Abstract
There is a great deal of interest in the possibility that complex nanoscale devices can be designed and engineered. Such devices will lead to the development of new materials, electronics and smart drugs. Producing complex nanoscale devices, however will present many challenges and the components of such devices will require a number of special features. Devices will be engineered to incorporate desired functionalities but, because of the difficulties of controlling matter precisely at the nanoscale with current technology, the nanodevice components must self-assemble. In addition, nanocomponents that are to have wide applicability in various devices must have enough flexibility to integrate into a large number of potentially very different environments. These challenges are daunting and complex, and artificial nanodevices have not yet been constructed. However, the existence of nanomachines in nature in the form of proteins (eg, enzymes) suggests that they will be possible to produce. As the material from which nature’s nanomachines are made, proteins seem ideal to form the basis of engineered components of such nanodevices. Initially, engineering projects may focus on building blocks such as rings, cages and tubes, examples of which exist in nature and may act as a useful start point for modification and further development. This review focuses on the recent research and possible future development of such protein building blocks.
Introduction
Since Richard Feynman’s famous 1959 talk at Caltech; “There’s Plenty of Room at the Bottom” (Feynman), it has been one of science’s most ambitious dreams to build complex, multi-functional, nanoscale devices, also known as nanomachines. There is much controversy in the scientific community as to whether complex nanodevices such as universal assemblers are feasible (CitationSmalley 2001; CitationBaum 2003). To date no one has succeeded in manufacturing a working, complex, artificial nanodevice. Obstacles to successful production include the materials used and the difficulty in manipulating nanometric components. Amongst non-biological approaches, carbon structures such as carbon nanotubes (CNTs) offer exciting possibilities (CitationBaughman et al 2002). However, a number of difficulties are associated with such nanotubes. It is worth considering these in some detail as many of the problems typify the general difficulties inherent in non-biological methods and highlight potential areas in which biological molecules, particularly proteins may be able to offer significant advantages.
Production of CNTs can be difficult and/or expensive and generally relies on one of three methods: arc discharge (CitationIijima 1991; CitationEbbesen and Ajayan 1992), laser ablation (CitationGuo et al 1995) or chemical vapor deposition (CitationJose-Yacaman et al 1993). While much progress has been made in the industrialization of multi-walled CNT production, production of single wall CNTs (SWNTs) whose diameters typically lie in the 0.4 to 3 nm range (CitationBaughman et al 2002) have proved much more difficult to scale up, and remain expensive. Other problems associated with CNT production are controlling precisely the length of the produced tubes and, in the case of SWNTs, avoiding contamination (CitationBaughman et al 2002). Defects in carbon nanotubes are common and ironically these are often necessary to allow modification of the tubes, providing convenient “hooks” for the addition of required molecules (CitationHirsch 2002). Problems in controlling exactly the length of produced CNTs are obstacles to their use in nanodevices where, as with components of macro-scale machines, precise dimensions engineered within fine tolerances may be required. Furthermore, in order to integrate such components into larger scale devices, modification to incorporate extra functionalities may be necessary. The small size of the basic component of the carbon nanotube (a single carbon atom) in which all the valence bonds are accounted for, has several implications: The basic component has no structure in itself and overall structure is determined by the covalent bonds with its neighbors. Thus, modification of carbon nanotubes without disrupting structure is difficult. Although progress has been made in this areas using, for example, non-covalent modification (CitationHirsch 2002; CitationSimmons et al 2007), the structure of the CNTs themselves places limits on the extent of modification available. Without the ability to modify CNTs to engineer in desired binding specificities it may be difficult for them, when mixed with other nanometric components of a device, to self-assemble to form the complex, final structure.
Biological molecules have long been recognized as potentially useful components of nanodevices because of their structural and sequence flexibility and because of their ability to self-assemble. Widely used biological molecules include peptides, DNA and proteins. There has been wide, longstanding research into the use of peptides to produce nanostructures having various applications (CitationAggeli et al 1997; CitationHolmes et al 2000; CitationRyadnov et al 2003). These structures include fibers (CitationTakahashi 2002; CitationMatsumura et al 2004) rings (CitationGhadiri et al 1993) and tubes (CitationGhadiri et al 1993; CitationSaviano et al 1994; CitationSeebach et al 1997; CitationClark et al 1998; CitationGao and Matsui 2005). A number of notable successes include the use of a modified beta-sheet forming 10-residue peptide that is able to form uniform tubes of 50 to 70 nm in diameter with an internal cavity 20 to 35 nm in diameter and which can reach many hundreds of nanometers in length (CitationMatsumura et al 2005). Peptide tubes can be patterned on surfaces (CitationReches and Gazit 2006) and coated with metal on the outer (CitationReches and Gazit 2006) or inner (CitationReches and Gazit 2003) cavity. The advantages of using peptides to make nanostructures are that they can easily be synthesized and in many cases the final structures can be predicted in advance. Disadvantages are that, as with carbon nanotubes, the basic unit is small and although they can be modified more readily than carbon nanotubes to give useful functionality (CitationMatsui et al 2001; CitationNuraje et al 2004) and shapes (CitationRyadnov 2007) they are nevertheless somewhat limited in the amount of modifications that can be tolerated before structure is adversely affected, with changes typically limited to alterations of individual peptide side chains and chemical modification. It is also difficult to precisely control the polymerization of the peptide tube structures, often resulting in polydispersity of length. Although it is possible to construct peptide tubes with small cavity sizes (CitationHartgerink et al 1998; CitationHorne et al 2003), this is not trivial and many cavities are considerably larger. While useful for some applications, for others such as acting as moulds for biomineralization of quantum wires, sub 10 nm diameters would be preferable.
DNA has also proven a useful tool for constructing nanoscale shapes. Most notably these include regular 2D tiling arrays (CitationFu and Seeman 1993; CitationWinfree et al 1998; CitationSeeman 2003; CitationPark et al 2006), 2D arrays of any desired pattern (CitationRothemund 2006), three-dimensional cages (CitationShih et al 2004) and tubes (CitationAldaye and Sleiman 2007). Advantages in using DNA are the ease in which it can be synthesized and the relative simplicity of having only 4 bases as the source of structural variability, meaning that predicting the overall shape is easier, allowing precise control.
It is clear that biological molecules offer exciting possibilities for construction of new complex, self assembled nanodevice components whose structures can be finely controlled. They potentially offer many advantages over non-biological systems. Biological molecules will not of course be able to replace non-biological nano-scale components in all cases. For example, while some biological molecules have impressive physical characteristics (the tensile strength of spider dragline silk, one of the strongest biological fibers known, is around 1.1 GPa (CitationVollrath and Knight 2001)) they cannot compete with those of nonbiological systems (the tensile strength of carbon nanotubes is at least an order of magnitude higher (CitationSinnott and Andrews 2001)). In electronics, carbon nanotubes and related materials have the potential to be used as semiconductors or superconductors (CitationKasumov et al 1999; CitationSinnott and Andrews 2001), something which biological molecules are unlikely to be capable of. Where biological molecules do show great promise is in the ability to self assemble extremely small yet regularly sized (often monodisperse) structures that can act as templates for the build-up of required structures from inorganic materials which, in the main, currently cannot self assemble. A second area where they may have an application is in the production of complex nanoscale devices that consist of numerous interlocking modules, something which has thus far proven difficult with inorganic materials yet which Nature does with ease. Thirdly, biological molecules may be able to act at the interface between inorganic systems and biological systems, for example in coating medical implants, or acting as detectors in a biological-silicon hybrid devices. Finally, many biological molecules can be produced in large (industrial) scale from bacterial expression systems relatively cheaply.
A major hurdle in constructing components of nanodevices is that of controlling self-assembly to give well defined shapes of precise dimensions and utilizing materials that can be easily modified to give new properties without losing overall structure.
Complex nanomachines found in nature, such as enzymes, are proteins and it is reasonable to suppose that this material is a good candidate for producing artificial nanodevices. When considering the design of nanoscale devices, a common problem is that of how to assemble the components, whose dimensions make them extremely difficult to manipulate. This problem is overcome by using a protein “building block” approach. In this system, protein components are used that naturally have, or can be engineered to have, affinities for other building blocks such that nanodevices can spontaneously self-assemble upon mixing of the individual components. Protein systems offer a number of other advantages such as synthesis under ambient conditions with no toxic by-products.
Protein cages, rings and tubes all offer useful starting points for further modification and have been engineered to produce semi-artificial structures which offer promise as basic components that may be built upon in future work. Recent work and future prospects of each of these proteins types is considered in more detail in the following sections.
Protein cages
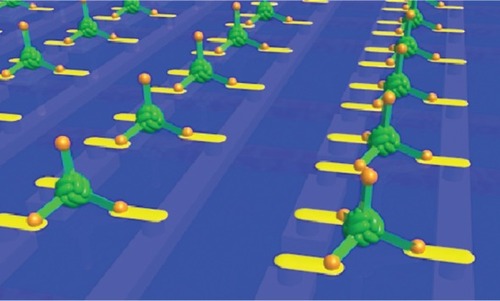
Protein cages are three-dimensional protein structures, usually roughly spherical in shape, which enclose a central hollow space into which materials can be deposited. Spherical cages have two surfaces: The inner surface and the outer surface. Protein cages self assemble from monomers in solution and one of their great advantages is that the central cavity is completely enclosed. This means that the interior of the cage can provide an isolated environment in which chemical reactions can take place shielded from bulk solution. The protein cage also acts to physically limit the size of particles produced in the cavity, making production of monodisperse nanoparticles relatively trivial. Protein cages of varying sizes are known. Dps (DNA-binding protein from starved cells, ; (CitationHarrison and Arosio 1996; CitationBozzi et al 1997)) from Listeria innocua, with an external diameter of 9 nm, is possibly the smallest. Dps is a bacterial protein produced in response to starvation and oxidative stress which has numerous reported functions including a role in protection of DNA from oxidative damage (CitationMann 1993). It also has a ferritin-like ability to biomineralize iron in its central cavity. Dps is an extremely small cage protein, with a central core approximately 4.5 nm in diameter (CitationStillman et al 2005). It has been shown that Dps can be used to biomineralize cobalt oxide in its central cavity (CitationAllen et al 2002). Furthermore, it has been used as part of a “ball-and-spike” supramolecule in which the C-terminal of gp5 protein (gp5c), part of the cell puncturing apparatus from bacteriophage T4 (CitationKanamaru et al 2002) was fused to the N terminus of Dps via a short (22 residue) linker peptide. Gp5c assembles as a trimer into a triangular prism. Because Dps is a dodecamer it has four 3-fold symmetry axes meaning that with a linker of the appropriate length (22 residues), four full gp5c trimers can be assembled with equal spacing around the surface of Dps, at positions corresponding to the vertices of a tetrahedron (). Dps can be filled with metal or semiconductor, which can function as a quantum dot. If gp5c can also be modified to act as a template for biomineralization, then it is hoped that biomineralized gp5c “spikes” could act as electrodes around a central Dps quantum dot, forming the basis of extremely small electronic components.
Figure 1 Crystal structures of a range of cage proteins. (a) Dps protein (pdb 2bjy (CitationIlari et al 2005)), diameter 9 nm; (b) Ferritin (pdb 2za6 (CitationYoshizawa et al 2007)) diameter 12 nm; (c) Cowpea chlorotic mottle virus (CCMV, pdb 1cwp (CitationSpeir et al 1995)), diameter 26 nm; and (d) Cowpea mosaic virus (1NY7 (CitationLin et al 1999)), diameter 28 nm. In (d), red spheres show the N-terminal glycine that points into the central cavity. Dark blue spheres show the C-terminal lysine on the external surface.
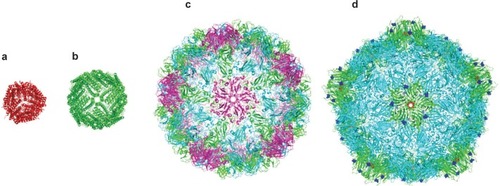
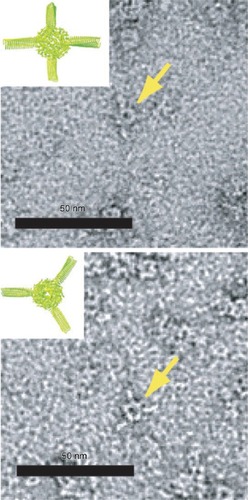
Figure 2 Transmission electron micrographs of “Ball-and-Spike” protein shown in two different orientations. Inset shows an interpretation of the micrograph using existing crystal structures of gp5c and Dps. Reproduced with permission from CitationSugimoto K, Kanamaru S, et al 2006. Construction of a ball-and-spike protein supramolecule13. Angew Chem Int Ed, 45:2725–8. Copyright ©. Wiley-VCH Verlag GmbH and Co. KGaA.
Dps is a ferritin-like protein and ferritin itself is one of the most widely studied and utilized cage proteins. Found in all domains of life, it is highly conserved, consisting of 24 identical protein subunits (CitationBanyard et al 1978; CitationYoshizawa et al 2007). In vivo its role is to act as a store of ferric oxide. Ferric iron in the cavity of ferritin is formed from Fe(II) in solution. Fe(II) is able to enter the internal cavity via a channel lined with acidic residues. Once inside, biomineralization begins at specific nucleation sites on the interior surface (CitationLawson et al 1991).
In higher animals, ferritin is made from two similar proteins; H and L type ferritin with only H type ferritin possessing a true catalytic site, known as a ferroxidase centre (CitationLawson et al 1989) although both types, when reconstituted as pure H or L type ferritin in vitro, are able to promote biomineralization of ferric iron (CitationTheil et al 2000). Work with ferritin was one of the earliest successes in biomineralization using proteins other than virus capsids. In pioneering work, Mann and colleagues were able to biomineralize a range of materials including manganese and iron oxides in the central cavity of purified ferritins (CitationMeldrum et al 1991, Citation1992, Citation1995; CitationMann 1993; CitationDouglas et al 1995). Ferritin was found to have the useful ability to inhibit biomineralization on its outside surface while catalyzing biomineralization in the cavity (the so-called “Janus effect”). Since then, significant work has been carried out by Yamashita and colleagues to extend the types of materials that ferritin can biomineralize (), and they have achieved success with numerous metals and semiconductors including nickel hydroxide (CitationOkuda et al 2003), cadmium selenide (CitationYamashita et al 2004), zinc selenide (CitationIwahori et al 2005) and gold sulfide (CitationYoshizawa et al 2006) while other groups have succeeded in minerlizaing further materials (CitationDouglas and Stark 2000; CitationKramer et al 2004). The Yamashita group have also developed systems of finely controlling the deposition of ferritin on various surfaces (CitationKumagai et al 2006; CitationYamashita et al 2006; CitationMatsui et al 2007) and have used ferritin-based quantum dots as the basis of prototype electronic devices (CitationYamashita 2001; CitationMiura et al 2006, Citation2007). It is anticipated that in this way, biomineralized ferritin may become an important component of future electronic devices. Indeed, it has already found a number of other applications, having been used as a catalyst for growth of polycrystalline silicon films (CitationKirimura et al 2005) and single wall carbon nanotubes (CitationBonard et al 2002) as well as a reporter protein in MRI (CitationCohen et al 2005).
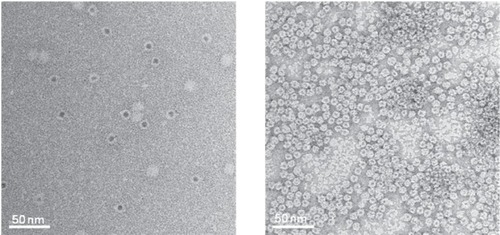
Figure 3 Transmission electron micrographs of ferritin (left) and Dps (right) both filled with CdS cores (CitationIwahori et al 2007; CitationIwahori and Yamashita 2007). Figure courtesy of Kenji Iwahori.
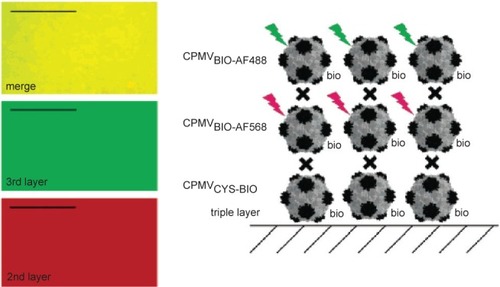
The other major class of protein cages used in bionanotechnology research are the virus capsids. Ranging in size from 18 to 500 nm in diameter for icosahedral viruses (), they offer the potential for use in a diverse range of applications (CitationDouglas and Young 2006). A particularly useful property of widely utilized capsids such as cowpea chlorotic mottle virus (CCMV) and cowpea mosaic virus (CPMV) is that they have N or C-termini on the outer or inner surfaces of the capsid and have additional external residues that can be addressed genetically or chemically. In a series of experiments by the Evans group, 180 exposed carboxylate groups on surface of CPMV were shown to be addressable and were attached to methyl(aminopropyl)viologen, which resulted in a redox active capsid nanoparticle (CitationSteinmetz et al 2006). Similarly, modification of surface exposed lysines with biotin and surface-exposed cysteines with fluorescent dyes, resulted in fluorescent particles that formed a dense monolayer on a gold surface. If the capsid layer was alternated with layers of streptavidin, multilayers of different fluorescently labeled particles could be achieved (; (CitationSteinmetz et al 2006, Citation2008)). In a similar way surface-exposed amine groups were used for attachment of redox active ferrocenecarboxylate molecules (CitationSteinmetz et al 2006). Plant viruses such as this are particularly attractive for use as potential in vivo therapeutic agents as they are not infectious to animals (although this may be a disadvantage if high efficiency of transport to the interior of target cells is required, see discussion of SV40 below) and they can be made and purified in large quantities from plant hosts.
Figure 4 CPMV capsids can be layered on a gold surface with each layer carrying a different modification. In this example, the biotinylated base layer is unlabeled, the second layer is labeled with a red fluorescent dye (AlexaFluor dye AF568) and a third layer with a green fluorescent dye (AF568). Layers are bridged by streptavidin (black cross). Fluorescence imaging microscopy (left) shows that each layer is homogenous. Scale bar is 10 μm. Reproduced with permission from CitationSteinmetz et al 2006a. Plant viral capsids as nanobuilding blocks: construction of arrays on solid supports. Langmuir, 22:10032–37. Copyright © 2006. American Chemical Society.
Like ferritin and Dps, the cavities of plant viruses offer potential uses as nanoreactors or as delivery systems. CCMV has been used to mineralize paratungstate, decavanadate and iron oxide in its cavity, taking advantage of the fact that pores in the capsid can be opened and closed via changes in pH, allowing for fine control of the reaction (CitationDouglas and Young 1998; CitationDouglas et al 2002).
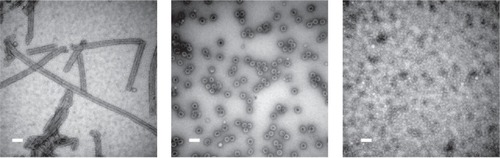
Another virus with potential for in vivo use is Simian virus 40 (CitationAnderer et al 1967; CitationLiddington et al 1991), a spherical virus with a diameter of 40–45 nm. SV40 is a tumorigenic DNA virus and is able to infect a variety of human cells with high efficiency. For this reason it is a potential candidate as a gene delivery system or as a method for transporting a variety of cargos to cellular targets. Furthermore, SV40 represents an interesting example of the flexibility and controllability inherent in many protein nanostructures. In vivo, the virus forms spherical particles constructed from 72 pentamers of VP1 protein, the major capsid protein, and 72 copies of minor proteins VP2 or VP3. VP1 alone is, however, enough to form a capsid and the morphology produced varies significantly depending on experimental conditions (). At pH 5.0, for example long, tubular structures are formed whereas at high salt concentrations, small (20 nm) particles predominate. A variety of switching mechanisms are also available to control structure: VP2 switches on assembly of spherical particles with VP1 at neutral pH (CitationKawano et al 2006) and the capsid can be reversibly disassembled by the addition of EGTA (CitationKanesashi et al 2003). Another layer of control can be affected by DNA, the presence of which is required for correct assembly of SV40 particles under physiological conditions and which is able to promote switching from tubular to spherical forms at pH5 (CitationTsukamoto et al 2007a). As well as its potential as a drug or gene delivery nanocapsule SV40 also, like other cage proteins, has potential for biomineralization of inorganic materials.
Figure 5 SV40 can form spherical or tubular structures depending on buffer conditions. Left: SV40VP1 pentamers assembled into tubular structure in vitro. Middle: SV40VP1 pentamers assembled into 40 nm spherical particles in vitro in the presence of DNA. Right: VP1 assembled into VLPs (Virus Like Particles) inside insect cells. In all cases, scale bar is 100 nm. Images courtesy of Hiroko Tsukamoto.
Protein rings
Protein rings are squat, three-dimensional tubes whose length is less than their diameter. Ring structures have a central hole and generally, four distinguishable surfaces; the surface lining the central hole, the corresponding outer surface and two “end” surface orthogonal to the hole axis. Ring proteins are common in nature where they perform numerous roles (). One well-known example is Rad52, an important protein involved in homologous recombination of DNA where it has a role in promoting annealing of single DNA strands. The protein forms a ring structure of 7 monomers (CitationStasiak et al 2000) and, if only the N-terminal half of the protein is present, it forms an 11-membered ring (CitationKagawa et al 2002; CitationSingleton et al 2002). The resulting structure is approximately 12 nm across with a central hole ranging from 2.5 to 5 nm in diameter and it is thought that DNA wraps around the outside of the ring. Another ring protein with similarity to RAD52 is the phage recombinase known as β. β is a 30 kDa protein that achieves single stranded DNA-annealing working in tandem with a partner protein, Exo (CitationPoteete 2001; CitationCourt et al 2002). In solution, β forms an approximately 12-membered ring but forms an approximately 12- to 18-membered ring if ssDNA is present (CitationPassy, Yu et al 1999).
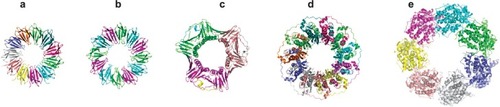
Figure 6 Crystal structures of various ring-shaped proteins. (a) wild-type TRAP protein (pdb 1qaw (CitationChen et al 1999), diameter approximately 8 nm); (b) mutant 12-membered TRAP protein (pdb 2zd0 (CitationWatanabe et al 2008)); (c) PCNA (pdb 1axc (CitationGulbis et al 1996)); (d) RAD52 (pdb 1kno (CitationKagawa et al 2002)), (e) GROEL (pdb 1grl (CitationBraig et al 1994)). All proteins shown approximately to scale.
The holes within ring proteins can be used to capture inorganic materials and array them on surfaces. The beta subunit of the HSP60 chaperonin protein from Sulfolobus shibatae, forms a barrel-like ring structure that has been successfully used to capture gold nanodots over the central core and arrange them into a well ordered 2D array (CitationMcMillan et al 2002).
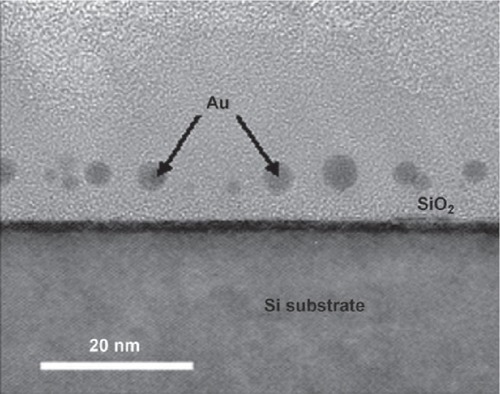
Recently TRAP (trp RNA-binding attenuation protein) has emerged as a potentially useful component for constructing bionano components. The protein, found in species of Bacillus, is involved in regulation of tryptophan synthesis (CitationBabitzke et al 1994, Citation1995; CitationBabitzke 1997, Citation2004; CitationGollnick et al 2005). The structure of the protein shows it to be a small ring consisting of 11 monomers, approximately 8.5 nm in diameter with a central hole approximately 2.5 nm in diameter (CitationAntson et al 1995). A further structure shows that the protein can bind single stranded RNA of a specific sequence around the circumference (CitationAntson et al 1999). TRAP is extremely thermostable (CitationBaumann et al 1997; CitationHeddle et al 2006) and is also tolerant to mutation. In our own work we have begun to use TRAP as an engineerable building block from which to construct more complex structures. For example, we were able to line the central hole with cytseine residues in order to capture gold nanodots (CitationHeddle et al 2007). The gold-binding protein ring was further modified with a titanium binding peptide (CitationSano and Shiba 2003) such that the gold bound ring was able to be specifically placed on a titanium or silicon oxide surface in a known orientation. Arraying gold nanodots in this way, we were able to construct a prototype MOS capacitor (; (CitationHeddle et al 2007)). Furthermore, by fusing together TRAP monomer genes in tandem it was possible to produce a TRAP protein that self-assembled not into the preferred 11mer form but a 12-mer form with 12-fold rotational symmetry which may be most suitable for formation of 2D crystals (; (CitationHeddle et al 2006; CitationWatanabe et al 2008)).
Figure 7 Cross-sectional TEM image of gold nanodots captured by TRAP and embedded in the SiO2 layer of a MOS capacitor. Reproduced with permission from CitationHeddle et al 2007. Using the ring-shaped protein TRAP to capture and confine gold nanodots on a surface. Small, 3:1950–6. Copyright © 2007. Wiley-VCH Verlag GmbH and Co. KGaA.
Proteins that interact with nucleic acids appear to commonly form ring structures: RuvB is another protein involved in homologous recombination, specifically resolution of Holliday junctions, and forms a hexameric ring 12 nm in diameter with a central hole 2–3 nm in diameter (CitationMiyata et al 2000; CitationYamada et al 2001) through which double-stranded DNA can bind. Other ring proteins, which also bind DNA include helicases such as gene 4 from bacteriophage T7 (CitationSingleton et al 2000) and recombinases such as Dmc1 (CitationPassy et al 1999). Proteins of shapes that may be useful in future nanodevices and also have the ability to bind DNA may be of particular interest as DNA itself is a well established, programmable nano-scaf-fold. It is possible to take advantage of the well-understood nature of Watson-Crick base-pairing in DNA molecules and the fact that DNA can be made with complimentary “sticky ends,” to construct “DNA tiles” that can be assembled into large-scale arrays (CitationSeeman 1999). More recently, a different approach, known as “DNA origami” has proved that it is possible to construct arbitrary two dimensional shapes using DNA (CitationRothemund 2006). It should be possible to construct DNA patterns containing specific areas for attachment of proteins. This offers the promise of three dimensional protein devices that can be templated onto surfaces in any given pattern using a DNA scaffold. Indeed, such experiments have already been carried out and include construction of a grid of RuvA protein arranged on a lattice of DNA Holliday junctions (CitationMalo et al 2005) and use of DNA nanoarrays containing aptamers specific for certain proteins to arrange those proteins into arbitrary patterns (CitationChhabra et al 2007).
There are many other protein rings of different sizes and characteristics which may provide useful building blocks for nanostructures. Naturally occurring protein rings may still offer surprising structures such as the catenated rings recently reported in crystals of mitochondrial peroxiredoxin III (CitationCao et al 2005).
Protein tubes
Tubes resemble elongated rings, where length is significantly greater than width. Like rings they have 4 addressable surfaces. Nanotubes have a number of potential uses, for example as containers for controlled release drug formulations, materials and electronics (CitationBong et al 2001; CitationSon et al 2006).
Many of these functions have of course already been suggested as possible uses for CNTs. We have seen that, while CNTs offer vast potential in a wide variety of fields, they may not be able to satisfy all the needs of future nanodevices. Nanometric protein structures may be able to fill many of the niches not available to CNTs and the two materials may complement each other.
The idea of using peptide rings stacked to form tubes has a long history, with a system for using cyclic peptides in this way being proposed as long ago as 1974 (CitationDe Santis et al 1974). More recently the use of proteins and protein rings to form tubes has been investigated. The use of larger proteins consisting of many amino acids gives a measure of redundancy to the system as these larger building blocks can be modified more extensively without a significant change in overall structure.
Naturally occurring protein tubes include microtubules, flagella and pili. While these may be useful they have a number of features which are not ideal. In some cases, naturally occurring tubes are made from multiple proteins, lack ease of modification, cannot be assembled easily in vitro and may have structural features that make addressing all 4 surfaces difficult. In addition, they may have a relatively short persistence length. For use in nanotechnological applications it would be preferable to be able to make a small diameter protein tube from a single subunit that can be expressed in large quantities and can be easily modified and assembled in solution in vitro. Such a tube should have all four surfaces accessible and it should be possible to program its length.
A protein that fulfills some of these requirements is tobacco mosaic virus (TMV). TMV consists of a tubular shaped ribonucleoprotein made from 2130 copies of the coat protein that assemble around a single-stranded RNA core. The resulting virus particle is approximately 300 nm in length (CitationShenton et al 1999). Longer tubes can be obtained by assembling the coat protein in the absence of RNA although in this instance, exact control of length is lost. The inner cavity has been used as a template for mineralization with nickel, cobalt, cobalt-platinum () and iron-platinum nanowires (CitationKnez et al 2003; CitationTsukamoto et al 2007b). The external surface has also been used as a template for the biomineralization of metals (CitationDujardin et al 2003; CitationGórzny et al 2008) and the insertion of cysteine residues on the surface via genetic engineering allowed the chemical attachment of fluorescent chromophores to form a self-assembling light-harvesting system which was able to collect light over a wide spectrum with high efficiency (CitationMiller et al 2007) and may be the basis for future components of optical devices.
Figure 8 Aurothioglucose-stained TEM image showing TMV with biomineralized Co-Pt forming a nanowire in its cavity. Scale bar is 50 nm. Reproduced with permission from CitationTsukamoto et al 2007b. Synthesis of CoPt and FePt3 nanowires using the central channel of tobacco mosaic virus as a biotemplate. Chem Mater, 19:2389–91. Copyright © 2007. American Chemical Society.
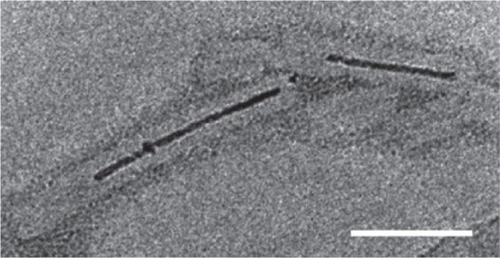
While the TMV tube consists of a building block of a single coat protein arranged in a helical pattern, other tubes of similar dimensions can be made by using stacked protein rings as building blocks. Recently, a number of groups have succeeded in using synthetic biology approaches to produce modified ring proteins that form self-assembled nanotubes, this exciting new area of research may result in new ways of producing nanowires, biosensors, drug delivery systems and structural components of future complex nanodevices.
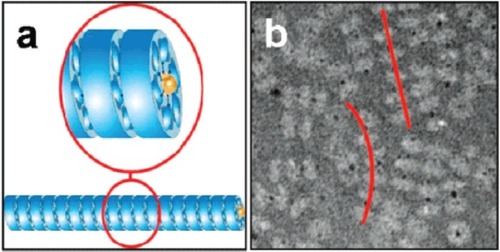
In one example, Stable Protein 1 (SP1) was used (CitationMedalsy et al 2008). This is a nano-ring made from 12 identical proteins, found in aspen plants (CitationWang et al 2002). The 12 proteins form a double-layered 6-membered ring which is 11 nm in diameter with a central hole 2 to 3 nm across (CitationDgany et al 2004). The central cavity was modified by addition of 6 copies of histidine to the N-terminus of each copy of the protein, lining the central hole. The histidine residues bound strongly to two 1.8 nm diameter Ni-NTA modified gold nanodots giving a total of two dots bound to SP1, one at each end of the double ring. The gold dots did not completely enter the hole; part of the dot protruded from each end of the double ring and so provided attachment points for further rings. In this way, the rings polymerized into tube-like chains with each double ring bridged by a gold nanodot (see ). The presence of the gold in the rings offers the potential that the tubes could be used as nanowires.
Figure 9 (a) A schematic showing the polymerization of SP1 rings (shown in blue) via interaction with gold nanodots (yellow) placed in the central cavity of the ring; (b) a TEM image of tube-like chains (highlighted by red lines) of SP1 rings (light grey) mediated by gold nanodots (black dots). Reprinted in part with permission from CitationMedalsy et al 2008. SP1 protein-based nanostructures and arrays. Nano Lett, 8:473–7. Copyright © 2008. American Chemical Society.
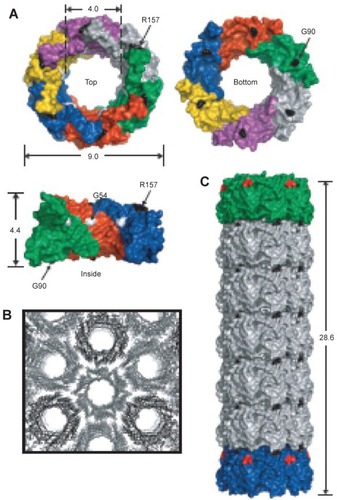
In a second example, hcp was used. Hcp is a homohexameric protein found in Pseudomonas aeruginosa, a protein ring that forms part of the bacteria’s type VI secretion system; it was modified to self-assemble into a tube (CitationBallister et al 2008). The protein naturally forms a ring approximately 9 nm in diameter with a central hole approximately 4 nm in diameter (CitationMougous et al 2006). To promote stacking of the ring into a tube, cysteine residues were engineered into the top and bottom faces so that interring disulfide bonds would form between opposing cysteines (see ). In this way the protein was able to polymerize into tubes of up to 100 nm. Furthermore, by introducing rings with only one face modified with cysteines, it was possible to cap the end of the growing tubes, a form of statistical length control. The capping rings could further be modified to plug the central hole such that the tubes became nanocapsules.
Figure 10 (A) Crystal structure of hcp (pdb 1y12 (CitationMougous, Cuff et al 2006)). Shown from the top, bottom and side. Dimensions are given in nanometers. Each monomer is shown in a different color and three monomers are removed for the side view. Based on the packing of the rings in the crystal structure into tubes (B) residues R157 and G90 were mutated to cysteine residues to facilitate tube formation via disulfide bond formation (C) Reproduced with permission from CitationBallister et al 2008. In vitro self-assembly of tailorable nanotubes from a simple protein building block. Proc Nat Acad Sci U S A, 105:3733–8. Copyright © 2008. National Academy of Sciences, USA.
Future prospects
Modifying proteins to produce new nanometric structures endowed with novel structures and properties is an innovative and exciting synthesis of bionanotechnology and synthetic biology and is already being used to make components of prototype nanodevices including nanocapsules (protein cages), nanorings, nanotubes and nanowires. As a catalogue of self-assembled nanoshapes is compiled, we may see the emergence of more complex nanodevices that consist of several such components that assemble together to make a complex, functioning system or “nanomachine”. A recent example demonstrating how this may be achieved is the “ball and spike protein” () a multicomponent device made from a single gene formed by the fusion of genes for Dps and gp5c (described above). This could be used as part of an electronic device with the biomineralized Dps cavity addressed by 4 “spikes” which, if decorated with appropriate materials, could play the roles of the source, drain and gate of a three dimensional, self assembling nanoscale transistor (illustrated in ). Applying this “building-block” approach to other engineered proteins could result in novel devices with applications as smart drugs and biosensors as well as nanoscale electronics and the field looks set to progress rapidly in coming years.
Acknowledgments
The author thanks Ichiro Yamashita, Kenji Iwahori and Mime Kobayashi for critical reading of the manuscript. The author is funded by MEXT Special Coordination Funds for Promoting Science and Technology and Grant-in-Aid for Young Scientists (WAKATE B-20710083) from the ministry of Education, Culture, Sports, Science and Technology, Japan.
Disclosures
The author has no conflicts of interest to declare.
References
- AggeliABellM1997Responsive gels formed by the spontaneous self-assembly of peptides into polymeric β-sheet tapesNature386259629069283
- AldayeFASleimanHF2007Modular access to structurally switchable 3D discrete DNA assembliesJ Am Chem Soc12913376717939666
- AllenMWillitsD2002Protein cage constrained synthesis of ferrimagnetic iron oxide nanoparticlesAdv Mater1415625
- AndererFASchlumbergerHD1967Structure of simian virus 40. II. Symmetry and components of the virus particleVirology32511234291308
- AntsonAADodsonEJ1999Structure of the trp RNA-binding attenuation protein, TRAP, bound to RNANature4012354210499579
- AntsonAAOtridgeJ1995The structure of trp RNA-binding attenuation proteinNature3746937007715723
- BabitzkeP1997Regulation of tryptophan biosynthesis: Trp-ing the TRAP or how Bacillus subtilis reinvented the wheelMol Microbiol26199383185
- BabitzkeP2004Regulation of transcription attenuation and translation initiation by allosteric control of an RNA-binding protein: the Bacillus subtilis TRAP proteinCurr Opin Microbiol7132915063849
- BabitzkePBearDG1995TRAP, the trp RNA-binding attenuation protein of Bacillus subtilis, is a toroid-shaped molecule that binds transcripts containing GAG or UAG repeats separated by two nucleotidesProc Nat Acad Sci U S A92791620
- BabitzkePStultsJT1994TRAP, the trp RNA-binding attenuation protein of Bacillus subtilis, is a multisubunit complex that appears to recognize G/UAG repeats in the trpEDCFBA and trpG transcriptsJ Biol Chem269165976047515880
- BallisterERLaiAH2008In vitro self-assembly of tailorable nanotubes from a simple protein building blockProc Nat Acad Sci U S A10537338
- BanyardSHStammersDK1978Electron density map of apoferritin at 2.8-A resolutionNature2712824563983
- BaughmanRHZakhidovAA2002Carbon nanotubes-the route toward applicationsScience2977879212161643
- BaumR2003Nanotechnology: Drexler and Smalley make the case for and against molecular assemblersChem Eng News813742
- BaumannCXirasagarS1997The trp RNA-binding attenuation protein (TRAP) from Bacillus subtilis binds to unstacked trp leader RNAJ Biol Chem2721986399242649
- BonardJMChauvinP2002Monodisperse multiwall carbon nanotubes obtained with ferritin as catalystNano Lett26657
- BongDTClarkTD2001Self-assembling organic nanotubesAngew Chem Int Ed409881011
- BozziMMignognaG1997A novel non-heme iron-binding ferritin related to the dna-binding proteins of the Dps family in Listeria innocuaJ Biol Chem2723259659013563
- BraigKOtwinowskiZ1994The crystal structure of the bacterial chaperonin GroEL at 2.8 ANature371578867935790
- CaoZRoszakAW2005Bovine mitochondrial peroxiredoxin III forms a two-ring catenaneStructure1316616416271889
- ChenXAntsonAA1999Regulatory features of the trp operon and the crystal structure of the trp RNA-binding attenuation protein from Bacillus stearothermophilusJ Mol Biol28910031610369778
- ChhabraRSharmaJ2007Spatially addressable multiprotein nanoarrays templated by aptamer-tagged DNA nanoarchitecturesJ Am Chem Soc12910304517676841
- ClarkTDBuehlerLK1998Self-assembling Cyclic β-peptide nanotubes as artificial transmembrane ion channelsJ Am Chem Soc1206516
- CohenBDafniH2005Ferritin as an endogenous MRI reporter for noninvasive imaging of gene expression in C6 glioma tumorsNeoplasia71091715802016
- CourtDLSawitzkeJA2002Genetic engineering using homologous recombinationAnnu Rev Genet363618812429697
- De SantisPMorosettiS1974Conformational analysis of regular enantiomeric sequencesMacromolecules75284837982
- DganyOGonzalezA2004The structural basis of the thermostability of SP1, a novel plant (Populus tremula) boiling stable proteinJ Biol Chem279515162315371455
- DouglasTDicksonDP1995Synthesis and structure of an iron(III) sulfide-ferritin bioinorganic nanocompositeScience26954717787702
- DouglasTStarkVT2000Nanophase cobalt oxyhydroxide mineral synthesized within the protein cage of ferritinInorg Chem3918283012526579
- DouglasTStrableE2002Protein engineering of a viral cage for constrained nano-materials synthesisAdv Mater144158
- DouglasTYoungM1998Host-guest encapsulation of materials by assembled virus protein cagesNature3931525
- DouglasTYoungM2006Viruses: making friends with old foesScience312873516690856
- DujardinEPeetC2003Organization of metallic nanoparticles using tobacco mosaic virus templatesNano Lett34137
- EbbesenTWAjayanPM1992Large-scale synthesis of carbon nanotubesNature3582202
- FeynmanRPA transcript of this talk is available online (www.zyvex.com/nanotech/feynman.html)
- FuTJSeemanNC1993DNA double-crossover moleculesBiochemistry323211208461289
- GaoXMatsuiH2005Peptide-based nanotubes and their applications in bionanotechnologyAdv Mater17203750
- GhadiriMRGranjaJR1993Self-assembling organic nanotubes based on a cyclic peptide architectureNature36632478247126
- GollnickPBabitzkeP2005Complexity in regulation of tryptophan biosynthesis in Bacillus subtilisAnnu Rev Genet39476816285852
- GórznyMWaltonAS2008Four-probe electrical characterization of Pt-coated TMV-based nanostructuresNanotechnology165704
- GulbisJMKelmanZ1996Structure of the C-terminal region of p21(WAF1/CIP1) complexed with human PCNACell872973068861913
- GuoTNikolaevP1995Self-assembly of tubular fullerenesJ Phys Chem99106947
- HarrisonPMArosioP1996The ferritins: molecular properties, iron storage function and cellular regulationBiochim Biophy Acta Bioenerg1275161203
- HartgerinkJDClarkTD1998Peptide nanotubes and beyondChem Eur J4136772
- HeddleJGFujiwaraI2007Using the ring-shaped protein TRAP to capture and confine gold nanodots on a surfaceSmall31950617935079
- HeddleJGYokoyamaT2006Rounding up: engineering 12-membered rings from the cyclic 11-Mer TRAPStructure149253316698553
- HirschA2002Functionalization of single-walled carbon nanotubesAngew Chem Int Ed4118539
- HolmesTCde LacalleS2000Extensive neurite outgrowth and active synapse formation on self-assembling peptide scaffoldsProc Nat Acad Sci U S A97672833
- HorneWSStoutCD2003A heterocyclic peptide nanotubeJ Am Chem Soc1259372612889966
- IijimaS1991Helical microtubules of graphitic carbonNature354568
- IlariALatellaMC2005The unusual intersubunit ferroxidase center of Listeria innocua Dps is required for hydrogen peroxide detoxification but not for iron uptake. A study with site-specific mutantsBiochemistry4455798715823016
- IwahoriKEnomotoT2007Cadmium sulfide nanoparticle synthesis in Dps protein from Listeria innocuaChem Mater19310511
- IwahoriKYamashitaI2007Fabrication of CdS nanoparticles in the bio-template, apoferritin cavity by a slow chemical reaction systemJournal of Physics: Conference Series492
- IwahoriKYoshizawaK2005Fabrication of ZnSe nanoparticles in the apoferritin cavity by designing a slow chemical reaction systemInorg Chem44639340016124819
- Jose-YacamanMMiki-YoshidaM1993Catalytic growth of carbon microtubules with fullerene structureAppl Phys Lett626579
- KagawaWKurumizakaH2002Crystal structure of the homologous-pairing domain from the human Rad52 recombinase in the undecameric formMol Cell103597112191481
- KanamaruSLeimanPG2002Structure of the cell-puncturing device of bacteriophage T4Nature415553711823865
- KanesashiSNIshizuK2003Simian virus 40 VP1 capsid protein forms polymorphic assemblies in vitroJ Gen Virol84189990512810885
- KasumovAYDeblockR1999Supercurrents through single-walled carbon nanotubesScience28415081110348734
- KawanoM-AInoueT2006The VP2/VP3 minor capsid protein of simian virus 40 promotes the in vitro assembly of the major capsid protein VP1 into particlesJ Biol Chem281101647316478732
- KirimuraKUraokaY2005Study of low-temperature crystallization of amorphous Si films obtained using ferritin with Ni nanoparticlesAppl Phys Lett86262106
- KnezMBittnerAM2003Biotemplate synthesis of 3–nm nickel and cobalt nanowiresNano Lett3107982
- KramerRMLiC2004Engineered protein cages for nanomaterial synthesisJ Am Chem Soc12613282615479082
- KumagaiSYoshiiS2006Electrostatic placement of single ferritin moleculesAppl Phys Lett88153103
- LawsonDMArtymiukPJ1991Solving the structure of human H ferritin by genetically engineering intermolecular crystal contactsNature34954141992356
- LawsonDMTreffryA1989Identification of the ferroxidase centre in ferritinFEBS Lett254207102776883
- LiddingtonRCYanY1991Structure of simian virus 40 at 3.8 resolutionNature354278841659663
- LinTChenZ1999The refined crystal structure of cowpea mosaic virus at 2.8 A resolutionVirology265203410603314
- MaloJMitchellJC2005Engineering a 2D protein-DNA crystalAngew Chem Int Ed44305761
- MannS1993Molecular tectonics in biomineralization and biomimetic materials chemistryNature365499505
- MatsuiHPorrataP2001Protein tubule immobilization on self-assembled monolayers on Au substratesNano Lett14614
- MatsuiTMatsukawaN2007Direct production of a two-dimensional ordered array of ferritin-nanoparticles on a silicon substrateJpn J Appl Phys46L713L15
- MatsumuraSUemuraS2004Fabrication of nanofibers with uniform morphology by self-assembly of designed peptidesChem Eur J1027899415195309
- MatsumuraSUemuraS2005Construction of biotinylated peptide nanotubes for arranging proteinsMol Biosyst1146816880977
- McMillanRAPaavolaCD2002Ordered nanoparticle arrays formed on engineered chaperonin protein templatesNat Mater12475212618787
- MedalsyIDganyO2008SP1 protein-based nanostructures and arraysNano Lett8473718193911
- MeldrumFCDouglasT1995Reconstitution of manganese oxide cores in horse spleen and recombinant ferritinsJ Inorg Biochem5859687738539
- MeldrumFCHeywoodBR1992Magnetoferritin: in vitro synthesis of a novel magnetic proteinScience25752231636086
- MeldrumFCWadeVJ1991Synthesis of inorganic nanophase materials in supramolecular protein cagesNature3496847
- MillerRAPresleyAD2007Self-assembling light-harvesting systems from synthetically modified tobacco mosaic virus coat proteinsJ Am Chem Soc1293104917319656
- MiuraAHikonoT2006Floating nanodot gate memory devices based on biominerlized inorganic nanodot array as a storage nodeJpn J Appl Phys45L1L3
- MiuraAUraokaY2007Bionanodot monolayer array fabrication for nonvolatile memory applicationSurf Sci601L81L5
- MiyataTYamadaK2000Two different oligomeric states of the ruvb branch migration motor protein as revealed by electron microscopyJ Struct Biol13183911042078
- MougousJDCuffME2006A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatusScience31215263016763151
- NurajeNBanerjeeIA2004Biological bottom-up assembly of antibody nanotubes on patterned antigen arraysJ Am Chem Soc1268088915225029
- OkudaMIwahoriK2003Fabrication of nickel and chromium nanoparticles using the protein cage of apoferritinBiotechnol Bioeng841879412966575
- ParkSHPistolC2006Finite-Size, fully addressable DNA tile lattices formed by hierarchical assembly proceduresAngew Chem Int Ed457359
- PassySIYuX1999Rings and filaments of beta protein from bacteriophage lambda suggest a superfamily of recombination proteinsProc Nat Acad Sci U S A96427984
- PassySIYuX1999Human Dmc1 protein binds DNA as an octameric ringProc Nat Acad Sci U S A96106848
- PoteeteAR2001What makes the bacteriophage lambda Red system useful for genetic engineering: molecular mechanism and biological functionFEMS Microbiol Lett20191411445160
- RechesMGazitE2003Casting metal nanowires within discrete self-assembled peptide nanotubesScience300625712714741
- RechesMGazitE2006Controlled patterning of aligned self-assembled peptide nanotubesNat Nanotech1195200
- RothemundPW2006Folding DNA to create nanoscale shapes and patternsNature44029730216541064
- RyadnovMG2007A self-assembling peptide polynanoreactorAngew Chem Int Ed4696972
- RyadnovMGCeyhanB2003Belt and braces: a peptide-based linker system of de novo designJ Am Chem Soc12593889412889969
- SanoKShibaK2003A hexapeptide motif that electrostatically binds to the surface of titaniumJ Am Chem Soc12514234514624545
- SavianoMLombardiA1994A structural two-ring version of a tubular stack of β-Rings in crystals of a cyclic D, L-hexapeptideJ Inclusion Phenom182736
- SeebachDMatthewsJL1997Cyclo-β-peptides: structure and tubular stacking of cyclic tetramers of 3-aminobutanoic acid as determined from powder diffraction data-peptidesHelv Chim Acta8017382
- SeemanNC1999DNA engineering and its application to nanotechnologyTrends Biotechnol174374310511701
- SeemanNC2003DNA in a material worldNature4214273112540916
- ShentonWDouglasT1999Inorganic-organic nanotube composites from template mineralization of tobacco mosaic virusAdv Mater112536
- ShihWMQuispeJD2004A 1.7-kilobase single-stranded DNA that folds into a nanoscale octahedronNature4276182114961116
- SimmonsJMInI2007Optically modulated conduction in chromophore-functionalized single-wall carbon nanotubesPhys Rev Lett9808680217359117
- SingletonMRSawayaMR2000Crystal structure of T7 gene 4 ring helicase indicates a mechanism for sequential hydrolysis of nucleotidesCell10158960010892646
- SingletonMRWentzellLM2002Structure of the single-strand annealing domain of human RAD52 proteinProc Nat Acad Sci U S A991349213497
- Sinnott SBAndrews R2001Carbon nanotubes: synthesis, properties, and applicationsCrit Rev Solid State Mater, Sci26145249
- SmalleyRE2001Of chemistry, love and nanobotsSci Am28576711524973
- SonSJBaiX2006Template synthesis of multifunctional nanotubes for controlled releaseJ Control Release1141435216870299
- SpeirJAMunshiS1995Structures of the native and swollen forms of cowpea chlorotic mottle virus determined by X-ray crystallography and cryo-electron microscopyStructure363787743132
- StasiakAZLarquetE2000The human Rad52 protein exists as a heptameric ringCurr Biol103374010744977
- SteinmetzNFBockE2008Assembly of multilayer arrays of viral nanoparticles via biospecific recognition: a quartz crystal microbalance with dissipation monitoring studyBiomacromolecules94566218197628
- SteinmetzNFCalderG2006aPlant viral capsids as nanobuild-ing blocks: construction of arrays on solid supportsLangmuir22100323717106996
- SteinmetzNFLomonossoffGP2006bCowpea mosaic virus for material fabrication: addressable carboxylate groups on a programmable nanoscaffoldLangmuir2234889016584217
- SteinmetzNFLomonossoffGP2006cDecoration of cowpea mosaic virus with multiple, redox-active, organometallic complexes13Small2530317193081
- StillmanTJUpadhyayM2005The crystal structures of Lactococcus lactis MG1363 Dps proteins reveal the presence of an N-terminal helix that is required for DNA bindingMol Microbiol5711011216091047
- SugimotoKKanamaruS2006Construction of a ball-and-spike protein supramolecule13Angew Chem Int Ed4527258
- TakahashiYUenoAMiharaH2002Amyloid architecture: complementary assembly of heterogeneous combinations of three or four peptides into amyloid fibrilsChembiochem36374212324997
- TheilECTakagiH2000The ferritin iron entry and exit problemInorg Chim Acta29724251
- TsukamotoHKawano2007aEvidence that SV40 VP1 DNA interactions contribute to the assembly of 40-nm spherical viral particlesGenes to Cells1212677917986010
- TsukamotoRMuraokaM2007bSynthesis of CoPt and FePt3 nanowires using the central channel of tobacco mosaic virus as a biotemplateChem Mater19238991
- VollrathFKnightDP2001Liquid crystalline spinning of spider silkNature410541811279484
- WangW-XPelahD2002Characterization of SP1, a stress-responsive, boiling-soluble, homo-oligomeric protein from aspenPlant Physiol1308657512376651
- WatanabeMMishimaY2008Intersubunit linker length as a modifier of protein stability: Crystal structures and thermostability of mutant TRAPProtein Sci1751852618287284
- WinfreeELiuF1998Design and self-assembly of two-dimensional DNA crystalsNature394539449707114
- YamadaKKunishimaN2001Crystal structure of the Holliday junction migration motor protein RuvB from Thermus thermophilus HB8Proc Nat Acad. Sci U S A9814427
- YamashitaI2001Fabrication of a two-dimensional array of nano-particles using ferritin moleculeThin Solid Films3931218
- YamashitaIHayashiJ2004Bio-template synthesis of uniform CdSe nanoparticles using cage-shaped protein, apoferritinChem Lett3311589
- YamashitaIKirimuraH2006Selective nanoscale positioning of ferritin and nanoparticles by means of target-specific peptides13Small211485217193580
- YoshizawaKIwahoriK2006Fabrication of gold sulfide nanoparticles using the protein cage of apoferritinChem Lett351192
- YoshizawaKMishimaY2007Effect of N-terminal residues on the structural stability of recombinant horse L-chain apoferritin in an acidic environmentJ Biochem (Tokyo)1427071317938140