Abstract
Premature ejaculation (PE) is a common male sexual disorder. Recent normative data suggest that men with an intravaginal ejaculatory latency time (IELT) of less than 1 minute have “definite” PE, while men with IELTs between 1 and 1.5 minutes have “probable” PE. Although there is insufficient empirical evidence to identify the etiology of PE, there is limited correlational evidence to suggest that men with PE have high levels of sexual anxiety and inherited altered sensitivity of central 5-HT (serotonin) receptors. Pharmacological modulation of the ejaculatory threshold using off-label daily or on-demand selective serotonin re-uptake inhibitors (SSRIs) offers patients a high likelihood of achieving improved ejaculatory control within a few days of initiating treatment, consequential improvements in sexual desire and other sexual domains and is well tolerated. Investigational drugs such as the ejaculo-selective serotonin transport inhibitors (ESSTIs) such as dapoxetine and UK-390,957 represent a major development in sexual medicine. These drugs offer patients the convenience of on-demand dosing, significant improvements in IELT, ejaculatory control, and sexual satisfaction with minimal adverse effects.
Introduction
Although premature ejaculation (PE) is one of the most common male sexual disorders and has been estimated to occur in 4%–39% of men in the general community, there is a lack of a universally accepted definition (CitationReading and Wiest 1984; CitationNathan 1986; CitationSpector and Boyle 1986; CitationSpector and Carey 1990; CitationGrenier and Byers 1997; CitationLaumann et al 1999; CitationRosen et al 2004). Medical literature contains several univariate and multivariate operational definitions of PE. The lack of agreement as to what constitutes PE has hampered basic and clinical research into the etiology and management of this condition. The World Health Organization (WHO) 2nd International Consultation on Sexual Health defined it as “... persistent or recurrent ejaculation with minimal stimulation before, on, or shortly after penetration, and before the person wishes it, over which the sufferer has little or no voluntary control which causes the sufferer and/or his partner bother or distress...” (CitationLue et al 2004). This multivariate definition encompasses the main dimensions of PE–ejaculatory latency, control, bother and sexual satisfaction.
Although PE may affect the level of sexual satisfaction of both men and/or their partners, few studies have examined the impact of PE on the man, his partner and/or their relationship (CitationRowland et al 2001; CitationByers and Grenier 2003). Many patients are reluctant to seek help and to discuss this issue with their physician out of embarrassment and uncertainty whether effective treatment options are available. In many relationships, PE causes few if any problems. Couples may reach an accommodation of the problem through various strategies – young men with a short refractory period may often experience a second and more controlled ejaculation during a subsequent episode of lovemaking. Frequently however, PE eventually leads to significant relationship problems with partners regarding the man as selfish and developing a pattern of sexual avoidance. This only worsens the severity of the prematurity on the occasions when intercourse does occur.
Epidemiology
Most community-based epidemiological studies are limited by their use of either diagnosis by patient self-report of PE or inconsistent and poorly validated definitions of PE. Furthermore, subjective complaints of PE may have differing meanings in different cultures and the attitude of the partner and, in heterosexual relationships, her culturally determined extent of emancipation may impact upon the subjective patient’s diagnosis of PE.
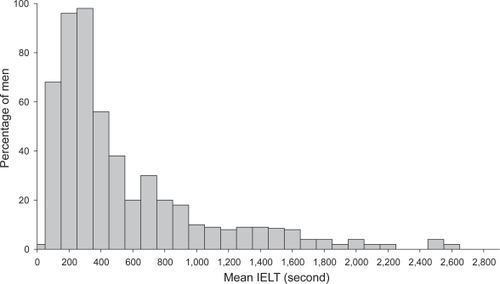
A recent multinational community-based age-ranging study of an unselected “normal” population of 500 heterosexual couples which involved stopwatch timing of the intravaginal ejaculatory latency time (IELT) during sexual intercourse has provided previously lacking normative data (CitationWaldinger, Quinn, et al 2005). This study demonstrated that the distribution of the IELT was positively skewed, with a median IELT of 5.4 minutes (range, 0.55–44.1 minutes) (). The median IELT decreased significantly with age, from 6.5 minutes in the 18–30 years group, to 4.3 minutes in the group older than 51 years (p < 0.0001). The median IELT varied between countries, with the median value for Turkey being the lowest, ie, 3.7 minutes (0.9–30.4 minutes), which was significantly different from each of the other countries. The median IELT value was not affected by condom use or circumcision status. The authors regarded the 0.5 and 2.5 percentiles as acceptable standards of disease definition in this type of skewed distribution, and proposed that men with an IELT of less than 1 minute (belonging to the 0.5 percentile) have “definite” PE, while men with IELTs between 1 and 1.5 minutes (between 0.5 and 2.5 percentile) have “probable” PE (CitationWaldinger, Zwinderman, et al 2005). Further community-based large-scale international studies using the same methodology but also exploring the dimensions of control, sexual satisfaction, and bother/distress are required to confirm and expand upon these initial findings.
Figure 1 Distribution of intravaginal ejaculatory latency times (IELT) values in a random cohort of 491 men. Reprinted with permission from CitationWaldinger MD, Quinn P, Dilleen M, et al. 2005. A multinational population survey of intravaginal ejaculation latency time. J Sex Med, 2:492–7. © 2005 Blackwell Publishing.
There are few published data on impact of birth country, religion, or culture on the prevalence of PE. Anecdotally, PE is more commonly reported by adolescents or young adults. An increased susceptibility to PE in men from the Indian subcontinent has been reported (CitationBhatia and Malik 1991; CitationVerma et al 1998). PE is more frequently reported by men in East Asia (China, Indonesia, Japan, Korea, and Malaysia), and less frequently by men in Middle Eastern and African countries (Algeria, Egypt, Morocco, and Turkey). However, a recent study reported a preponderance of men from Middle Eastern and Asian backgrounds living in the United Kingdom presenting for treatment of PE which exceeded the representation of these ethnic groups in the local population (CitationRichardson and Goldmeier 2005). The demonstrated variance in IELT between countries may be related to genetic or sociocultural factors but additional studies are required to confirm these findings.
The premise that PE is a psychosomatic disturbance and due to a psychologically overanxious personality was first suggested by CitationSchapiro in 1943. He classified PE as either primary (lifelong) or secondary (acquired) (CitationSchapiro 1943). The behavioristic view that chronic PE was the result of performance anxiety related to a disturbing initial episode of PE was first proposed by CitationMasters and Johnson (1966). Most of the behavioral treatments currently used are based on this premise.
In a study of 1326 consecutive men with PE, lifelong PE was present in 736 men (74.4%) and acquired PE was present in 253 men (25.6%) (CitationMcMahon 2002). Men with PE appear younger than those without, and after adjusting for concomitant erectile dysfunction (ED) the risk of PE significantly decreased with aging (CitationFasolo, Mirone, et al 2005). Higher levels of education and divorce appear to increase the risk of PE. A decreased risk of PE has been reported in men with treated diabetes, and no association was found with hypertension, cardiac disease, hypercho-lesterolemia, and peripheral or central neuropathy. Men with self-reported PE have a lower frequency of sexual intercourse, higher levels of intercourse-related anxiety, and note greater impairment in intercourse satisfaction, and sexual relationship satisfaction compared to men without PE (CitationPerelman et al 2004). However, they do not report a reduced quality of life, reduced sexual desire or a reduced ability to become sexually aroused (CitationLue et al 2004; CitationPerelman et al 2004).
Over the past 15 years, an increasing number of publications have reported the pharmacological treatment of PE with a variety of different medications, which act centrally or locally to retard the psycho-neurological control of ejaculation and subsequent orgasm (CitationMontague et al 2004). It is well established that major tranquillizers and selective serotonin re-uptake inhibitor drugs (SSRIs) retard ejaculation significantly and will, in a small percentage of men, result in an ejaculation (CitationKotin et al 1976; CitationMonteiro 1987; CitationDeveaugh-Geiss 1989). The efficacy of SSRIs in delaying ejaculation combined with the low side-effect profile make them first-line agents for PE administered either on a daily or an “on-demand basis” (CitationWaldinger, Hengeveld, et al 1998b; CitationMcMahon and Touma 1999).
Physiology of ejaculation
Ejaculation is a reflex comprising sensory receptors and areas, afferent pathways, cerebral sensory areas, cerebral motor centers, spinal motor centers, and efferent pathways. There are three basic mechanisms involved in normal antegrade ejaculation – emission, ejection, and orgasm (CitationLipshultz et al 1981). Emission is the result of a sympathetic spinal cord reflex initiated by genital and/or cerebral erotic stimuli and involves the sequential contraction of accessory sexual organs. Considerable initial voluntary control of emission progressively decreases until the point of ejaculatory inevitability (CitationYeates 1987). Ejection also involves a sympathetic spinal cord reflex upon which there is little or no voluntary control. Ejection involves bladder neck closure, rhythmic contractions of bulbocavernous, bulbospongiosus and other pelvic floor muscles, and relaxation of the external urinary sphincter (CitationYeates 1987). Orgasm is the result of cerebral processing of pudendal nerve sensory stimuli resulting from increased pressure in the posterior urethra, sensory stimuli arising from the veramontanum, and contraction of the urethral bulb and accessory sexual organs.
The ejaculatory reflex is predominantly controlled by a complex interplay between central serotonergic and dopaminergic neurons with secondary involvement of cholinergic, adrenergic, nitrergic, oxytocinergic, galanergic, and GABAergic neurons. The cerebral events which occur during ejaculation and the abnormalities present in men with PE have not been clearly defined with PET and fMRI brain imaging techniques. Seminal emission and ejection are integrated into the complex pattern of copulatory behavior by several forebrain structures including the medial preoptic area (MPOA) and the nucleus paragigantocellularis (nPGi) (CitationRobinson and Mishkin 1966; CitationYells et al 1992). Descending serotonergic pathways from the nPGI to the lumbosacral motor nuclei tonically inhibit ejaculation (CitationYells et al 1992). Disinhibition of the nPGI by the MPOA facilitates ejaculation. A population of lumbar spinothalamic neurons has been identified in male rats (LSt cells) that constitutes an integral part of the generation of ejaculation. LSt cells send projections to the autonomic nuclei and motor neurons involved in the emission and expulsion phase, and they receive sensory projections from the pelvis (CitationTruitt and Coolen 2002). Several brain areas are activated after ejaculation by ascending fibers from the spinal cord and may have a possible role in satiety and the post-ejaculatory refractory time.
Our understanding of the neurobiology of both normal ejaculation and ejaculatory dysfunction, the supraspinal and spinal neuroanatomical pathways (CitationPfaus and Heeb 1997; CitationVeening and Coolen 1998; CitationTruitt and Coolen 2002; CitationTruitt et al 2003), and the multiple brain neurotransmitters and/or neuropeptides involved has evolved as a result of several animal studies where the sexual behavior of laboratory rats was investigated during pharmacological challenge and/or stimulation of specific brain areas (CitationLarsson and Ahlenius 1999; CitationPfaus 1999). These studies have attributed a serotonergic basis and possible genetic etiology to PE (CitationOlivier et al 1998; CitationWaldinger, Rietschel, et al 1998; CitationWaldinger and Hengeveld 2000; CitationWaldinger and Olivier 2001). Male rat studies demonstrate that serotonin and 5-HT receptors are involved in the ejaculatory process. The speed of ejaculation appears to be determined by 5-HT2C and 5-HT1A receptors. Stimulation of 5-HT2C receptors with non-selective 5-HT2C agonists delays ejaculation in male rats whereas stimulation of postsynaptic 5-HT1A receptors resulted in shorter ejaculation latency (CitationAhlenius et al 1981). Administration of SSRIs results in active blockade of presynaptic membrane 5-HT transporters, and the resultant higher synaptic cleft levels of 5-HT activate post-synaptic 5-HT2C and 5-HT1A receptors and delay ejaculation (CitationOlivier et al 1998; CitationWaldinger, Berendsen et al 1998).
Diagnosis
Diagnosis of PE in clinical practice is not difficult and is based on patient self-report, clinical history, and examination findings alone. Men with PE should be evaluated with a detailed medical and sexual history, a physical examination and appropriate investigations to establish the true presenting complaint and identify any obvious biological causes such as genital or lower urinary tract infection. However, treating physicians must interpret patient self-report of PE with some caution as the estimation of ejaculatory latency by men and women may correlate poorly with stopwatch measured latency times. It is clinically relevant to distinguish lifelong and acquired PE. Usually lifelong PE has a global manifestation whereas acquired PE may occur situationally (CitationGrenier and Byers 1992).
However in a research setting, objective measurement of the intravaginal ejaculatory latency time (IELT) by stopwatch and subjectively validated, reliable and consistent patient reported outcome measures (PROs) of ejaculatory control, sexual satisfaction, and bother/distress are essential (CitationSymonds et al 2002; CitationRust and Golombok 1986; CitationYuan et al 2004). Each of the three criteria above has been operationalized, although not always with consistency (CitationRowland et al 2001). Clinical trial outcome measures include the following.
Intravaginal ejaculatory latency time (IELT)
The length of time between penetration and ejaculation, the IELT forms the basis of most current clinical studies on PE (CitationWaldinger et al 2004). The IELT is measured with a stopwatch operated by the female partner, is expressed in seconds or minutes, and in case of ante-portal ejaculation, is equal to zero. Waldinger et al reported IELTs of less than 30 seconds and less than 60 seconds in 77% and 90% of 110 men with PE, respectively (CitationWaldinger, Hengeveld, et al 1998a). McMahon et al reported similar results in 1346 consecutive men with PE and a mean IELT of 43.4 seconds (CitationMcMahon 2002). Predominant ante-portal ejaculation (during foreplay) occurred in 5.6% of men. However, recent normative data derived from a multinational, community-based, age-ranging study have provided previously lacking normative data suggesting that men with IELTs of less than 60 seconds and between 60 and 90 seconds have “definite” PE and “probable” PE, respectively (CitationWaldinger, Quinn, et al 2005).
Voluntary control
Kaplan and other authors have suggested that an inability to voluntarily defer ejaculation defines PE (CitationKaplan et al 1974; CitationZilbergeld 1978; CitationVandereycken 1986; CitationMcCarthy 1988). This definition has yet to be adequately operationalized to allow comparison across subjects or across studies (CitationGrenier and Byers 1997; CitationWaldinger, Hengeveld, et al 1998a; CitationPatrick, Althof, et al 2005). Patrick et al reported ratings of “very poor” or “poor” for control over ejaculation in 72% of men with PE compared with 5% in a group of normal controls (CitationPatrick et al 2005). The patient’s feeling of ejaculatory control is a subjective measure and difficult to translate in quantifiable terms. Although feelings of ejaculatory control are part of the ejaculation process, diminished feelings of ejaculatory control are not exclusive for men suffering from PE and some men with a brief IELT report adequate ejaculatory control (CitationGrenier and Byers 1997).
Sexual satisfaction
Men with PE report lower levels of sexual satisfaction than do men with normal ejaculatory latency. A recent observational study reported sexual satisfaction ratings of “very poor” or “poor” in 31% of men with PE, compared with 1% in a group of normal controls (CitationPatrick et al 2005).
Distress
Existing definitions of PE include distress as an important dimension of PE (APA 1994; CitationLue et al 2004; CitationMontague et al 2004). However, the word “distress” has negative social implications and its existence is denied by most men with PE. This dimension of PE is better captured by the word “bother.” The extent of bother defines the severity of PE. One study reported that 64% of men with PE rated their extent of personal distress as “quite a bit” or “extremely” compared to 4% in a group of normal controls (CitationPatrick et al 2005). Although partner distress is perhaps the most common reason for men with PE to seek treatment, there is limited information regarding the effect of PE on the partner. Patrick et al reported that 44% of partners of men with PE rated their extent of personal distress as “quite a bit” or “extremely” compared to 3% in a group of partners of normal controls (CitationPatrick et al 2005).
The results of PE drug clinical trials are only reliable, interpretable, and capable of being generalized to patients with the disorder studied when conducted in well-defined and consistent populations, using a double-blind, placebo-controlled study design with consistent objective physiological measures or sensitive, validated outcome assessment instruments as study endpoints (CitationLue et al 2004). The dimensions of latency, control, satisfaction, and distress/bother must be well-defined, measured, and analyzed as continuous variables without arbitrary cut-off values. Subjective patient reported outcomes (PROs) of ejaculatory control, sexual satisfaction and bother/distress may be important additional efficacy endpoints and can be evaluated using validated patient reported outcome instruments (Symonds et al; CitationRust and Golombok 1986; CitationYuan, Xin et al 2004). However, a meta-analysis of 35 drug treatment studies has confirmed that the variability of answers of spontaneous reports and questionnaire studies on the IELT are significantly higher than stopwatch assessments (CitationWaldinger, Zwinderman, et al 2004).
Etiology of premature ejaculation
Historically, attempts to explain the etiology of PE has included a diverse range of biological and psychological theories. Most of these proposed etiologies are not evidence-based and are speculative at best. Psychological theories include the effect of early experience and sexual conditioning, anxiety, sexual technique, the frequency of sexual activity, and psychodynamic explanations. Biological explanations include evolutionary theories, penile hypersensitivity, central neurotransmitter levels and receptor sensitivity, degree of arousability, the speed of the ejaculatory reflex, and the level of sex hormones.
There is little empirical evidence to suggest a causal link between PE and any of the factors thought to cause PE. There is, however, limited correlational evidence to suggest that lifelong PE is due to altered sensitivity of central 5-HT receptors and acquired PE is due to high levels of sexual anxiety, ED or lower urinary tract infection.
Ejaculatory latency time is probably a biological variable, which is genetically determined and may differ between populations and cultures, ranging from “extremely rapid” through “average to slow” ejaculation. Hyposensitivity of the 5-HT2C and/or hypersensitivity of the 5-HT1A receptors have been suggested as a possible explanation of lifelong PE (CitationWaldinger, Berendsen et al 1998; CitationWaldinger 2002). Men with low 5-HT neurotransmission and probable 5-HT2C receptor hyposensitivity may have their ejaculatory threshold genetically “set” at a lower point and ejaculate quickly and with minimal stimulation whereas men with a higher set-point men can sustain more prolonged and higher levels of sexual stimulation and can exert more control over ejaculation. Men with a very high set-point may experience delayed or absent ejaculation despite achieving a full erection and prolonged sexual stimulation. Treatment with an SSRI class drug activates the 5-HT2C receptor, elevates the ejaculatory threshold set-point and delays ejaculation. The extent of ejaculatory delay may vary widely in different men according to the dosage and frequency of administration of SSRI and the genetically determined ejaculatory threshold set-point. Cessation of treatment results in re-establishment of the previous set-point within 5–7 days in men with lifelong PE.
Anxiety has been reported as a cause of PE by multiple authors and is entrenched in the folklore of sexual medicine as the most likely cause of PE despite scant empirical research evidence to support any causal role (CitationSchapiro 1943; CitationKaplan et al 1974; CitationWilliams 1984). Several authors have suggested that anxiety activates the sympathetic nervous system and reduces the ejaculatory threshold as a result of an earlier emission phase of ejaculation (CitationKaplan et al 1974; CitationWilliams 1984). The possibility that high levels of anxiety, and excessive and controlling concerns about sexual performance and potential sexual failure might distract a man from monitoring his level of arousal and recognizing the prodromal sensations that precede ejaculatory inevitability, has been suggested as a possible cause of PE by several authors (CitationZilbergeld 1978; CitationKockott et al 1980; CitationKaplan 1983; CitationVandereycken 1986; CitationKaplan 1989; CitationZilbergeld 1992). The causal link between anxiety and PE is speculative, is not supported by any empirical evidence and is, in fact, contrary to empirical evidence from some researchers (CitationStrassberg et al 1990).
Recent data demonstrate that almost half of men with ED also experience PE (CitationLaumann et al 2005). Men with early ED may intentionally “rush” sexual intercourse to prevent premature loss of their erection and ejaculate with a brief latency. This may be compounded by the presence of high levels of performance anxiety related to their ED which serves only to worsen their prematurity. In the absence of a thorough sexual history, these men may be incorrectly diagnosed as suffering from PE and not the underlying ED.
Pharmacological treatment
Pharmacological modulation of ejaculatory threshold represents a novel and refreshing approach to the treatment of PE and a radical departure from the psychosexual model, previously regarded as the cornerstone of treatment. The introduction of the selective serotonin reuptake inhibitors SSRIs has revolutionized the approach to and treatment of PE. SSRIs encompass five compounds: citalopram, fluoxetine, fluvoxamine, paroxetine, and sertraline, with a similar pharmacological mechanism of action. Although the methodology of the initial drug treatment studies was rather poor, later double-blind and placebo-controlled studies replicated the genuine effect of clomipramine and SSRIs to delay ejaculation. In spite of a development towards more evidence-based drug treatment research, the majority of studies still lack adequate design and methodology (CitationWaldinger 2003). A recent meta-analysis of all drug treatment studies demonstrated that only 14.4% had been performed according to the established criteria of evidence-based medicine, and that open design studies and studies using subjective reporting or questionnaires showed a higher variability in ejaculation delay than double-blind studies in which the ejaculation delay was prospectively assessed with a stopwatch (CitationWaldinger 2003).
Daily treatment with SSRIs
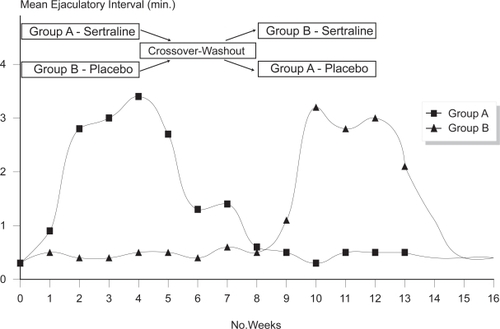
Daily treatment can be performed with paroxetine 20–40 mg, clomipramine 10–50 mg, sertraline 50–100 mg, and fluoxetine 20–40 mg. Paroxetine appears to exert the strongest ejaculation delay, increasing IELT approximately 8.8 fold over baseline (CitationWaldinger 2003). Ejaculation delay usually occurs within 5–10 days but may occur earlier () (CitationMcMahon 1998). Adverse effects are usually minor, start in the first week of treatment, gradually disappear within 2–3 weeks and include fatigue, yawning, mild nausea, loose stools or perspiration. Diminished libido or mild ED is infrequently reported. Significant agitation is reported by a small number of patients and treatment with SSRIs should be avoided in men with a history of bipolar depression.
Figure 2 Selective serotonin re-uptake inhibitors produce ejaculatory delay within 5–10 days (CitationMcMahon 1998).
On-demand treatment with SSRIs
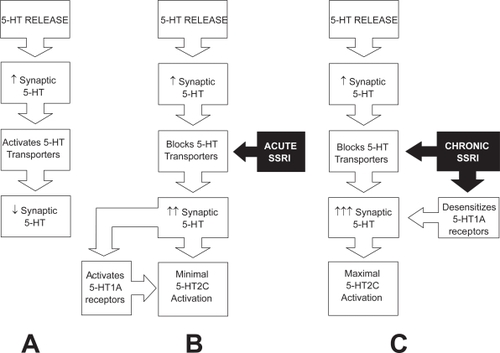
Administration of clomipramine, paroxetine, sertraline, fluoxetine 4–6 hours before intercourse is efficacious and well-tolerated but is associated with less ejaculatory delay than daily treatment. Daily administration of an SSRI is associated with superior fold increases in IELT compared with on-demand administration due to greatly enhanced 5-HT neurotransmission resulting from several adaptive processes which may include presynaptic 5-HT1a and 5-HT1b/1d receptor desensitization () (CitationWaldinger, Berendsen, et al 1998). On-demand treatment may be combined with either an initial trial of daily treatment or concomitant low dose daily treatment (CitationKim and Paick 1999; CitationMcMahon and Touma 1999; CitationStrassberg et al 1999).
Figure 3 A. Synaptic cleft 5-HT and 5-HT neurotransmission are regulated by somatodendritic 5-HT1A autoreceptors, presynaptic 5-HT1B/1D autoreceptors and a 5-HT transporters re-uptake system. As 5-HT is released into the synaptic cleft from pre-synaptic axonal vesicles, 5-HT transporters re-uptake and remove 5-HT from the synaptic cleft, preventing over-stimulation of the postsynaptic receptors. B. After blockage of 5-HT transporters by acute administration of selective serotonin re-uptake inhibitor class drugs (SSRIs) synaptic cleft 5-HT increases but is counteracted by activation of 5-HT1A autoreceptors which inhibit further 5-HT release. C. Chronic administration of selective serotonin re-uptake inhibitor class drugs (SSRIs) results in greatly enhanced 5-HT neurotransmission due to several adaptive processes which may include presynaptic 5-HT1A and 5-HT1B/1D receptor desensitisation (CitationOlivier et al 1998).
Ejaculo-selective serotonin transport inhibitors (ESSTIs)
A number of rapid-acting short half-life SSRIs (dapoxetine, Johnson & Johnson, UK-390, 957-Pfizer) are under investigation as on-demand treatments for PE.
Dapoxetine
Dapoxetine is the first compound specifically developed for the treatment of PE. Dapoxetine is a potent SSRI (pKi = 8 nM), structurally similar to fluoxetine () (CitationSorbera et al 2004). Equilibrium radioligand binding studies using human cells demonstrate that dapoxetine binds to 5-HT, norepinephrine (NE) and dopamine (DA) re-uptake transporters and inhibits uptake in the following rank order of potency: NE < 5-HT >> DA (CitationGengo et al 2005). Brain PET studies have demonstrated significant displaceable binding of radiolabeled dapoxetine in the cerebral cortex and subcortical grey matter (CitationLivni et al 1994).
Figure 4 Molecular structure of dapoxetine: (+)-(S)-N, N-dimethyl-(α)-[2(1naphtha lenyloxy)ethyl]-benzenemethanamine hydrochloride.
![Figure 4 Molecular structure of dapoxetine: (+)-(S)-N, N-dimethyl-(α)-[2(1naphtha lenyloxy)ethyl]-benzenemethanamine hydrochloride.](/cms/asset/06ac1901-3a95-4ffc-9149-b8cadd6458aa/dndt_a_24489_f0004_b.jpg)
Pharmacokinetics
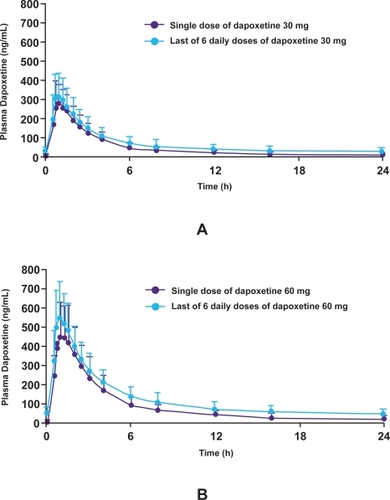
Dapoxetine undergoes rapid absorption and elimination resulting in minimal accumulation and has dose-proportional pharmacokinetics, which are unaffected by multiple dosing (). The pharmacokinetic profile of dapoxetine suggest that it is a good candidate for on-demand treatment of PE. The pharmacokinetics of both single doses and multiple doses over 6–9 days (30, 60, 100, 140, or 160 mg) of dapoxetine were been evaluated.
Figure 5 Plasma concentration profiles of dapoxetine after administration of a single dose or multiple doses of dapoxetine 30 mg (A) and dapoxetine 60 mg (B) (CitationModi et al 2005b).
In a randomized, double-blind, placebo-controlled trial, single doses and multiple doses over 6 days of dapoxetine (60, 100, 140, or 160 mg) were administered to 77 healthy male volunteers (CitationDresser, Lindert, et al 2004). Dapoxetine has a Tmax of 1.4–2.0 hours and rapidly achieves peak plasma concentration (Cmax) following oral administration. Both plasma concentration and area under the curve (AUC) are dose dependent up to 100 mg. The mean half-life of dapoxetine after a single dose is 0.5–0.8 hours and plasma concentrations rapidly decline to about 5% of Cmax at 24 hours. The terminal half-life of dapoxetine was 15–19 hours after a single dose and 20–24 after multiple doses.
In a subsequent pharmacokinetic study, single doses and multiple doses over 9 days of dapoxetine (30, 60 mg) were evaluated in a randomized, open-label, 2-treatment, 2-period, crossover study of 42 healthy male volunteers (CitationModi et al 2005a). Subjects received a single dose of dapoxetine 30 mg on Day 1 (single-dose phase) and on Days 4–9 (multiple-dose phase); and a single dose of dapoxetine 60 mg on Day 1 and on Days 4–9. Dapoxetine was rapidly absorbed, with mean maximal plasma concentrations of 297 and 349 ng/mL noted 1.01 and 1.27 hours after single doses of dapoxetine 30 and 60 mg, respectively (). Elimination of dapoxetine was rapid and biphasic, with an initial half-life of 1.31 and 1.42 hours, and a terminal half-life of 18.7 and 21.9 hours following single doses of dapoxetine 30 and 60 mg, respectively. The pharmacokinetics of dapoxetine and its metabolites were not affected by repeated daily dosing and state plasma concentrations were reached within 4 days, with only modest accumulation of dapoxetine (approximately 1.5 fold).
Table 1 Pharmacokinetics of single doses of dapoxetine (30, 60 mg) and effect of food on pharmacokinetics.
Food does not have a clinically significant effect on dapoxetine pharmacokinetics. Mean maximal plasma concentrations of dapoxetine decrease slightly after a high-fat meal, from 443 ng/mL (fasted) to 398 ng/mL (fed), and are delayed by approximately 0.5 hours following a high-fat meal (1.30 hours fasted, 1.83 hours fed) (CitationDresser, Modi, et al 2005). The rate of absorption is modestly decreased, but there is no effect of food on the elimination of dapoxetine or the exposure to dapoxetine, assessed by the area under the plasma concentration-versus-time curve (AUC). The frequency of nausea is decreased after a high-fat meal (24% [7/29] of fasted subjects and 14% [4/29] of fed subjects, respectively).
Dapoxetine is extensively metabolized to multiple metabolites including desmethyldapoxetine, didesmethyl-dapoxetine, and dapoxetine-N-oxide, all of which have much lower plasma concentrations compared with dapoxetine (CitationDresser et al 2004). Details of the specific pathways and isoenzymes involved in metabolism, the volume of drug distribution and details on excretion are not currently available (CitationDresser et al 2004).
Animal studies
Animal studies using rat experimental models have demonstrated that acute treatment with oral, subcutaneous, and IV dapoxetine inhibits ejaculation at doses as low as 1 mg/kg.
Clement et al reported the effects of IV dapoxetine on the emission and expulsion phases of ejaculation using p-chloroamphetamine (PCA)-induced ejaculation as an experimental model of ejaculation in anesthetized rats (CitationClement et al 2006). Intraseminal vesicle pressure (SVP) and electromyograms of bulbospongiosus muscles (BS) were used as physiologic markers of the emission and expulsion phases, respectively. At all doses, dapoxetine significantly reduced the proportion of rats displaying PCA-induced ejaculation in a dose-dependent manner, from 78% of rats with vehicle to 33%, 22%, and 13% of rats following IV dapoxetine 1, 3, and 10 mg/kg, respectively. Dapoxetine significantly decreased the AUC of PCA-induced SVP increases and BS contractile bursts by 78% at all doses, and by 91% following dapoxetine 1 mg, respectively.
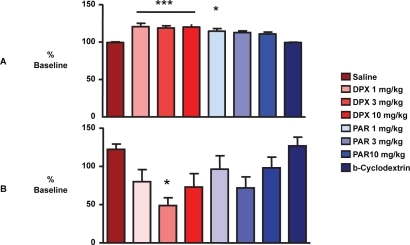
Using a different animal experimental model of the ejaculatory reflex in rats, Giuliano et al measured the latency, amplitude and duration of pudendal motoneuron reflex discharges (PMRD) elicited by stimulation of the dorsal nerve of the penis before and after IV injection of vehicle, dapoxetine or paroxetine (1, 3, and 10 mg/kg) (CitationGiuliano et al 2006). At the 3 doses of dapoxetine tested, the latency of PMRD following stimulation of dorsal nerve of the penis was significantly increased and the amplitude and duration of PMRD decreased from baseline values (). Acute IV paroxetine appeared less effective than dapoxetine.
Figure 6 The latency, amplitude and duration of pudendal motoneuron reflex discharges (PMRD) elicited by stimulation of the dorsal nerve of the penis in a rat esperimental model of ejaculation before and after IV injection of vehicle, dapoxetine or paroxetine (1, 3, and 10 mg/kg). (***p < 0.001, *p < 0.05 compared to vehicle) (CitationGiuliano et al 2006).
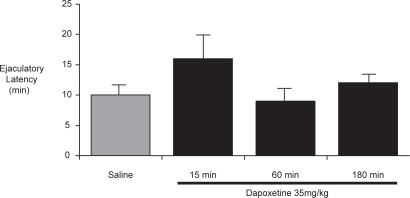
In a behavioral study of sexually experienced rats, Gengo et al reported that treatment with subcutaneous or oral dapoxetine significantly delayed ejaculation compared to saline control (16 ± 4 min (subcut.) vs 10 ± 1 min. In saline controls, p < 0.05) when administered 15, but not 60 or 180 minutes prior to exposure to receptive females () (CitationGengo et al 2006). The greatest delay in ejaculatory latency was observed in animals with shorter baseline latencies and oral dapoxetine did not affect the latency in rats with a baseline latency longer than 10 minutes.
Figure 7 Ejaculatory latency in male rats that received dapoxetine 35 mg/kg, 15 to 180 minutes before testing (CitationGengo et al 2006).
Human clinical trials
The results of two phase 2 and two phase 3 trials have been published in abstract form.
Dapoxetine dose-finding data have been derived from two multicenter Phase 2 studies and used to determine the appropriate doses for Phase 3 studies. Both studies used a randomized, placebo-controlled, double-blind, 3-period, crossover study design and randomized heterosexual men with PE diagnosed according to DSM-IV criteria and a baseline IELT of ≤2 minutes. Study drug was administered 1–2 hours prior to planned sexual intercourse activity and subjects were required to attempt intercourse at least twice a week. The primary outcome measure was IELT measured by partner-operated stopwatch.
In study 1 128 of 157 randomized subjects completed the study (CitationHellstrom et al 2005). Subjects were randomized to receive dapoxetine 20 mg, dapoxetine 40 mg, or placebo for 4 weeks with no washout period between treatment arms. Baseline IELT (mean baseline ILET = 1.34 min) was estimated by patient recall. In study 2 130 of 166 randomized subjects completed the study (CitationHellstrom et al 2004). Subjects were randomized to receive dapoxetine 60 mg, dapoxetine 100 mg, or placebo for 2 weeks, separated by a 3-day washout period. Baseline IELT (mean baseline IELT = 1.01 min) was measured by partner-operated stopwatch.
The intention-to-treat analysis of both studies demonstrated that all four doses of dapoxetine are effective, superior to placebo and increased IELT 2.0–3.2 fold over baseline in a dose-dependent fashion () (CitationHellstrom et al 2004; CitationHellstrom et al 2005). The magnitude of effect of dapoxetine 20 mg on IELT was small. The most commonly reported adverse events were nausea, diarrhea, headache, dizziness. The incidence of most adverse events appeared to be dose-dependent. The most common adverse event was nausea and occurred in 0.7%, 5.6%, and 16.1% of subjects with placebo, dapoxetine 60 mg and dapoxetine 100 mg, respectively. Overall, dapoxetine 60 mg was better tolerated than dapoxetine 100 mg. The most common reason for study withdrawal was nausea in patients receiving dapoxetine 100 mg. Based on these results, doses of 30 mg and 60 mg were chosen for further investigation in Phase 3 efficacy and safety studies.
Table 2 Results of dapoxetine Phase 2 studies (CitationHellstrom et al 2004; CitationHellstrom et al 2005).
In two Phase 3 multicenter studies using a randomized, placebo-controlled, double-blind study design, Pryor et al evaluated the efficacy and safety of dapoxetine 30 mg and dapoxetine 60 mg in 2614 men diagnosed with PE according to DSM-IV criteria and a baseline IELT of ≤2 minutes (CitationPryor et al 2005). Following a 2 week baseline run-in period, patients were randomized to receive 12 weeks treatment with either dapoxetine 30 mg, dapoxetine 60 mg, or placebo. Study drug was administered 1–3 hours prior to planned sexual intercourse activity. The primary outcome measure was IELT measured by partner-operated stopwatch and secondary outcome measures included the patient reported outcomes (PROs) of perception of Control over Ejaculation and perception of Satisfaction with Sexual Intercourse using a 5-point scoring scale (0 = very poor to 5 = very good).
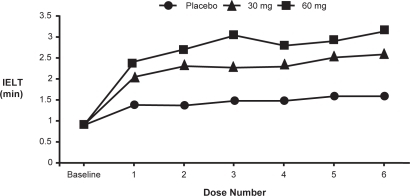
In both studies, dapoxetine (30/60 mg) significantly improved primary and secondary outcome measures compared to placebo (). Of significance was the report that dapoxetine (30/60 mg) was more effective than placebo in increasing IELT on the initial dose and maintained that efficacy with subsequent dosing (). Intravaginal ejaculatory latency time (IELT) increased from 0.91 minutes at baseline to 2.78 and 3.32 minutes at study end with dapoxetine 30 and 60 mg, respectively. In comparison, the placebo group increased from a baseline of 0.90–1.75 minutes.
Figure 8 A. Dapoxetine increased intravaginal ejaculatory latency time (IELT) from 0.91 minutes at baseline to 2.78 and 3.32 minutes at study end with dapoxetine 30 and 60 mg respectively. B. % of subjects rating Control Over Ejaculation as fair, good, or very good increased from 3.1% at baseline to 51.8% and 58.4% at study end with dapoxetine 30 and 60 mg respectively. C. % of subjects rating Sexual Satisfaction as fair, good or very good increased from 53.6% at baseline to 70.9% and 79.2 % with dapoxetine 30 mg and 60 mg respectively (rating scale 0–5 scale, 0 = very poor, and 5 = very good) (CitationPryor et al 2005).
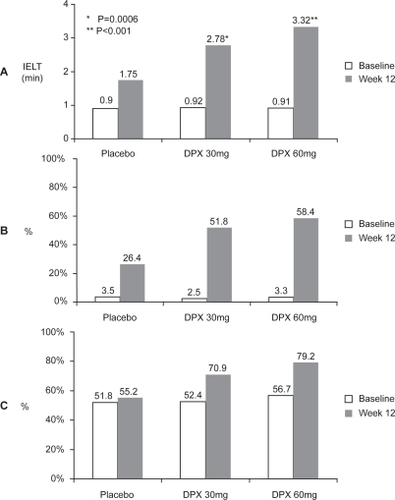
Figure 9 Dapoxetine (30/60 mg) was more effective than placebo in increasing IELT on the initial dose and maintained that efficacy with subsequent dosing (CitationPryor et al 2005).
Mean patient rating of Control Over Ejaculation as fair, good, or very good increased from 2.8% at baseline to 51.8% and 58.4% at study end with dapoxetine 30 and 60 mg, respectively. Similar results were seen with Satisfaction of Sexual Intercourse with changes from baseline of 52.4%– 70.9% with dapoxetine 30 mg, 56.7% to 79.2% with dapoxetine 60 mg, and 51.8%–55.2% with placebo ().
Treatment-related side–effects were uncommon, dose dependent, included nausea, diarrhea, headache, and dizziness, and were responsible for study discontinuation in 4% (30 mg) and 9.5% (60 mg) of subjects. Nausea and headache were the most common adverse events reported with both doses of dapoxetine. Nausea was reported by 1.9%, 8.7%, and 20.1% of patients with placebo, dapoxetine 30 mg group and dapoxetine 60 mg. Headache was reported by 4.0%, 5.9%, and 6.8% of patients with placebo, dapoxetine 30 mg and dapoxetine 60 mg, respectively. Diarrhea and dizziness were also more common with dapoxetine 60 mg compared with placebo and dapoxetine 60 mg, occurring in 6.8% and 6.2% of subjects. Erectile dysfunction was reported by 2.9% and 3.8% of subjects taking dapoxetine 30 mg and dapoxetine 60 mg.
Although no direct comparator trials of dapoxetine and other SSRIs have been reported, daily administration of paroxetine is likely to produce a greater fold increase in IELT than on-demand dapoxetine due to the greatly enhanced 5-HT neurotransmission resulting from several adaptive processes in the serotonergic neuron which occur with daily dosing () (CitationWaldinger, Berendsen, et al 1998).
Table 3 Comparison of fold increases in IELT with meta-analysis data for daily paroxetine, sertraline, fluoxetine, clompipramine (CitationWaldinger, Zwinderman, et al 2004) and phase 3 data for on-demand dapoxetine (CitationPryor et al 2005).
Adverse effects
The most common adverse events seen in Phase 2 and 3 trials of dapoxetine of were nausea, headache, diarrhea, and dizziness and were dose-dependent (CitationHellstrom et al 2004; CitationPryor et al 2005). Although nausea was reported by 20.1% of subjects taking dapoxetine 60 mg, its incidence attenuated with continued use and its severity was assessed as mild by most patients with vomiting occurring in only 2.2% of subjects with this dose. Overall, 6.7% of subjects discontinued dapoxetine due to adverse events, predominantly nausea. There are currently no reports of any cardiovascular, hepatic, renal or hematological adverse events associated with dapoxetine.
Drug interactions
No drug–drug interactions associated with dapoxetine have been reported. Co-administration of dapoxetine with ethanol did not produce significant changes in dapoxetine pharmacokinetics (CitationModi, et al 2005). Mean peak plasma concentrations of dapoxetine, its metabolites and ethanol did not significantly change with co-administration and there were no clinically significant changes in ECGs, clinical laboratory results, physical examination and no serious adverse events. Drug interactions studies demonstrate that tadalafil, a phosphodiesterase-5 inhibitors used in the treatment of ED, did not affect the pharmacokinetics of dapoxetine, whereas sildenafil increased the dapoxetine AUC by 22% (CitationDresser, Jazrawi, et al 2005). However, this was not regarded as clinically important. Dapoxetine did not appear to affect the pharmacokinetics of tadalafil or sildenafil.
Dosage and administration
Large Phase 3 studies demonstrate that doses of 30 mg and 60 mg administered on-demand 1 to 3 hours before planned sexual intercourse are appropriate for clinical use.
Future of PE drug development
Several in vitro and animal studies have demonstrated that the desensitization of 5-HT1A receptors, increased activation of postsynaptic 5-HT2C receptors, and the resultant higher increase in synaptic 5-HT neurotransmission seen in daily dosing of SSRI class drugs can be acutely achieved by blockade of these receptors by administration of an on-demand SSRI and a 5-HT1A receptor antagonist (CitationCremers et al 2000; CitationWilliamson et al 2003; Citationde Jong et al 2005). Drug combinations such as this or single agents that target multiple receptors may form the foundation of more effective future on-demand medication.
Conclusion
The off-label use of SSRIs and clomipramine, along with the development of new on-demand drugs for the treatment of PE, has drawn new attention to this common and often ignored sexual problem. Recent epidemiological and observational research has provided new insights into the problem and the associated negative psychosocial effects of this dysfunction. However, until the neurobiological, physiological, and psychological mechanisms responsible for PE are better understood, ideal treatment outcomes may remain elusive. Ejaculation-specific selective serotonin reuptake inhibitors such as dapoxetine and UK-390,957 represent a major development in sexual medicine, and offer patients the convenience of on-demand dosing, significant improvements in IELT, ejaculatory control, and sexual satisfaction with minimal adverse effects. Drug combinations or single agents that target multiple 5-HT receptors may represent the next stage of PE drug development.
Disclosures
Dr McMahon is an investigator, member of an advisory board, and speaker’s panel for Johnson & Johnson, Janssen-Cilag, Pfizer, Icos-Lilly, and Bayer.
References
- AhleniusSLarssonKSvenssonL1981Effects of a new type of 5-HT receptor agonist on male rat sexual behaviorPharmacol Biochem Behav15785926458826
- [APA] American Psychiatric Association1994Diagnostic and Statistical Manual of Mental Disorders, DSM-IV4th edWashington D.CAPA
- BhatiaMSMalikSC1991Dhat syndrome–a useful diagnostic entity in Indian cultureBr J Psychiatry15969151756347
- ByersESGrenierG2003Premature or rapid ejaculation: heterosexual couples’ perceptions of men’s ejaculatory behaviorArch Sex Behav3232617012807298
- ClementPBernabePGengoP2006Dapoxetine inhibits p-Chloroamphetamine-induced ejaculation in Anesthetized Rats., Book of Abstracts - 8th Congress of the European Society for Sexual MedicineJ Sex Med55abstract P-02–159
- CremersTIDe BoerPLiaoY2000Augmentation with a 5-HT1A, but not a 5-HT1B receptor antagonist critically depends on the dose of citalopramEur J Pharmacol397637410844100
- de JongTRPattijTVeeningJG2005Citalopram combined with WAY 100635 inhibits ejaculation and ejaculation-related Fos immunoreactivityEur J Pharmacol5094959Epub 2005 Jan 21.15713429
- Deveaugh-GeissJLandauPKatzR1989Preliminary results from a multicentre trial of clomipramine in obsessive compulsive disorderPsychopharmacol Bull2536402672070
- DresserMJJazrawiRPDesaiD2005Dapoxetine for the treatment of premature ejaculation: Lack of interaction with PDE5 inhibitorsJ Urol174201-abstract 739.
- DresserMJLindertKLinD2004Pharmacokinetics of single and multiple escalating doses of dapoxetine in healthy volunteersClin Pharmacol Ther75113(abstract p1).
- DresserMModiNBStaehrP2005The effect of food on the pharmacokinetics of dapoxetine, a new on-demand treatment for premature ejaculationJ Sex Med20053Suppl 125abstract 37.
- FasoloCBMironeVGentileV2005Premature ejaculation: prevalence and associated conditions in a sample of 12,558 men attending the Andrology Prevention Week 2001-A study of the Italian Society of Andrology (SIA)J Sex Med233768216422869
- GengoRJGiulianoFMcKennaKE2005Monoaminergic transporter binding and inhibition profile of dapoxetine, a medication for the treatment of premature ejaculationJ Urol1734230 -abstract 878.
- GengoPJMarsonLGravittA2006Actions of dapoxetine in ejaculation and sexual behaviour in rats. Book of Abstracts - 8th Congress of the European Society for Sexual MedicineJ Sex Med28abstract MP-01–07416409215
- GiulianoFBernabeJGengoP2006pudendal motoneuron reflex discharges in anesthetized rats are dealayed by dapoxetine. Book of Abstracts - 8th Congress of the European Society for Sexual MedicineJ Sex Med12abstract PS-03–027.16409214
- GrenierGByersS1992Evaluating models of premature ejaculation: a reviewCan J Hum Sex311522
- GrenierGByersES1997The relationships among ejaculatory control, ejaculatory latency, and attempts to prolong heterosexual intercourseArch Sex Behav2627479015578
- HellstromWJAlthofSGittelmanM2005Dapoxetine for the treatment of men with premature ejaculation (PE): dose-finding analysisJ Urol173238-abstract 877.
- HellstromWJGittelmanMAlthofS2004Dapoxetine HCl for the treatment of premature ejaculation: A Phase II, randomised, double-blind, placebo controlled studyJ Sex Med1Suppl 159097
- KaplanH1983The evaluation of sexual disorders: The urologic evaluation of ejaculatory disordersNew YorkBrunner/Mazel
- KaplanHS1989PE: How to overcome premature ejaculationNew YorkBrunner/Mazel
- KaplanHSKohlRNPomeroyWB1974Group treatment of premature ejaculationArch Sex Behav3443524413604
- KimSWPaickJS1999Short-term analysis of the effects of as needed use of sertraline at 5 PM for the treatment of premature ejaculationUrology54544710475369
- KockottGFeilWRevenstorfD1980Symptomatology and psychological aspects of male sexual inadequacy: results of an experimental studyArch Sex Behav9457757458655
- KotinJWilbertDEVerburgD1976Thioridazine and sexual dysfunctionAm J Psychiatry1338251247127
- LarssonKAhleniusS1999Brain and sexual behaviorAnn N Y Acad Sci87729230810415656
- LaumannEONicolosiAGlasserDB2005Sexual problems among women and men aged 40–80 y: prevalence and correlates identified in the Global Study of Sexual Attitudes and BehaviorsInt J Impot Res17395715215881
- LaumannEOPaikARosenRC1999Sexual dysfunction in the United States: prevalence and predictorsJAMA2815374410022110
- LipshultzLIMcConnellJBensonGS1981Current concepts of the mechanisms of ejaculation. Normal and abnormal statesJ Reprod Med264995077310763
- LivniESatterleeWRobeyRL1994Synthesis of [11C]dapoxetine. HCl, a serotonin re-uptake inhibitor: biodistribution in rat and preliminary PET imaging in the monkeyNucl Med Biol21669759234326
- LueTFBassonRRosenRC2004Sexual medicine–sexual dysfunctions in men and womenParis, FranceHealth Publications
- MastersWHJohnsonVE1966Human sexual responseBostonLittle Brown
- McCarthyB1988Cognitive-behavioral strategies and techniques in the treatment of early ejaculationPrinciples and practices of sex therapy: update for the 1990’sLeiblumSRRosenRNew YorkGuilford Press14167
- McMahonCG1998Treatment of premature ejaculation with sertraline hydrochloride: a single-blind placebo controlled crossover studyJ Urol159193589598491
- McMahonCG2002Long term results of treatment of premature ejaculation with selective serotonin re-uptake inhibitorsInt J Impot Res14Suppl 3S1912161764
- McMahonCGToumaK1999Treatment of premature ejaculation with paroxetine hydrochloride as needed:2 single-blind placebo controlled crossover studiesJ Urol16118263010332446
- ModiNBDresserMDesaiD2005aDapoxetine, a new on-demand treatment for premature ejaculation exhibits rapid single and multiple-dose pharmacokinetics. Book of Abstracts - 8th Congress of the European Society for Sexual MedicineJ Sex Med56abstract P-02–162.
- ModiNBDresserMJDesai2005Dapoxetine for the treatment of premature ejaculation: lack of interaction with ethanolJ Urol173239-Abstract 879.
- MontagueDKJarowJBroderickGA2004AUA guideline on the pharmacologic management of premature ejaculationJ Urol172290415201797
- MonteiroWONoshirvaniHFMarksIM1987Anorgasmia from clomipramine in obsessive-compulsive disorder: A controlled trialBr J Psychiatry151107123315086
- NathanSG1986The epidemiology of the DSM-III psychosexual dysfunctionsJ Sex Marital Ther12267813493350
- OlivierBvan OorschotRWaldingerMD1998Serotonin, serotonergic receptors, selective serotonin reuptake inhibitors and sexual behaviourInt Clin Psychopharmacol13Suppl 6S9149728669
- PatrickDLAlthofSEPryorJL2005Premature ejaculation: an observational study of men and their partnersJ Sex Med2586716422908
- PerelmanMAMcCullochARBullS2004The impact of self-reported premature ejaculation n other aspects of sexual functionJ Sex Med1Suppl 159O98.
- PfausJG1999Neurobiology of sexual behaviorCurr Opin Neurobiol9751810607643
- PfausJGHeebMM1997Implications of immediate-early gene induction in the brain following sexual stimulation of female and male rodentsBrain Res Bull443979370204
- PryorJLAlthofSESteidleC2005Efficacy and tolerability of dapoxetine in the treatment of premature ejaculationJ Urol173201740.
- ReadingAWiestW1984An analysis of self-reported sexual behavior in a sample of normal malesArch Sex Behav13696712463
- RichardsonDGoldmeierD2005Premature ejaculation–does country of origin tell us anything about etiology?J Sex Med25081216422845
- RobinsonBWMishkinM1966Ejaculation evoked by stimulation of the preoptic area in monkeysPhysiol Behav126972
- RosenRCPorstHMontorsiF2004The premature ejaculation, prevalence and attitudes (PEPA) survey-A multinational surveyJ Sex Med1Suppl1O92
- RowlandDLCooperSESchneiderM2001Defining premature ejaculation for experimental and clinical investigationsArch Sex Behav302355311330115
- RustJGolombokS1986The GRISS: a psychometric instrument for the assessment of sexual dysfunctionArch Sex Behav15157653718204
- SchapiroB1943Premature ejaculation, a review of 1130 casesJ Urol503749
- SorberaLACastanerJCastanerRM2004Dapoxetine hydrochlorideDrugs Future2912015
- SpectorIPCareyM1990Incidence and prevalence of the sexual dysfunctions: a critical review of the empirical literatureArch Sex Behav193892205172
- SpectorKRBoyleM1986The prevalence and perceived aetiology of male sexual problems in a non-clinical sampleBr J Med Psychol5935183801344
- StrassbergDSde Gouveia BrazaoCARowlandDL1999Clomipramine in the treatment of rapid (premature) ejaculationJ Sex Marital Ther258910110327378
- StrassbergDSMahoneyJMSchaugaardM1990The role of anxiety in premature ejaculation: a psychophysiological modelArch Sex Behav1925172360874
- SymondsTAlthofSERosenRC2002Questionnaire assessment of ejaculatory control: development and validation of a new instrumentPresented at the Fifth Congress of the European Society for Sexual and Impotence ResearchHamburg, Germany
- TruittWACoolenLM2002Identification of a potential ejaculation generator in the spinal cordScience2971566912202834
- TruittWAShipleyMTVeeningJG2003Activation of a subset of lumbar spinothalamic neurons after copulatory behavior in male but not female ratsJ Neurosci233253112514231
- VandereyckenW1986Towards a better delineation of ejaculatory disordersActa Psychiatr Belg8657633962693
- VeeningJGCoolenLM1998Neural activation following sexual behavior in the male and female rat brainBehav Brain Res92181939638960
- VermaKKKhaitanBKSinghOP1998The frequency of sexual dysfunctions in patients attending a sex therapy clinic in north IndiaArch Sex Behav27309149604119
- WaldingerM2003Towards evidenced based drug treatment research on premature ejaculation: a critical evaluation of methodologyJ Impot Res1530913
- WaldingerMHengeveldMZwindermanA1998aAn empirical operationalization of DSM-IV diagnostic criteria for premature ejaculationInt J Psychiatry Clin Pract228793
- WaldingerMDHengeveldMWZwindermanAH1998bEffect of SSRI antidepressants on ejaculation: a double-blind, randomized, placebo-controlled study with fluoxetine, fluvoxamine, paroxetine, and sertralineJ Clin Psychopharmacol18274819690692
- WaldingerMOlivierB2001Hersenonderzoek en farmacologie: serotonine, seks en agressieHet Brein Belicht: Opstellen over Niet-Aangeboren HersenletselWolters-SchweitzerHJBeugerCLUtrechtUitgeverij Lemma5563
- WaldingerMD2002The neurobiological approach to premature ejaculationJ Urol16823596712441918
- WaldingerMDBerendsenHHBlokBF1998Premature ejaculation and serotonergic antidepressants-induced delayed ejaculation:the involvement of the serotonergic systemBehav Brain Res92111189638953
- WaldingerMDHengeveldM2000Neuroseksuologie en seksuele psychofarmacologieTijdschr Psychiatr8585
- WaldingerMDQuinnPDilleenM2005A multinational population survey of intravaginal ejaculation latency timeJ Sex Med2492716422843
- WaldingerMDRietschelMNothenMM1998Familial occurrence of primary premature ejaculationPsychiatr Genet837409564687
- WaldingerMDZwindermanAHOlivierB2005Proposal for a definition of lifelong premature ejaculation based on epidemiological stopwatch dataJ Sex Med249850716422844
- WaldingerMDZwindermanAHSchweitzerDH2004Relevance of methodological design for the interpretation of efficacy of drug treatment of premature ejaculation: a systematic review and meta-analysisInt J Impot Res163698114961051
- WilliamsW1984Secondary premature ejaculationAust N Z J Psychiatry18333406596940
- WilliamsonIJTurnerLWoodsK2003The 5-HT1A receptor antagonist robalzotan enhances SSRI-induced ejaculation delay in the ratBrit J Pharmacol Biochem Behav138Suppl 1PO32
- YeatesWK1987AndrologyLondonButterworths
- YellsDPHendricksSEPrendergastMA1992Lesions of the nucleus paragigantocellularis: effects on mating behavior in male ratsBrain Res5967391468004
- YuanYMXinZCJiangH2004Sexual function of premature ejaculation patients assayed with Chinese Index of Premature EjaculationAsian J Androl6121615154086
- ZilbergeldB1978Male sexualityTorontoBantam
- ZilbergeldB1992The new male sexualityNew YorkBantam