Abstract
Specific memory deficits, reduced intellectual processing speed, and a variety of social and behavioral problems have been implicated as long-term effects of cranial radiation therapy (CRT). These deficits are thought to be related to changes in brain cytology and structure associated with microvascular aberrations. N-3 fatty acids may serve as protectants in pediatric patients who receive CRT for brain tumors. Timed-pregnant rat dams were fed one of four diets that were identical in all respects, except for their essential fatty acid content. The dams were placed on these diets at the beginning of the third trimester of gestation and their pups remained on them throughout the study. The rats’ behavioral response as judged by acoustic startle response (ASR) and neurocognitive response (performance in a radial maze, RM) were evaluated in relation to diet, gender, and CRT. The following hypotheses were tested: (1) female rats will show greater CRT-induced neurocognitive and behavioral deficits; (2) dietary n-3 fatty acids will diminish CRT-induced neurocognitive and behavioral deficits; (3) gender-specific differences would be dampened by n-3 fatty acids in the diet. All three hypotheses were partially supported. These findings are discussed in light of the potential neuroprotective effects of n-3 fatty acids.
Introduction
The standard treatment for pediatric brain tumors has been a combination of neurosurgery, chemotherapy, and cranial radiation therapy (CRT). The radiation is generally thought to work by destroying tumor cells as they divide (CitationArmstrong and Mulhern 1999). Five-year survival rates range between 60% and 80% following the use of radiation therapy (CitationStewart and Cohen 1998). Six-months to several years post-irradiation, deleterious side-effects manifest in the central nervous system (CNS). The most often implicated effects (demyelination and microvascular changes) are also thought causative (CitationSchultheiss et al 1995). Interestingly, the female gender has increased risk and this has been related to the differences in male and female myelination (CitationSchlieper et al 1989; CitationWaber et al 1990; CitationMulhern et al 1991). Late effects include demyelination with histopathologic loss of white matter, neurocognitive deficits of memory and intellectual processing speed, and a variety of social and behavioral problems including impaired social functioning and lowered quality of life (CitationKun and Mulhern 1983; CitationCarpentieri et al 1993; CitationDennis et al 1996; CitationMulhern et al 1998; CitationMulhern et al 1999; CitationFuemmeler et al 2002).
The difficulty in finding a way to prevent CRT-induced neurologic deficits lies in the fact that the neuroprotectant should not also protect the tumor. Investigators have tried varying either the dose of CRT or varying when (during the regimen of chemotherapy and surgery) the child receives CRT. These studies clearly show that the severity of late-effect neurocognitive deficit is directly related to the intensity of CRT; however, even after very low doses of CRT, the neurocognitive deficits persist (CitationMulhern et al 1998). Certain drugs are being investigated in a preclinical manner for pharmaceutical neuroprotection but crossing the blood–brain barrier remains a problem for this avenue of intervention (CitationCapizzi 1999).
In this study, we take another approach to neuroprotection – dietary essential fatty acids. Essential fatty acids readily cross the blood–brain barrier and the accumulation of the omega-3 (n-3) essential fatty acids are considered necessary for proper development of brain and retina (CitationBenolken et al 1973; CitationAnderson et al 1990; CitationAgostoni et al 1995; CitationBirch et al 2000). Importantly, the n-3 fatty acids perturb development of tumors (CitationJurkowski and Cave 1985; CitationCave 1991; CitationZeriyga et al 1996), improve learning (CitationSuzuki et al 1998; CitationGamoh et al 1999), and they are at the same time neuroprotective. The n-3 fatty acids inhibit neuronal apoptosis (CitationRotstein et al 1997; CitationKim et al 2000) and they reduce the damage that follows middle cerebral artery occlusion and pharmacologically induced excitotoxic damage (CitationBourre et al 1989; CitationRelton et al 1993; CitationOkada et al 1996).
To model this situation, we used rats that were fed one of four diets that were identical in all respects, except for their essential fatty acid content. We tested the following hypotheses: (1) female rats will show greater CRT-induced neurocognitive and behavioral deficits; (2) dietary n-3 fatty acids will diminish CRT-induced neurocognitive and behavioral deficits; (3) gender-specific differences would be dampened by n-3 fatty acids in the diet.
Materials and methods
Rats
All experimental animals (Sprague-Dawley rats) were handled in accordance with protocols approved by the University of Oklahoma Health Sciences Center (OUHSC) Institutional Animal Care and Use Committee. The rats were housed under 12 hours light/dark cyclic light in an AAALAC-approved colony on the OUHSC campus. Experimental rats were maintained on one of four semisynthetic diets (Dyets Inc., Bethlehem, PA, USA). They were born of timed-pregnant dams that were fed the same diets since the third trimester of pregnancy. Contents of protein, carbohydrate, vitamins, and fat were identical for all the diets; the only difference between them was their fatty acid composition. Fat content was 7% (g/g) of the diet. The majority of dietary fat was provided with soybean oil (SO). The fatty acid content of diet 1 was 100% SO. Diet 2 was made with 95% SO and 5% DHASCO® (n-3 docosahexaenoic acid, DHA). Diet 3 was 95% SO and 5% ARASCO® (n-6 arachidonic acid, AA). Diet 4 was 90% SO, 5% DHASCO®, and 5% ARASCO®). Rats were fed ad libitum except during the maze task when they were maintained on only 10–15 g of their respective diet (given approximately 1–2 hours after each testing session).
Radial maze (RM) task
In a room 3–4 m square, an 8-arm RM was constructed entirely of 1.6-cm plywood. Each arm (runway) was 93 cm long, 14 cm wide, and had walls 11 cm tall. The entire RM was painted black. It sat on a tabletop (painted white) 72-cm tall and 80-cm square. One side of the maze was positioned flush with the north wall. Visual cues including wall posters, shelves, counters, and lights, and experimenters were maintained in constant positions around the maze. Test sessions were run in standard illumination conditions, ie, overhead fluorescent lights. Sugar-coated cereal was used as bait for the runways.
Rats were tested on their abilities to learn a working–reference memory task. Reference memory was evaluated by assessing the rats’ abilities to avoid entering un-baited arms and to enter only the baited arms. Working memory was assessed by the rats’ abilities to enter each baited arm only once during the test. To perform well, rats must remember whether they visit and retrieve bait from a particular arm during that session. Maze arms were either baited (4 arms) with a sugared cereal or unbaited (4 arms). For each rat, either the odd (1, 3, 5, 7) or the even numbered arms (2, 4, 6, 8) contained bait. These assignments remained the same for each rat throughout the study. At the beginning of each session, rats were placed in the center of the maze, always facing north and away from the experimenter. Rats could freely explore all 8 arms of the maze, retrieving and eating baits as they found them. Training and testing sessions ran for 10 minutes or until all of the baits were retrieved.
The maze study had 3 phases: habituation, acquisition, and testing. Habituation was for two days when rats were allowed to freely explore the maze for 10 min. So the rats would associate the apparatus with food, sugar coated cereal was scattered randomly throughout the maze on the first day. On the second day, food was placed in the food cups at the end of each arm. The acquisition of the maze task began on day 3 and proceeded for 23 days thereafter, one session/day. Rats were always placed into the maze facing the same direction. They explored the maze until all baits were retrieved or 10 minutes had elapsed, whichever came first. The rats were then returned to their home cages. The testing phase of the RM task for CRT-treated rats began 3 weeks after CRT and 6 weeks (41 days) after the retention tests. Rats not receiving CRT were tested in the same conditions as before irradiation. Retention and savings of the task was assessed as a function of dietary condition, gender, and irradiation. Retention was assessed by measuring performance on the first day of re-training. Savings were measured by performance in re-learning the task.
Acoustic startle response (ASR)
The ASR apparatus had four simultaneously operating startle cubicles, each controlled by a DOS-compatible computer and Med Associates startle software. The computer connects to the startle cubicles through a serial microcontroller and rack mount interface. Rats were placed in cylindrical lucite containers which rested upon a startle platform consisting of a transducer, amplifier, and 60-Hz line noise filter. Auditory stimuli were produced with a program acoustic stimulator (1000–4000 Hz, 58–130 db). Startle signals were processed through a high-speed analog/digital converter (all startle hardware from Med Associates).
Sessions took place in a dark room but a dim red light illuminated each startle chamber. A 72 db white noise background was provided in each chamber. Startle and pre-pulse stimuli consisted of white noise bursts (115/82 db, 50/20 ms duration, respectively, 5 ms rise/fall time). Each session consisted of 3 blocks of 20 stimulus trials (60 trials total). Blocks 1 and 3 each consisted of 4 different stimulus conditions (5 trials each): no stimulus (N), PP alone (P), startle alone (S), or startle + pre-pulse (SP), which were randomly intermixed within blocks. Block 2 consisted of N and S stimuli (10 trials each). Trials were separated by variable, randomly-determined interstimulus intervals ranging from 10 to 30 sec. Rats were tested before and 18 weeks after CRT in sessions lasting for 3 consecutive days.
Cranial irradiation protocol
The rats were anesthetized with a mixture of ketamine (120 mg/kg body weight) and xylazine (6 mg/kg body weight) administered by intraperitoneal injection. Each anesthetized rat underwent treatment field localization using a conventional simulator (Kermath Simulator TSL). Customized Cerrobend® alloy shielding was designed to protect nasopharyngeal, oral cavity, and oropharygeal structures. Daily shielding would allow the rats to eat normally by minimizing acute mucosal reactions. A Varian 6/100 c linear accelerator (Varian Medical Systems, Palo Alto, CA) was used. The treatment regimen used right and left lateral ports to provide a total of 20 Gy delivered in 4-Gy fractions over 5 consecutive days. All of the tested rats underwent a biparietal thickness measurement prior to the start of therapy. A single dosimetric calculation was used for all subjects since the cranial measurements varied by only +/− 3 mm. The treatment dose was calculated at dmax for the 6 MV accelerator (1.5 cm). A solid state system for measuring ionizing radiation was used to verify the dose delivered. Lithium fluoride chips (TLDs) positioned at central axis points on either side of the cranium were exposed to the calculated number of monitor units required to provide a total of 400 cGy. The TLDs were processed within 24 hours of exposure. Results showed a difference of +/− 2% of the calculated dose. Port films were also obtained prior to the start of therapy to document proper alignment of the Cerrobend® shields.
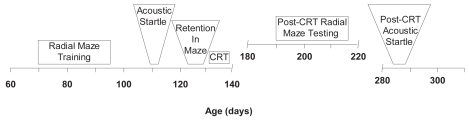
Time line
Testing in the RM maze and for ASR began prior to CRT (). The CRT was followed by another set of RM and ASR tests. Rats were 70 days old when the pre-CRT testing was begun in the RM. These tests lasted for 26 days. The ASR tests were begun approximately 40 days later and lasted for 3 days. Five days of retention tests in the RM were administered 1 week later. This was followed immediately by 5 days of CRT. Post-CRT testing in the RM began 5 weeks after CRT (27 weeks old). Post-CRT testing for ASR began 13 weeks later when the rats were 41 weeks old. Rats not receiving CRT (n = 12) passed through the acquisition phase of the RM with those that did get CRT (n = 12). The non-CRT group was tested at 21 weeks (150 days and 6 weeks after their retention tests). The CRT group was tested at 27 weeks of age (192 days of age and 6 weeks after their retention tests).
Design and analysis
Data for both were subjected to between and within analyses of variance (ANOVA) and were collected in two distinct phases, ie, RM and ASR. For both phases, between-group factors included diet, sex, and age. Dependent variables for the RM phase were measures of latency and errors. Reference memory errors consisted of any entries into un-baited maze arms. Working memory errors consisted of re-entries into maze arms that have already been visited once. Errors were subdivided and analyzed according to whether they consisted of immediate re-entry into the arm last visited (perseveration) or visitation to other erroneous arms (other). These error analyses were used to assess whether specific memory processes were related to erroneous selection or random errors on the part of the animals. Number and location of arm entries were recorded as a measure of baseline performance and general locomotor activity.
Results
RM tests
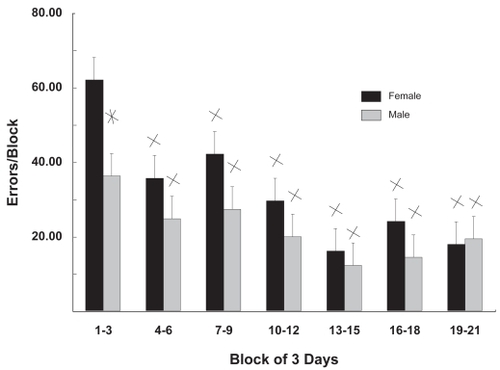
The rats used in this study were fed fatty acid-supplemented diets from the beginning of the third trimester of development. The same group of rats was tested for ASR and their behavior in the RM. Tests were administered before and after CRT. In the aquisition phase of the RM tests (), mean errors per day were summed into blocks of 3 days. Female rats tended to make more errors than males and in block 1 (days 1–3) made 63% more errors than males. A statistically significant difference between males and females was seen only in block 1. The number of errors decreased relative to block 1 in subsequent testing blocks. No significant effects of diet were observed in this phase (acquisition) of the test; therefore, values in are the means for all animals tested irrespective of diet.
Figure 2 Gender differences in maze acquisition. Mean errors/day as a function of 3-day acquisition blocks are shown for males and females. Female rats made more errors than males in the first 3 days of testing; subsequently their performances were similar. No significant effects of diet were observed so values are the means for all animals tested.
Values are mean ± standard error.
*Gender specific difference; p < 0.006; Student’s t tests; n = 12.
XDifferent from day 1–3; p < 0.02; Student’s t tests; n = 12.
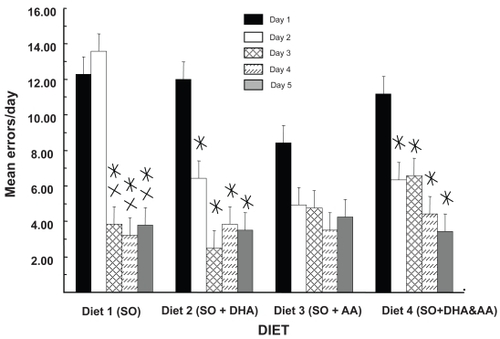
Rats were tested twice for their retention of the RM. Each of these tests ran for 5 days. The first retention tests were ~7 weeks after the acquisition tests, and the second retention tests were administered ~6 weeks after CRT (~13 weeks after the acquisition tests). The values in are the means for all animals tested in the first retention test – both male and female. These data show that diet had an effect. Rats made more errors on day 1 than on days 3, 4, and 5. There were also no diet-associated differences in performance at day 1, but enhanced savings of the task was indicated by the fact that rats fed the DHA supplement (diets 2 and 4) had significantly fewer errors at day 2 compared with day 1.
Figure 3 DHA facilitates maze retention. The data reveal the interaction of diet and testing day without regard for gender and cranial radiation therapy. Values are mean errors/day. In DHA-fed rats (diets 2 and 4), there are significant decreases in errors from day 1 to day 2, but not in diets 1 and 3. There were no significant effects of diet at day 1.
Values are mean ± standard error.
*Different from day 1; p < 0.03; simple effects analysis; Newman-Keuls; n = 12.
XDifferent from day 2; p < 0.02; simple effects analysis; Newman-Keuls; n = 12.
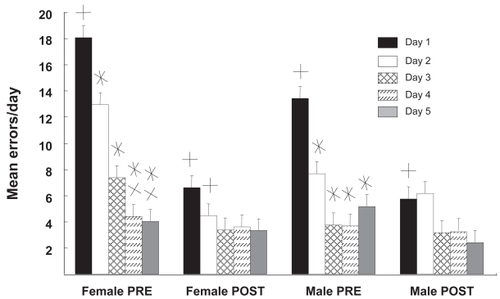
Effects of gender and CRT in RM retention are revealed in where data from both retention tests are presented. Differences between the first and second retention tests showed that the rats improved in their overall ability to run the RM and there was only a minor effect of CRT. The tests revealed that the gender differences seen in the first days of acquisition testing were retained in the retention phases of the test, ie, females made 33% more errors in the first retention test. The second retention test (post-CRT) suggests that RM performance was reaching asymptote because there was no statistical difference between days 1–5. It is interesting to note, however, that there were significant differences between days 1 and 2 for both males and females in the pre-CRT tests and these were lost in the post CRT-test. This could be an indicator of CRT-induced impairment, though this finding would require further studies to document. None of the diets seemed to influence this post-CRT effect.
Figure 4 Sex differences in radial maze retention. Mean errors/day for males and females as a function of testing phase and day are shown. The “PRE” retention tests were given ~7 weeks after the initial acquisition. The “POST” tests were given ~13 weeks later which was also ~6 weeks after cranial radiation therapy (CRT). During the PRE-CRT retention test, males but not females reached a performance asymptote by day 2. The number of errors made by females on days 1 and 2 were significantly higher in the PRE-CRT test than in the POST-CRT test; this was seen in males at day 1 only. Although females tended to make more errors on the first day of PRE-CRT testing than males, the difference was not significant. There were no significant differences in error rates for males or females at any day during the POST-CRT tests.
Values are mean ± standard error.
*Different from day 1; p < 0.03; simple effects analysis; Newman-Kules; n = 12.
XDifferent from day 2; p < 0.02; simple effects analysis; Newman-Kules; n = 12.
+Different from corresponding day pre- vs post-; p < 0.01; planned comparisons test; n = 12.
ASR tests
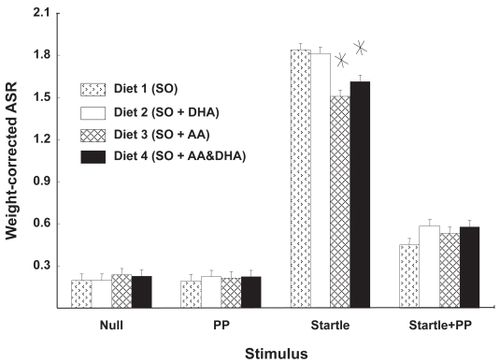
The ASR values used in these experiments were corrected for the weight of the rats in this study, ie, the magnitude of the response was divided by the rat’s weight. shows the effects of diet on the ASR. There were no differences between the “null” condition and the prepulse condition, nor were there any affects of diet in these conditions. As expected, there was significantly more response after the 115-db startle stimulus and administration of an 82-db prepulse stimulus significantly diminished these startle responses. Rats fed AA (diets 3 and 4) tended to have lower startle responses. Comparison of diets in the startle-plus-prepulse (SP) condition suggested a tendency for diet 1 to show lowered ASR with respect to diets 2, 3, and 4 combined. However, this trend did not achieve significance (p < 0.07).
Figure 5 Rats fed AA had diminished ASR. Weight-corrected ASR magnitudes are shown as function of diet and stimulus condition. The response to acoustic startle was not significant for any single dietary supplement, but when the responses of AA-fed rats were compared with those that did not get AA, a decreased response to startle stimulus was evident in the rats that were fed AA.
Values are mean ± standard error.
*Different from diets 1 and 2; p < 0.006; planned comparisons; n = 24.
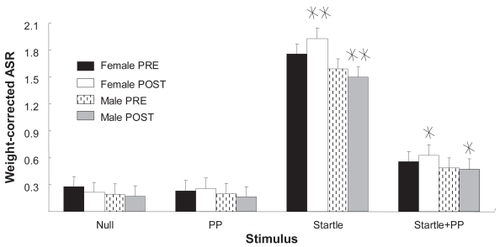
shows the effects of CRT and gender in ASR. Overall ASR magnitude in the S condition was significantly higher than in the SP condition, and both were significantly higher than N or P. Planned comparisons between males and females before and after CRT showed that male rats responded similarly to females prior to CRT. However, following CRT, the male S and the male SP responses were diminished in comparison with those of the females. As in , the dietary regimens employed here could not attenuate the affects of CRT. There were also no significant gender differences in the pre-CRT phase, but females did tend to have higher ASR, suggesting that CRT heightened the differences between males and females.
Figure 6 Gender differences in startle and startle + prepulse. Weight-corrected ASR magnitudes are shown as function of sex, treatment phase, and stimulus condition. In the startle and the startle-plus-pre-pulse conditions, female ASR magnitude after cranial radiation therapy was significantly greater than that of males.
Values are mean ± standard error.
*Gender-specific difference; p < 0.02; planned comparisons; n = 12.
**Gender-specific difference; p < 0.004; planned comparisons; n = 12.
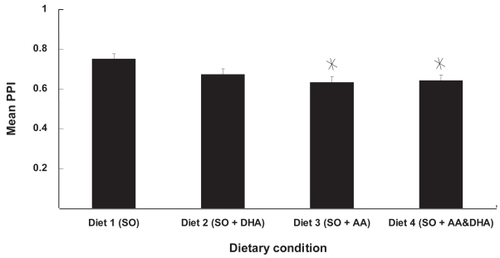
Pre-pulse inhibition (PPI) is the percent inhibition of startle induced by a prepulse. shows the effect of diet on prepulse inhibition. There was no effect of CRT on prepulse inhibition, so values in combine the scores of all rats – male and female, before and after CRT. Rats that were fed AA had diminished prepulse inhibition relative to those fed either soy alone (diet 1) or soy plus DHA (diet 2). There was also a tendency for rats supplemented with docosahexaenoic acid (DHA) to have lower PPI scores than the SO only group, but this difference was not significant. Lower PPI scores were primarily determined by slightly elevated SP responding () in diets 2, 3, and 4, combined with relatively depressed S responding in diets 3 and 4.
Figure 7 AA supplementation decreased PPI. PPI scores are shown as function of diet. Rats fed diets supplemented with AA had significantly lower PPI scores than rats fed SO only.
Values are mean ± standard error.
*Different from control (diet 1); p < 0.04; Newman-Kules; n = 24.
Collectively, the results show that except for the loss of a difference between days 1 and 2, there were no effects of CRT on rats in the RM tests but effects we seen in the ASR test; therefore values in (RM retention) are the combined result of rats tested before. There were effects for diet and gender in maze retention. In DHA-fed rats (diets 2 and 4), there are significant decreases in errors from day 1 to day 2, but not in diets 1 and 3. There were no significant effects of diet at day 1.
Discussion
The neurocognitive loss of children that is seen after CRT prompted us to use the RM and ASR tests to assess the impacts that dietary essential fatty acid supplements and gender might have on neurological changes after CRT. The RM test has been used to assess the roles of DHA in neurocognitive performance (CitationGamoh et al 1999) while the ASR evaluates more fundamental levels of behavior that are related to stress–stimulus reactivity and reflex excitability (CitationDavis 1980). Results are conflicting regarding the effect of n-6 and n-3 fats in water maze performance. Because one study shows that dietary n-6/n-3 ratio fatty acids had no effect on rat performance in the Morris water maze (CitationWainwright et al 1999) and another indicates circulating cortisol and stress induced deficits in a Morris water maze are diminished when the n-6/n-3 ratio is reduced (CitationYehuda et al 2000), we decided to evaluate stress using the ASR test.
This randomized, clinical trial of the effects of different diets on neuroprotection from the damaging effects of CRT in rats showed partial support for our hypotheses. Specifically: Hypothesis 1 predicted that female gender would be associated with significantly more neurocognitive and behavioral deficits in irradiated rats. Our results demonstrated partial support for this hypothesis. Female rats showed significantly worse abilities in the ASR paradigm following CRT; however, there were no significant changes in either males or females following CRT in the RM paradigm. It may be that female rats are not differentially affected by CRT. Also, we may not have allowed enough time to elapse between CRT and testing, thus masking findings that would otherwise have declared themselves at a later date. However, these mixed findings raise interesting questions about the sensitivity and specificity of the two neurocognitive and behavioral paradigms that were used in this study.
It may be that the RM test is not as sensitive at detecting CRT-induced damage in rats. We decided to use the RM test due to its ability to tap visual memory in animals.
The literature is replete with reports establishing the RM paradigm as a standard measure for animal behavior and memory. However, the clinical literature indicates that children who receive CRT show more deficits in the areas of verbal memory and processing speed. Therefore, the RM may not be an ideal paradigm to test for these deficits. Future studies utilizing multiple neurocognitive and behavioral tests are necessary.
Hypothesis 2 stated that omega-3 fatty acids would be associated with significantly less neurocognitive and behavioral deficits in irradiated rats. Again, our findings indicated limited support for this hypothesis. On the ASR task (but again not on the RM task), rats fed some form of AA showed significantly increased PPI after CRT than those rats fed either DHA or standard chow. This indicates that AA, but not DHA, offered some protection from CRT on the ASR task, but not the RM task. The arguments put forward above bear repeating here, namely that RM may not be an ideal task to use in testing for CRT-induced neurocognitive and behavioral damage.
Beyond this finding, it is intriguing that AA and not DHA showed significant protective abilities after CRT. Future studies should attempt to replicate this initial finding, and test for possible neurobiological explanations for this effect. It may be that AA and not DHA protect those aspects of the brain that are involved in PPI on the ASR. However, before rats were given CRT, those animals in the RM task which received DHA showed fewer errors from day 1 to day 2. Similarly (ie, pre-CRT), those rats in the ASR task which received AA also had lower startle responses.
Hypothesis 3 predicted a significant gender by diet interaction, ie, female irradiated rats fed omega-3 fatty acids would have significantly fewer neurocognitive and behavioral deficits. Our findings offered no direct support for this hypothesis. While we did see significant gender effects of CRT on certain neurocognitive and behavioral measures, we did not see any protective effects of these specific diets on gender.
Overall, our findings demonstrate more deleterious effects of CRT on female rats on certain measures but not others. Specifically, ASR as opposed to RM seemed to show more pronounced measurable effects. It may be that some of the subtle neurocognitive changes associated with CRT are better detected using the ASR rather than the RM. These subtle changes may involve cortical inhibition, as opposed to primarily visual memory. However, it is curious that one of the reported areas of deficit in the clinical (human) literature is visual/spatial memory. This lack of significant effect on the RM may be related to the intensity of CRT (CitationKondziolka et al 1996) or different rates and neuronal development – locations of arborization and pruning in males and females. Another hypothesis that may account for these findings relates to the basic difference between human and rat in the sensory inputs used to form spatial memory. Laboratory rats are practically blind, and navigate (form spatial memory) based on tactile input from whiskers, while humans base spatial memory on visual input. It could be that neuronal areas associated with visual-spatial deficits following CRT in humans may not be affected in rats; conversely, neuroprotectants associated with preserved or improved visual functioning (such as DHA and AA) may not have an effect in rats, as these animals tend to use other modalities for forming spatial memory. Our findings raise the necessity of further studies using other measures of other abilities believed to be affected by CRT.
These findings should be interpreted within the limitations of this study. Our data indicate that the RM, a standard measure of animal behavior and memory did not reveal CRT-induced deficits. As discussed above, the measures of neurocognitive and behavioral abilities may not have efficiently tapped brain areas associated with CRT in rats. Similarly, we may not have given the animals enough time before testing them to see a demonstrable effect from CRT and diet. The amount of diet given may have not been enough to show an effect. Finally, it may be that there is simply no effect of CRT on the RM in rats.
It is our hope that any significant findings from this early, preclinical trial can be eventually translated to human populations in order to lessen the late effects of cranial radiation therapy. Ultimately, the goal of this study is to improve to quality of life for children diagnosed and treated for pediatric brain tumors.
Acknowledgments
This research was supported by NIDA 5T32 DA07248, Presbyterian Health Foundation Equipment Grant (F.A. Holloway). Presbyterian Health Foundation Seed Grant (R.E. Martin). Oklahoma Center for Advancement of Science and Technology (R.E. Martin). DHASCO® and ARASCO® were donated by Martek Biosciences Corp.
References
- AgostoniCRivaETrojanS1995Docosahexaenoic acid status and developmental quotient of healthy term infantsLancet3466387651024
- AgostoniCTrojanSBelluR1995Neurodevelopmental quotient of healthy term infants at 4 months and feeding practice:the role of long-chain polyunsaturated fatty acidsPediatr Res3826267478826
- AndersonGJConnorWECorlissJD1990Docosahexaenoic acid is the preferred dietary n-3 acid for the development of the brain and retinaPediatr Res2789972136947
- ArmstrongFDMulhernRKBrownRT1999Acute lymphoblastic leuykemias and brain tumorsCognitive aspects of chronic illness in childrenNew YorkGuilford4777
- BenolkenRMAndersonREWheelerTG1973Membrane fatty acids associated with the electrical response in visual excitationScience182125344752217
- BirchEEGarfieldSHoffmanDR2000A randomized controlled trial of early dietary supply of long-chain polyunsaturated fatty acids and mental development in term infantsDev Med Child Neurol421748110755457
- BourreJMFrancoisMYouyouA1989The effects of dietary a-linolenic acid on the composition of nerve membranes, enzymatic activity, amplitude of electrophysiological parameters, resistance to poisons and performance of learning tasksJ Nutr1191880922576038
- CapizziRL1999The preclinical basis for broad-spectrum selective cyto-protection of normal tissues from cytotoxic therapies by amifostineSemin Oncol2632110348255
- CarpentieriSCMulhernRKDouglasS1993Behavioral resiliency among children surviving brain tumors: A longitudinal studyJ Clin Child Psychol2223646
- CaveWT1991Dietary n-3 (omega-3) polyunsaturated fatty acid effects on animal tumorigenesisFASEB J5216061673664
- DaigleJLHongJHChiangCS2001The role of tumor necrosis factor signaling pathways in the response of murine brain to irradiationCancer Res88596511751409
- DavisM1980Neurochemical modulation of sensory-motor reactivity: acoustic and tactile startle reflexesNeurosci Biobehav Rev4241636106916
- DennisMSpieglerBJHetheringtonCR1996Neuropsychological sequelae of the treatment of children with medulloblastomaJ Neurooncol29911018817420
- FuemmelerBFElkinTDMullinsLL2002Survivors of childhood brain tumors:Behavioral, emotional, and social adjustmentClin Psychol Rev225478512094511
- GamohSHashimotoMSugiokaK1999Chronic administration of docosahexaenoic acid improves reference memory-related learning ability in young ratsNeuroscience32374110430487
- GlantzMJBurgerPCFriedmanAH1994Treatment of radiation-induced nervous system injury with heparin and warfarinNeurology44202077969953
- JurkowskiJJCaveWT1985Dietary effects of menhaden oil on the growth and membrane lipid composition of rat mammary tumorsJ Natl Cancer Inst741145503858582
- KimHYAkbarMLauA2000Inhibition of neuronal apoptosis by docosahexaenoic acid (22:6n-3):Role of phosphatidylserine in antiapoptotic effectJ Biol Chem275352152310903316
- KondziolkaDSomazaSComeyC1996Radiosurgery and fractionated radiation therapy:comparison of different techniques in an in vitro rat glioma modelJ Neurosurg84103388847568
- KunLEMulhernRK1983Neuropsychologic function in children with brain tumors:II. Serial studies of intellect and time after treatmentAm J Clin Oncol6651656637877
- MulhernRKFaircloughDOchsJ1991A prospective comparison of neuropsychologic performance of children surviving leukemia who received 18-Gy, 24-Gy or no cranial irradiationJ Clin Oncol7166062809681
- MulhernRKKepnerJLThomasPR1998Neuropsychologic functioning of survivors of childhood medulloblastoma randomized to receive convnetional or reduced-dose cranial-spinal irradiation: A Pediatric Oncology Group studyJ Clin Oncol16172389586884
- MulhernRKReddickWEPalmerSL1999Neurocognitive deficits in medulloblastoma survivors and white matter lossAnn Neurol468344110589535
- OkadaMAmamotoTTomonagaM1996The chronic administration of docosahexaenoic acid reduces the spatial cognitive deficit following transient forebrain ischemia in ratsNeuroscience7117258834389
- ReltonJKStrijbosPJLMCooperAL1993Dietary N-3 fatty acids inhibit ischaemic and excitotoxic brain damage in the ratBrain Res Bull3222368374800
- RisMDNollRB1994Long-term neurobehavioral outcome in pediatric brain-tumor patients:Review and methodological critiqueJ Clin Exp Neuropsychol1621428150888
- RotsteinNPAveldañoMIBarrantesFJ1997Apoptosis of retinal photoreceptors during development in vitro:Protective effect of docosahexaenoic acidJ Neurochem69504139231708
- SchlieperAEEsseltineDWTarshisMA1989Cognitive function in long-term survivors of childhood acute lymphoblastic leukemiaPediatr Hematol Oncol6192641693
- SchultheissTEKunLEAngKK1995Radiation response of the central nervous systemInt J Radiat Oncol Biol Phys3110931127677836
- StewartESCohenDG1998Central nervous system tumors in childrenSemin Oncol Nurs1434429503513
- SuzukiHParkSJTamuraM1998Effect of the long-term feeding of dietary lipids on the learning ability, fatty acid composition of brain stem phospholipids and synaptic membrane fluidity in adult mice:a comparison of sardine oil diet with palm oil dietMech Ageing Dev101119289593318
- WaberDPUrionDKTarbellNH1990Late effects of central nervous system treatment of acute lymphocytic leukemia in childhood are sex-dependentDev Med Child Neurol32238482311827
- WainwrightPEXingHCWardGR1999Water maze performance is unaffected in artificially reared rats fed diets supplemented with arachidonic acid docosahexaenoic acidJ Nutr12910798910222403
- YehudaSRabinovitzSCarassoRL2000Fatty acid mixture counters stress changes in cortisol, cholesterol, and impair learningInt J Neurosci101738710765992
- ZerougaMStillwellWStoneJ1996Phopholipid class as a determinant in docosahexaenoic acid’s effect on tumor cell viabilityAnticancer Res16286388917399