Abstract
There has been considerable interest in polyelectrolyte multilayer nanofilms, which have a variety of applications ranging from optical and electrochemical materials to biomedical devices. Polyelectrolyte multilayer nanofilms are constructed from aqueous solutions using electrostatic layer-by-layer self-assembly of oppositely-charged polyelectrolytes on a solid substrate. Multifunctional polyelectrolyte multilayer nanofilms have been studied using charged dyes, metal and inorganic nanoparticles, DNA, proteins, and viruses. In the past few years, there has been increasing attention to developing polyelectrolyte multilayer nanofilms as drug delivery vehicles. In this mini-review, we present recent developments in polyelectrolyte multilayer nanofilms with tunable drug delivery properties, with particular emphasis on the strategies in tuning the loading and release of drugs in polyelectrolyte multilayer nanofilms as well as their applications.
Introduction
Polyelectrolyte multilayer nanofilms are typically prepared using electrostatic layer-by-layer self-assembly (LbL), which has been widely used as a versatile technique for fabricating nanofilms with controlled structures and compositions.Citation1 Due to the high degree of control over film properties, flexible choice of assembly components, and ease of processing, polyelectrolyte multilayer nanofilms have a variety of applications ranging from optical and electrochemical materials to biomedical devices.Citation2–Citation4 Most studies to date have focused on engineering the specifics of polyelectrolyte multilayer nanofilms in terms of efficiency of ion-pairing, internal thin film architecture, ion permeability, porosity, wetting/dewetting properties, and surface roughness as well as mechanical properties. The biocompatibility of polyelectrolyte multilayer nanofilms has also been investigated. In vitro and in vivo studies have shown that some polyelectrolyte multilayer nanofilms are biocompatible, and may enhance adhesion and growth of cells like osteoblasts,Citation5,Citation6 chondrocytes,Citation7,Citation8 myoblasts,Citation9 chondrosarcomas,Citation10 and smooth muscle cells.Citation11
Over the past few years, there has been increasing interest in developing drug-carrying polyelectrolyte multilayer nanofilms for biomedical applications, which may offer the ability to control the structure and concentration of incorporated drugs in precise-scale. A variety of drugs such as proteins and DNA have been incorporated into polyelectrolyte multilayer nanofilms (), retained their bioactivity,Citation12,Citation13 and showed promise in treating diseases including biomedical device-associated infection.Citation7,Citation14–Citation18 Here we briefly review the research progress in polyelectrolyte multilayer nanofilms as tunable drug delivery systems and their applications.
Table 1 Examples of polyelectrolyte multilayer nanofilms as tunable drug delivery systems
Tunable drug loading and release in polyelectrolyte multilayer nanofilms
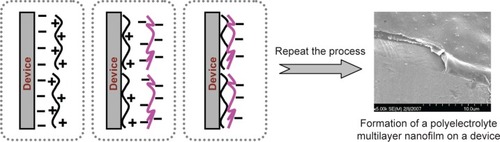
Polyelectrolyte multilayer nanofilms are prepared using LbL nanotechnology, which is currently one of the most powerful methods for preparing nanofilms of controlled molecular architecture. LbL technology, developed in the 1990s,Citation1 involves the repetitive sequential dipping of a charged substrate into solutions of oppositely-charged polyelectrolytes, and the adsorption of the oppositely-charged polyelectrolytes leads to the formation of a multilayer nanofilm on the substrate, eg, a device (). Compared to other coating techniques, such as the plasma technique, LbL provides a rather simple aqueous-based means of varying film composition while providing enormous design flexibility. The properties of polyelectrolyte multilayer nanofilms can easily be manipulated.Citation19 The physical basis of LbL is primarily electrostatic attraction; however, other forces, eg, hydrophobic, van der Waals, and hydrogen bonding or acid-base type, may also play a significant role under certain conditions. These forces can be used to control drug loading and release from polyelectrolyte multilayer nanofilms.
Tunable drug loading in polyelectrolyte multilayer nanofilms
In the last decade, polyelectrolyte multilayer nanofilms have attracted great interest as potential vehicles for controlled drug delivery. In general, drugs can be incorporated within polyelectrolyte multilayer nanofilms through a variety of means including pH-induced drug loading, integrating drugs as an LbL-coating component, or covalent bonding of the drug to nanofilms.
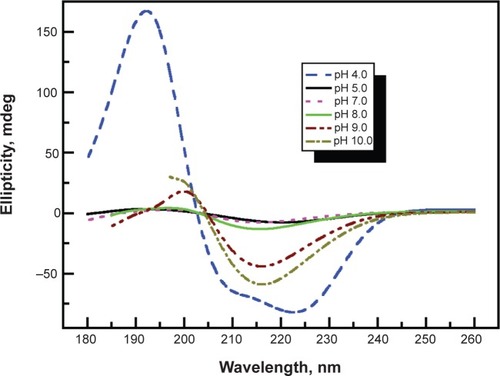
We recently reported the loading of small charged drug molecules (eg, cefazolin and gentamicin) into polypeptide multilayer nanofilms, and we found that the loading of small charged drug molecules could be finely tuned by the number of film layers, drug incubation time, and application of heat treatment after film formation.Citation17 Moreover, we showed that drug loading was pH-dependent and could be tuned by drug-loading solution pH as well as film preparation pH. We created “binding-sites” or net charges within multilayer nanofilms for binding of oppositely-charged drug molecules by controlling the LbL process pH and the drug-loading solution pH. As a result, we achieved tunable loading of antibiotics in polypeptide multilayer nanofilms.Citation17 More recently, we found that the pH (in the range of 4.0–10.0) of the LbL process also influenced the secondary structure of polypeptide multilayer nanofilms. We observed more β-sheet structure in poly(L-lysine)/poly(L-glutamic acid) or PLL/PLGA multilayer nanofilms prepared at a lower (eg, pH 4.0) or higher pH (eg, pH 10.0) than at pH 7.0 (). The secondary structure of nanofilms may play a role in tuning drug loading as well as release in polyelectrolyte multilayer nanofilms although no such evidence has been reported yet. In other studies, amphiphilic block copolymer micelles,Citation20,Citation21 hydrogel,Citation22,Citation23 and nanostructured poresCitation24 were also introduced in polyelectrolyte multilayer nanofilms to control drug-loading and release.
Figure 2 Effects of pH at which nanofilms were prepared on secondary structure of (PLL/PLGA)10 nanofilms. (PLL/PLGA)10 nanofilms were assembled on quartz slides at pHs 4.0, 5.0, 7.0, 8.0, 9.0, and 10.0.
Moreover, by integrating drug molecules as an LbL coating component, a variety of charged macromolecular drugs, eg, proteins, polysaccharides, enzymes, nucleic acids, and DNA, have been immobilized into polyelectrolyte multilayer nanofilms.Citation25–Citation29 In these studies, the macromolecular drug molecules served as both functional drugs and film components. As a coating component, interleukin-12 p70 (IL-12 p70; a key cytokine for cell-mediated immune response), together with a protein carrier (ie, bovine serum albumin), was recently incorporated in polypeptide multilayer nanofilms and it was found that its loading and release were tunable by controlling the number of film layers as well as IL-12 p70 concentration.Citation18
In addition, drug molecules can be covalently bonded to polyelectrolyte multilayer nanofilms.Citation12,Citation30,Citation31 A drug delivery platform in hyaluronan/chitosan or HA/CHI multilayer nanofilms was developed. Paclitaxel, a chemotherapeutic agent, was conjugated on the HA chain within the nanofilms, and its drug bioactivity was found to be retained.Citation12 Werner and colleaguesCitation31 coupled an adhesion peptide, derived from laminin, in PLL/PLGA multilayer nanofilms, and observed enhanced epithelial cell adhesion and better in vivo cell colonization.
Tunable drug release from polyelectrolyte multilayer nanofilms
Drug release behavior in polyelectrolyte multilayer nanofilms depends on the permeability, erosion or degradation of multilayer nanostructure, as well as the interaction between drug molecules and nanofilm components. As a result, the release of drugs can be tuned by control of environment pH, ionic strength, temperature, and deconstruction or dissolution of polyelectrolyte multilayer nanofilms.
Our studies showed that environment pH could be used to tune the release of electrostatically-bound drug molecules, eg, cefazolin and gentamicin, and the pH-triggered drug release was due to the change of net charges of weak polyelectrolytes within the nanofilms as pH changes.Citation17 The change in net charges results in attraction or repulsion between charged drug molecules and polyelectrolyte film components thereby leading to the tuning of drug release. Polyelectrolyte multilayer nanofilms composed of poly(allylamine hydrochloride)/poly(acrylic acid) also had pH-responsive release of small charged drug molecules.Citation32 In addition, drug release could be controlled by introducing pH-degradable components in polyelectrolyte multilayer nanofilms, as pH-induced degradation and drug release were observed in multilayer nanofilms assembled with degradable poly(β-amino ester).Citation33
Similarly, ionic strength can be utilized to tune drug release, since changing the ionic strength of the drug release medium can influence the electrostatic interactions between polyelectrolyte coating components and charged drug molecules. It was found that high concentrations of salt (eg, 0.6 M) weakened the electrostatic interactions between adjacent layers of polyelectrolytes and caused the destruction of polyelectrolyte multilayer nanofilms.Citation34,Citation35
Temperature-triggered drug release is another attractive approach to tune drug release from polyelectrolyte multilayer nanofilms. Polyelectrolyte multilayer nanofilms were constructed by introducing a thermo-responsive hydrogel, eg, poly(N-isopropylacrylamide), as a drug delivery vehicle.Citation36 The poly(N-isopropylacrylamide) hydrogel undergoes a reversible volume phase transition at its critical point temperature, at which it shrinks or expands. This reversible shrinking/expanding structure therefore leads to tunable drug-loading and release. Quinn and colleagues also studied polyelectrolyte multilayer nanofilms assembled with poly(styrene-alt-maleic acid) and poly(ethylene oxide); the latter is thermo-responsive.Citation36 Rhodamine B, a commonly-used dye, was used as a drug model and loaded into the nanofilms. It was found that the release of Rhodamine B was temperature-dependent.Citation36
Finally, introducing crosslinking, hydrolysis, or enzymatic degradation can tune drug release from polyelectrolyte multilayer nanofilms. Amphiphilic block copolymer micelles were integrated as nanometer-sized vehicles for hydrophobic drug delivery, and sustained release of triclosan was obtained from polyelectrolyte multilayer nanofilms after introducing crosslinking.Citation37 Hydrolysis of the ester linkage between HA and paclitaxel under physiological conditions also showed controlled release of paclitaxel from HA/CHI multilayer nanofilms.Citation12 Tunable release of procoagulant agents and DNA was observed in enzyme hydrolysable polyelectrolyte multilayer nanofilms.Citation38,Citation39
Applications of polyelectrolyte multilayer nanofilms as tunable drug delivery systems
Polyelectrolyte multilayer nanofilms have been widely used to modify the surface properties of biomedical devices. Drug-carrying polyelectrolyte multilayer nanofilms have recently attracted attention for their antibacterial applications, inflammation alleviation, and tissue engineering, to name a few examples.
The surface of biomedical devices is a common site of bacterial and fungal adhesion, the first step in the formation of a biofilm that frequently leads to infections. In order to prevent such infections, several physical and chemical modifications of device surface have been studied, and the LbL process is one promising technique. Our studies showed that PLL/PLGA multilayer nanofilms with tunable antibiotic loading prevented the colonization of Staphylococcus aureus on orthopedic implants and demonstrated controllable antibacterial properties in vitro against S. aureus.Citation17 Cefazolin and gentamicin-loaded PLL/PLGA nanofilms with different numbers of layers and prepared at different solution pHs showed different antibacterial properties. Also, our developed IL-12 p70 incorporated polypeptide multilayer nanofilm proved its efficacy in preventing biomedical device-associated infection; IL-12 p70 on stainless steel Kirschner wires substantially decreased bacterial infection in an open femur fracture rat model.Citation18 Also, CHI, a commonly used antibacterial dressing, has received attention in fabrication of antibacterial polyelectrolyte multilayer nanofilms. CHI-containing multilayer nanofilms showed high resistance to bacterial adhesion and led to a substantial decrease in Escherichia coli adhesion compared to control bare substrates.Citation7,Citation14,Citation15 Moreover, CHI-containing multilayer nanofilms prepared at different ionic strengths showed different bacterial resistance properties,Citation7 and the LbL process solution pH had a remarkable effect on the antibacterial properties of polyelectrolyte multilayer nanofilms.Citation14 Antibacterial nanoparticles such as silver nanoparticles could also be immobilized in polyelectrolyte multilayer nanofilms and enabled the nanofilms antibacterial properties against methicillin-resistant S. aureus or MRSA.Citation16 In addition, antifungal polyelectrolyte multilayer nanofilms were developed by incorporating chromogranin A-derived antifungal peptide in PLL/PLGA multilayer nanofilms, and in vitro studies demonstrated the ability of the nanofilms in inhibiting growth of the yeast Candida albicans and the filamentous fungus Neurospora crassa.Citation40
Polyelectrolyte multilayer nanofilms incorporated with drugs may also alleviate inflammation associated with biomedical devices. Schultz and colleaguesCitation29 conjugated a synthetic analog of an anti-inflammatory peptide, alpha-melanocyte-stimulating hormone (alpha-MSH) to PLGA, and their developed PLL/PLGA multilayer nanofilms showed reduced inflammatory response in vivo.
Further, polyelectrolyte multilayer nanofilms have been investigated to functionalize biomedical device surfaces for tissue engineering applications. Controlled delivery of growth factors and cytokines from biomedical device surfaces may offer the potential to concentrate the drugs and deliver them locally, as opposed to topical administration. Immobilization of growth factors and cytokines within polyelectrolyte multilayer nanofilms may also protect the drugs from degradation by enzymes in tissue fluids. Localized and sustained drug delivery systems were designed for recombinant human bone morphogenetic-protein 2 (rhBMP-2) in polyelectrolyte multilayer nanofilms.Citation13 Cross-linked PLL/HA multilayer nanofilms were prepared as reservoirs for rhBMP-2 delivery to myoblasts and induced their differentiation into osteoblasts in a dose-dependent manner. The amount of rhBMP-2 loaded in the nanofilms was controlled by varying the deposition conditions and the film thickness, and the immobilized growth factor was found to be bioactive. Such polyelectrolyte multilayer nanofilms could have potential applications in local delivery of growth factors for tissue engineering constructs.
Conclusions and outlook
Polyelectrolyte multilayer nanofilms with tunable drug delivery have attracted great interest and shown promise for the development of coatings for implant devices and tissue engineering scaffolds. A variety of drugs including antibiotics, cytokines, and growth factors have been incorporated in polyelectrolyte multilayer nanofilms. The incorporation and release of drugs can be finely tuned by environmental conditions, including pH, temperature, and ionic strength; by the history of nanofilm preparation, including physical binding or chemical bonding of drug molecules within polyelectrolyte multilayer nanofilms, and pH, concentration, and ionic strength of polyelectrolyte self-assembly solutions and drug-loading solutions; as well as by post-preparation treatments (eg, heat treatment).
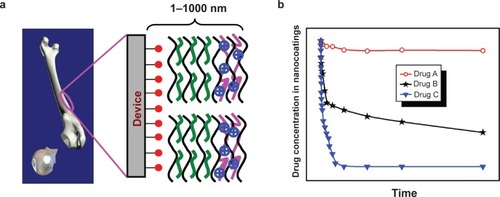
Future development in this area could be polyelectrolyte multilayer nanofilms possessing multifunctions including combined delivery of multiple therapeutic agents or combined delivery of therapeutic and diagnostic agents. Multiple therapeutic and/or diagnostic agents could be immobilized on the surface of one biomedical device (eg, an orthopedic implant) via formation of polyelectrolyte multilayer nanofilms, and different drug-loading strategies described in this paper could be used to incorporate multiple drugs in polyelectrolyte multilayer nanofilms (). Depending on the means that drugs are incorporated, multiple drug delivery patterns, eg, burst release, sustained release, and constant concentration, could be obtained in one device (). Such devices could achieve ideal treatment outcomes, eg, achieving infection prevention and wound healing simultaneously or combining diagnosis and treatment in a single device.
Figure 3 a) Multifunctional polyelectrolyte multilayer nanofilm on a device. ![]()
Acknowledgments
Financial support from National Science Foundation (OISE-0737735), AO Foundation, Osteosynthesis and Trauma Care Foundation, National Aeronautics and Space Administration West Virginia Experimental Program to Stimulate Competitive Research (NASA WV EPSCoR), and West Virginia University Research Corporation, is acknowledged. Project S-07-43L was supported by the AO Research Fund of the AO Foundation. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the funding agencies. We also thank Suzanne Smith for proofreading the manuscript.
References
- DecherGFuzzy nanoassemblies: Toward layered polymeric multicompositesScience199727712321237
- HammondPTForm and function in multilayer assembly: New applications at the nanoscaleAdv Mater20041612711293
- BaronRWillnerBWillnerIBiomolecule-nanoparticle hybrids as functional units for nanobiotechnologyChem Commun200728323332
- SahooSKParveenSPandaJJThe present and future of nanotechnology in human health careNanomedicine20073203117379166
- PicartCElkaimRRichertLPrimary cell adhesion on RGD-functionalized and covalently crosslinked thin polyelectrolyte multilayer filmsAdv Funct Mater2005158394
- LikibiFJiangBBLiBBiomimetic nanocoating promotes osteoblast cell adhesion on biomedical implantsJ Mater Res2008231232223228
- RichertLLavallePPayanELayer by layer buildup of polysaccharide films: Physical chemistry and cellular adhesion aspectsLangmuir2004b2044845815743090
- RichertLSchneiderAVautierDImaging cell interactions with native and crosslinked polyelectrolyte multilayersCell Biochem Biophys20064427328516456228
- VazquezCPBoudouTDulongVVariation of polyelectrolyte film stiffness by photo-cross-linking: A new way to control cell adhesionLangmuir2009253556356319275180
- SchneiderARichertLFranciusGElasticity, biodegradability and cell adhesive properties of chitosan/hyaluronan multilayer filmsBiomed Mater200724551
- RichertLBoulmedaisFLavallePImprovement of stability and cell adhesion properties of polyelectrolyte multilayer films by chemical cross-linkingBiomacromolecules2004a528429415002986
- ThierryBKujawaPTkaczykCDelivery platform for hydrophobic drugs: Prodrug approach combined with self-assembled multilayersJ Am Chem Soc20051271626162715700982
- CrouzierTRenKNicolasCLayer-by-layer films as a biomimetic reservoir for rhBMP-2 delivery: Controlled differentiation of myoblasts to osteoblastsSmall2009559860819219837
- FuJJiJYuanWShenJConstruction of anti-adhesive and antibacterial multilayer films via layer-by-layer assembly of heparin and chitosanBiomaterials2005266684669215946736
- FuJHJiJFanDZConstruction of antibacterial multilayer films containing nanosilver via layer-by-layer assembly of heparin and chitosan-silver ions complexJ Biomed Mater Res200679A665674
- YuDGLinWCYangMCSurface modification of poly(L-lactic acid) membrane via layer-by-layer assembly of silver nanoparticle-embedded polyelectrolyte multilayerBiocon Chem20091815211529
- JiangBBLiBTunable drug incorporation and release from polypeptide multilayer nanofilmsInt J Nanomedicine20094375319421369
- LiBJiangBBBoyceBMLindseyBAMultilayer polypeptide nanoscale coatings for the prevention of biomedical device associated infectionsBiomaterials2009302552255819215980
- ZhongYLiBHaynieDTFine tuning of physical properties of designed polypeptide multilayer films by control of pHBiotechnol Prog200622112613216454502
- QiBTongXZhaoYLayer-by-layer assembly of two different polymer micelles with polycation and polyanion coronasMacromolecules20063957145719
- ZhongYWhittingtonCFHaynieDTStimulated release of small molecules from polyelectrolyte multilayer nanocoatingsChem Commun20071414151417
- QuinnJFCarusoFThermoresponsive nanoassemblies: Layer-by-layer assembly of hydrophilic-hydrophobic alternating copolymersMacromolecules20053834143419
- WangLWangXXuMFLayer-by-layer assembled microgel films with high loading capacity: Reversible loading and release of dyes and nanoparticlesLangmuir2008241902190918205423
- BergMCZhaiLCohenRERubnerMFControlled drug release from porous polyelectrolyte multilayersBiomacromolecules2006735736416398536
- ScrantonABRangarajanBKlierJBiomedical applications of polyelectrolytesAdv Polym Sci1995122154
- ChlubaJVoegelJCDecherGPeptide hormone covalently bound to polyelectrolytes and embedded into multilayer architectures conserving full biological activityBiomacromolecules2001280080511710034
- JesselNAtalarFLavallePBioactive coatings based on a polyelectrolyte multilayer architecture functionalized by embedded proteinsAdv Mater200315692695
- CaiKYRechtenbachAHaoJYPolysaccharide-protein surface modification of titanium via a layer-by-layer technique: Characterization and cell behavior aspectsBiomaterials2005265960597115913761
- SchultzPVautierDRichertLPolyelectrolyte multilayers functionalized by a synthetic analogue of an anti-inflammatory peptide, alpha-MSH, for coating a tracheal prosthesisBiomaterials2005262621263015585265
- LuZZWuJSunTMBiodegradable polycation and plasmid DNA multilayer film for prolonged gene delivery to mouse osteoblastsBiomaterial200829733741
- WernerSHuckOFrischBThe effect of microstructured surfaces and laminin-derived peptide coatings on soft tissue interactions with titanium dental implantsBiomaterials2009302291230119168216
- ChungAJRubnerMFMethods of loading and releasing low molecular weight cationic molecules in weak polyelectrolytes multilayer filmsLangmuir20021811761183
- WoodKCBoedickerJQLynnDMTunable drug release from hydrolytically degradation layer-by-layer thin filmsLangmuir2005211603160915697314
- SukhishviliSAGranickSLayered, erasable, ultrathin polymer filmsJ Am Chem Soc200012295509551
- DubasSTFarhatTRSchlenoffJBMultiple membranes from “true” polyelectrolyte multilayersJ Am Chem Soc20011235368536911457408
- QuinnJFCarusoFFacile tailoring of film morphology and release properties using layer-by-layer assembly of thermoresponsive materialsLangmuir200420202215744991
- KimBSParkSWHammondPTHydrogen-bonding layer-by-layer-assembled biodegradable polymeric micelles as drug delivery vehicles from surfacesACS Nano2008238639219206641
- SerizawaTYamaguchiMAkashiMAlternating bioactivity of polymeric layer-by-layer assemblies: Anticoagulation vs procoagulation of human bloodBiomacromolecules2002372473112099816
- SerizawaTYamaguchiMAkashiMTime-controlled desorption of ultrathin polymer films triggered by enzymatic degradationAngew Chem Int Ed20034211151118
- EtienneOGasnierCTaddeiCAntifungal coating by biofunctionalized polyelectrolyte multilayered filmsBiomaterials2005266704671215992921