaminergic system in Parkinson’s diseaseArch Neurol521183907492293
- BatesMEConvitACalevA1999Neuropsychology and neuroimaging of alcohol and illicit drug abuseNeuropsychological functions in psychiatric disordersWashington, D.CAmerican Psychiatric Press373446
- BecharaA2004The role of emotion in decision-making: Evidence from neurological patients with orbitofrontal damageBrain and cognition (Special Issue: Development of orbitofrontal function)553040
- BentonALHamsherK1989Multilingual aphasia examinationIowa City, IOAJA Associates
- BergEA1948A simple objective technique for measuring flexibility in thinkingJ Gen Psychol39152218889466
- BooijJTissinghGBoerGJ1997[123I]FP-CIT SPECT shows a pronounced decline of striatal dopamine transporter labeling in early and advanced Parkinson’s diseaseJ Neurol Neurosurg Psychiatry62133409048712
- CarpenterMB1991Core text of neuroanatomyBaltimore, MDWilliams and Wilkins
- DeLucaJ1993Predicting neurobehavioral patterns following anterior communicating artery aneurysmCortex29639478124940
- DiamondBJDeLucaJKelleySM1997Memory and executive functions in amnesic and non-amnesic patients with aneurysms of the anterior communicating arteryBrain1201015259217685
- DunkerROHarrisAB1976Surgical anatomy of the proximal anterior cerebral arteryJ Neurosurg4425972
- FellowsLKFarahMJ2005Dissociable elements of human foresight: a role for the ventromedial frontal lobes in framing the future, but not in discounting future rewardsNeuropsychologia4312142115817179
- FolsteinMFFolsteinSEMcHughPR1975‘Mini-mental State.’ A practical method for grading the cognitive state of patients for the clinicianJ Psychiatric Res118998
- FreedmanM1990Object alternation and orbitofrontal system dysfunction in Alzheimer’s and Parkinson’s diseaseBrain Cognit14134432285509
- FreedmanMBlackSEbertP1998Orbitofrontal function, object alternation and perseverationCereb Cortex818279510382
- FreedmanMOscar-BermanM1986aBilateral frontal lobe disease and selective delayed response deficits in humansBehav Neurosci100337423730139
- FreedmanMOscar-BermanM1986bSelective delayed response deficits in Alzheimer’s and Parkinson’s diseaseArch Neurol43886903741206
- FusterJM1997The prefrontal cortex3rd edNYRaven Press
- HamiltonMA1960A rating scale for depressionJ Neurol Neurosurg Psychiatry23566214399272
- HappaneyKZeazoPDStussDT2004Development of orbitofrontal function: Current themes and future directionsBrain Cognit (Special Issue: Development of orbitofrontal function)55110
- HeatonRKCheluneGJTalleyJL1993Wisconsin Card Sorting Test (WCST) Manual revised and expandedOdessa, FLPsychological Assessment Resources
- HoakenPNSGiancolaPRPhilRO1998Executive cognitive functions as mediators of alcohol-related aggressionAlcohol Alcohol3347549539177
- HoehnMMYahrMD1967Parkinsonism: onset, progression and mortalityNeurology17427426067254
- JornJLRybarczykB1995Interpreting the confabulations of geriatric medical inpatients: two case studiesClinical Gerontologist165962
- KempsterPAGibbWRSternGM1989Asymmetry of substantia nigra neuronal loss in Parkinson’s disease and its relevance to the mechanism of levodopa related motor fluctuationsJ Neurol Neurosurg Psychiatry527262709038
- LeendersKLSalmonEPTyrrellP1990The nigrostriatal dopa-minergic system assessed in vivo by positron emission tomography in healthy volunteer subjects and patients with Parkinson’s diseaseArch Neurol47129082123623
- LevinHSEisenbergHMBentonAL1991Frontal lobe function and dysfunctionNYOxford University Press
- LevinBELlabreMMWeinerWJ1989Cognitive impairments associated with early Parkinson’s diseaseNeurology39557612927680
- LezakMD1995Neuropsychological assessment3rd edNew York, NYOxford University Press
- LichterDGCummingsJL2001Frontal-subcortical circuits in psychiatric and neurological disordersNew York, NYGuilford
- MelgaardBHenriksonLAhlgrenP1990Regional cerebral blood flow in chronic alcoholics measured by single photon emission computerized tomographyActa Neurol Scand8287932256449
- MiddletonFAStrickPLLichterDGCummingsJL2001A revised neuroanatomy of frontal-subcortical circuitsFrontal-subcortical circuits in psychiatric and neurological disordersNew York, NYGuilford4458
- MoselhyHFGeorgiouGKahnA2001Frontal lobe changes in alcoholism: A review of the literatureAlcohol Alcohol363576811524299
- MountainMASnowWG1993Wisconsin Card Sorting Test as a measure of frontal pathology: A reviewClin Neuropsychol710818
- MozleyPDSchneiderJSActonPD2000Binding of [99mTc] TRODAT-1 to dopamine transporters in patients with Parkinson’s disease and in healthy volunteersJ Nucl Med41584910768556
- NIAAA1993Eighth special report to the US Congress on alcohol and health NIH Publication No. 94–3699
- Oscar-BermanMNoronhaAEckardtMJWarrenK2000Neuropsychological vulnerabilities in chronic alcoholismReview of NIAAA’s neuroscience and behavioral research portfolio National Institute on Alcohol Abuse and Alcoholism Research Monograph – 34Bethesda, MDThe Institute14958
- Oscar-BermanMBardenhagenFTrosterA1998Nonhuman animal models of memory dysfunction in neurodegenerative diseaseMemory in neurodegenerative diseaseNYCambridge University Press320
- Oscar-BermanMEvertDNussbaumPD1997Alcoholic Korsakoff’s syndromeHandbook of neuropsychology and agingNYPlenum Press20115
- Oscar-BermanMKirkleySMGanslerDA2004Comparisons of Korsakoff and non-Korsakoff alcoholics on neuropsychological tests of prefrontal brain functioningAlcohol Clin Exp Res286677515100620
- Oscar-BermanMMarinkovicK2003Alcoholism and the brain: An overviewAlcohol Res Health271253315303622
- Oscar-BermanMZola-MorganSM1980Comparative neuropsychology and Korsakoff’s syndrome. II: Two-choice visual discrimination learningNeuropsychologia18513266449681
- PfefferbaumASullivanEVMathalonDHLimKO1997Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholicsAlcohol Clin Exp Res2152199161613
- ReitanRM1958Validity of the Trail Making Test as an indicator of organic brain damagePercept Mot Skills82716
- ReitanRMWolfsonD1993The Halstead Reitan neuropsychological test battery: Theory and clinical interpretationTucson, AZNeuropsychology Press
- RobinsLHelzerJCottlerL1989NIMH Diagnostic Interview Schedule: Version III revisedSt. Louis, MOWashington University
- RollsET2004The functions of the orbitofrontal cortexBrain Cognit55112915134840
- RoyallDRLauterbackECCummingsJL2002Executive control function: A review of its promise and challenges for clinical researchJ Neuropsychiatry Clin Neurosci1437740512426407
- Saint-CyrJA2003Frontal-striatal circuit functions: Context, sequence, and consequenceJ Internat Neuropsychological Soc910327
- SandsonJAlbertM1984Varieties of perseverationNeuropsychologia22715326084826
- ShalliceTStussDTKnightRT2002Fractionation of the supervisory systemPrinciples of frontal lobe functionNYOxford26177
- SpreenOStraussE1998A compendium of neuropsychological tests2nd edNew York, NYOxford University Press
- StussDTAlexanderMPShalliceT2005Multiple frontal systems controlling response speedNeuropsychologia4339641715707616
- SullivanEVDeshmukhADesmondJE2000Cerebellar volume decline in normal aging, alcoholism, and Korsakoff’s syndrome: Relation to ataxiaNeuropsychology143415210928737
- TaylorAESaint-CyrJALangAE1986Frontal lobe dysfunction in Parkinson’s diseaseBrain109845833779372
- TissinghGBergmansPBooijJ1998Drug-naïve patients with Parkinson’s disease in Hoehn and Yahr stages I and II show a bilateral decrease in striatal dopamine transporters as revealed by [123I]beta-CIT SPECTJ Neurol24514209457623
- VictorMRopperA2001Principles of neurology,New York, NYMcGraw-Hill
- WechslerD1997Wechsler Adult Intelligence Scale-IIINew York, NYThe Psychological Corporation
- WechslerD1997Wechsler Memory Scale-IIINYThe Psychological Corporation