Abstract
The a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) comprise a family of secreted zinc metalloproteinases with a precisely ordered modular organization. These enzymes play an important role in the turnover of extracellular matrix proteins in various tissues and their dysregulation has been implicated in disease-related processes such as arthritis, atherosclerosis, cancer, and inflammation. ADAMTS-7 and ADAMTS-12 share a similar domain organization to each other and form a subgroup within the ADAMTS family. Emerging evidence suggests that ADAMTS-7 and ADAMTS-12 may play an important role in the development and pathogenesis of various kinds of diseases. In this review, we summarize what is currently known about the roles of these two metalloproteinases, with a special focus on their involvement in chondrogenesis, endochondral ossification, and the pathogenesis of arthritis, atherosclerosis, and cancer. The future study of ADAMTS-7 and ADAMTS-12, as well as the molecules with which they interact, will help us to better understand a variety of human diseases from both a biological and therapeutic standpoint.
Introduction
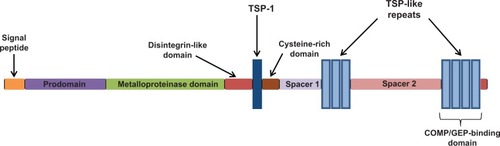
The a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) are zinc matrix metalloproteinases (MMPs) with a precisely ordered modular organization. ADAMTS comprises a family of secreted proteinases, many of which bind to and modulate extracellular matrix proteins. The ADAMTSs are translated initially as inactive pre-proenzymes, whose structure includes a signal peptide, pro-domain, catalytic domain, disintegrin-like domain, a central thrombospondin type I-like (TSP) repeat, a cysteine-rich domain, a spacer region, and a variable number of C-terminal TSP repeats. ADAMTSs can occur in multiple isoforms due to alternative splicing.Citation1,Citation2
First identified in 1997, members of the ADAMTS family are involved in diseases ranging from coagulation disorders to malignancy ().Citation3–Citation6 ADAMTS-13 plays a role in the development of the coagulation disorder, thrombotic thrombocytopenic purpura.Citation5,Citation7–Citation9 Patients with Ehler–Danlos syndrome type 7C, a genetic disorder of collagen synthesis, have mutations in the ADAMTS-2 gene.Citation10,Citation11 These mutations have also been associated with bovine dermatopraxis, an inherited disorder characterized by severe skin fragility.Citation10 ADAMTS-1 exhibits angioinhibitory properties and is crucial for the development and function of the urogenital system.Citation12–Citation14 ADAMTS-1 may also contribute to atherosclerosis by cleaving versican, a component of extracellular matrix (ECM).Citation15 Indeed, mutations in ADAMTS-1 have been associated with an increased risk of coronary artery disease.Citation16 Other ADAMTS, including ADAMTS-4 and 8, have also been implicated in the formation of atherosclerotic plaque and atherothrombotic disease.Citation17,Citation18 ADAMTS-5 has also been associated with osteoarthritis and other inflammatory joint diseases due to its ability to degrade aggrecan.Citation19–Citation22 In addition, other ADAMTS, including ADAMTS-1, 4, 8, 9, 12, 16, and 18 have also been shown to cleave aggrecan in vitro.Citation20,Citation23–Citation27
Table 1 Biological roles of ADAMTS metalloproteinases
Two recently discovered members of the ADAMTS family, ADAMTS-7 and ADAMTS-12, form a subgroup within the ADAMTS family based on their shared domain organization (). Emerging evidence suggests that ADAMTS-7 and ADAMTS-12 may play a key role in the pathogenesis of important diseases, such as arthritis, atherosclerosis, and cancer.Citation3,Citation28,Citation29 In this review, we summarize what is currently known about the roles of ADAMTS-7 and ADAMTS-12 in the pathogenesis of these diseases as well as in other important biological processes.
Figure 1 Schematic representation of the domain organization of ADAMTS-7/-12. The C-terminal COMP/GEP-binding TSP1 motifs are indicated.
Role in arthritis
The notion that MMPs and ADAMTSs play an important role in osteoarthritis and rheumatoid arthritis has been well established.Citation29–Citation36 In one study, ADAMTS-7 was found to be significantly upregulated in arthritic cartilage and synovium compared with normal controls.Citation37 Quantitative real-time polymerase chain reaction (PCR) has revealed that while ADAMTS-7 and -12 are both significantly upregulated in RA cartilage, only ADAMTS-12 is significantly upregulated in OA cartilage (unpublished data).Citation37,Citation38
The inflammatory cytokines, tumor necrosis factor (TNF) and interleukin (IL)-1β have been previously shown to induce the expression of a number of MMPs involved in the development and progression of arthritis.Citation39–Citation43 Real-time PCR analysis of cultured human cartilage explants show that both TNF and IL-1β strongly induce ADAMTS-7 and ADAMTS-12 expression.Citation44 Interestingly, this induction does not occur for ADAMTS-12 in human fetal fibroblasts, suggesting that there may be some tissue specificity for this effect.Citation45
Interaction with COMP
Arthritis is a disease process characterized by the proteolytic degradation of ECM components with subsequent loss of articular cartilage and bone. Cartilage oligomeric matrix protein (COMP), a 524 kDa disulfide-bonded multidomain glycoprotein composed of five 110 kDa subunits, is a prominent noncollagenous component of cartilage ECM.Citation46 Mutations in the human COMP gene have been linked to the development of pseudoachondroplasia and multiple epiphyseal dysplasia, which are autosomal-dominant forms of short-limb dwarfism.Citation47–Citation50 Although the function of COMP is not completely understood, it appears to mediate chondrocyte attachment via an integrin receptor.Citation51,Citation52 Accumulating evidence suggests that COMP may function to stabilize the ECM of articular cartilage by specific interactions with matrix components including collagen type II and IX, aggrecan, and fibronectin.Citation53–Citation56 Fragments of COMP have been detected in the diseased cartilage, synovial fluid, and serum of patients with post-traumatic knee injuries, primary osteoarthritis (OA) and rheumatoid arthritis (RA).Citation57,Citation58 This suggests that COMP degradation may play a key role in these disease processes. Furthermore, several recent studies have suggested that monitoring COMP levels in joint fluid and/or serum may be useful in assessing the progression of arthritis in a clinical setting.Citation59–Citation64 Thus, the study of COMP-degradative enzymes is of potential significance; both to elucidate the mechanism of disease as well as for the development of novel approaches to the diagnosis and therapy of arthritis.
Purified COMP is digested by several MMPs in vitro, including MMP-1, MMP-3, MMP-9, MMP-13, MMP-19, and MMP-20.Citation65,Citation66 A member of the ADAMTS family, ADAMTS-4, has also been reported to cleave COMP in vitro.Citation67 Despite these findings, the exact role of MMPs in COMP degradation has yet to be confirmed by in vivo animal studies.
The relationship between ADAMTS-7, ADAMTS-12, and COMP was first established in our lab via a functional genetic study involving the yeast-hybrid system, which identified both ADAMTS-7 and -12 as binding partners of COMP.Citation37,Citation68 This result has also been confirmed by coimmunoprecipitation studies suggesting that ADAMTS-7 and -12 bind specifically to COMP in vivo. Furthermore, an analysis of ADAMTS-7 and -12 deletion mutants has revealed that four C-terminal thrombospondin type-1 repeats are conserved in both enzymes and are required for binding to the EGF-like domain of COMP and subsequent COMP cleavage.Citation37,Citation68 These findings are in accordance with the notion that C-terminal domains of metalloproteinases are important for determining substrate specificity.Citation69
ADAMTS-7 is expressed in bone, cartilage, synovium, tendon, and ligament, all of which contain COMP.Citation46,Citation51 Although northern blot analysis has found ADAMTS-12 expression only in the fetal lung, real-time PCR analysis has detected ADAMTS-12 in cartilage, synovium, tendon, skeletal muscle, and fat.Citation45,Citation68 ADAMTS-7 is also detectable in meniscus, skeletal muscle, and fat.Citation37 Through immunostaining analysis, we know that ADAMTS-7 and -12 co-localize with COMP both in the cytoplasm and on the surface of human chondrocytes.Citation37,Citation68 These studies also suggest that the interaction between ADAMTS-7 and -12 with the chondrocyte membrane may be mediated by COMP. Immunohistochemistry assays performed on embryonic murine limbs show significant overlap between COMP, ADAMTS-7, and ADMATS-12 expression patterns in vivo.Citation37,Citation68
Subsequent studies involving recombinant enzyme, conditioned medium, and purified protein have demonstrated that ADAMTS-7 and -12 can both digest COMP in vitro. An analysis of COMP fragments taken from in vitro assays suggests that ADAMTS-7 may cleave COMP at multiple sites.Citation57 Interestingly, COMP fragments taken from the cartilage explants of osteoarthritis patients are of similar size to those found with in vitro studies (110 kDa).Citation44 This highlights the possible role that the digestion of COMP by ADAMTS-7 and -12 may play in degenerative joint disease.
Since inflammatory cytokines TNF-α and IL-1β have been shown to induce the expression of ADAMTS-7 and -12, these cytokines would also be expected to induce COMP degradation by upregulating these enzymes. Indeed, cells treated with both cytokines give rise to abundant levels of 110 kDa COMP fragments.Citation44 Furthermore, these fragments are completely eliminated in the presence of anti-ADAMTS-7 and ADAMTS-12 antibodies, providing strong evidence to suggest that ADAMTS-7 and -12 serve as key links between inflammatory cytokines and disease progression.Citation37 These results have been further confirmed via small interfering RNA silencing of ADAMTS-7 and -12 in human chondrocytes.Citation44 The next logical step would be to validate these findings in vivo by generating ADAMTS-7 or -12-null mice in an arthritis model. Previous findings involving ADAMTS-5 and aggrecan degradation in osteoarthritis and inflammatory arthritis mouse models have demonstrated the efficacy of this approach.Citation24,Citation26
Interaction with GEP
A recent study has found that COMP associates with a growth factor named granulin-epithelin precursor (GEP), which is strongly upregulated in the synovium of both OA and RA patients.Citation70 GEP is also highly expressed in chondrocytes.Citation70 First purified in the early 1990s, GEP is an 80 kDa secreted glycoprotein, which contains seven and a half repeats of a cysteine-rich motif.Citation71–Citation74 Acting as an autocrine growth factor, GEP undergoes proteolytic processing with the liberation of ∼6 kDa repeating units known as granulins, which retain at least some of the biologic activity of GEP.Citation75 These peptides are active in cell growth assays and may be mediators of inflammation.Citation76,Citation77 GEP is also known by the names PC-cell derived growth factor, progranulin, proepithelin, and acrogranin.
The finding that COMP associates with both ADAMTS-7Citation37 and GEPCitation70 prompted us to determine whether GEP binds to ADAMTS-7 and whether ADAMTS-7, COMP, and GEP form a protein-protein interaction network. Data from our yeast-2-hybrid and coimmunoprecipitation assays show that ADAMTS-7 does indeed bind to GEP.Citation78 Further experiments have found that the four C-terminal TSP repeats of ADAMTS-7 are required for this interaction.
GEP has been shown to exhibit a potent antiprotease activity; it is an inhibitor of TNF-induced protease and GEP-derived granulin inhibits the protease thrombin.Citation79,Citation80 Unpublished data from our lab demonstrate that GEP specifically inhibits the ability of ADAMTS-7 and -12 to degrade COMP. Co-expression of GEP and ADAMTS-7 in a COMP-stable cell line results in a dose-dependent blockade of ADAMTS-7-mediated COMP degradation (Guo et al, unpublished data). Additionally, data from an in vitro digestion assay show that GEP prevents ADAMTS-12 from degrading COMP (Guo et al, unpublished data). Further data show that ADAMTS-7 can also be categorized as a GEP convertase, since it is involved in the proteolytic processing of GEP with the liberation of small fragments.Citation78
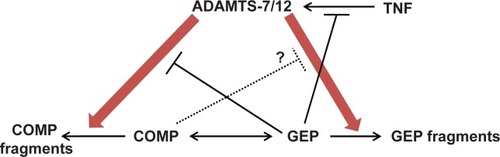
The available data suggest that GEP inhibits the action of ADAMTS-7 via two distinct mechanisms. First, GEP inhibits the induction of ADAMTS-7 by inflammatory cytokines such as TNF-α. Second, it disrupts the association between ADAMTS-7 and COMP via a direct protein-to-protein interaction.Citation29 Thus, ADAMTS-7 and -12 metalloproteinases, COMP extracellular matrix protein, GEP growth factor, and TNF inflammatory cytokine all act in concert to form an key interaction and interplay network in the pathogenesis of arthritis ().
Figure 2 An interaction network among ADAMTS-7/-12, COMP, and GEP. Arrows indicate a stimulatory effect. Crossed lines indicate an inhibitory effect. A dotted line indicates that the relationship is based on unpublished data.
Role in chondrogenesis
Chondrogenesis is a well orchestrated process mediated by interactions between cellular receptors, growth factors, and surrounding matrix proteins. These extracellular enzymes, which include the MMPs, lead to the activation of cell signaling pathways and gene expression in a temporal-spatial-specific manner. Both ADAMTS-7 and -12 are expressed in musculoskeletal tissues, including cartilage, and are thus poised to play key roles in chondrogenesis.Citation46,Citation68,Citation81 ADAMTS-7 and ADAMTS-12 are also highly expressed in the proliferative and pre-hypertrophic zones of growth.Citation78,Citation82 Initial real-time PCR data involving micromass cultures of a mouse embryonic mesenchymal stem cell line show that ADAMTS-7 is highly induced during the terminal stage of chondrogenic differentiation, which is accompanied by the increase of collagen-X expression.Citation78 However, immunohistochemistry performed on mouse embryos show that ADAMTS-7 is abundantly expressed in both the early and late stages of cartilage development, as well as in chondrocytes throughout the mature growth plate.Citation78 This suggests that ADAMTS-7 may play a significant role in chondrogenesis, and may influence various stages of cartilage development. ADAMTS-12 is prominently expressed in proliferating and prehypertrophic chondrocytes in the embryonic growth plate.Citation82
Given the expression pattern of ADAMTS-7 and -12 during various stages of chondrogenesis, their role in the process of chondrogenic differentiation has also been elucidated. Overexpression of either ADAMTS-7 or 12 in murine mesenchymal stem cells results in the potent inhibition of chondrocyte differentiation, specifically during the stage of chondrocyte hypertrophy.Citation78,Citation82 This effect can also be observed in fetal mouse metatarsal explants, where chondrocyte hypertrophy, mineralization, and bone length are significantly inhibited by ADAMTS-7-rich conditioned medium. Experiments with ADAMTS-12 in human mesenchymal stem cells have also led to similar results.Citation82 Further experimentation has established that the chondrogenic inhibitory effect of ADAMTS-7 and -12 depends specifically on four C-terminal thrombospondin motifs.Citation78,Citation82
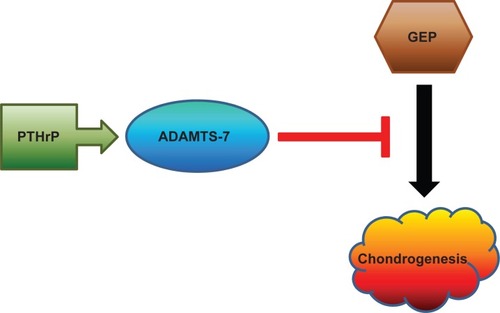
Once the inhibitory effect of ADAMTS-7 and -12 on chondrogenesis had been established, the focus was then shifted to finding the relevant upstream regulatory molecules of the signaling pathway. Both PTHrP and IHH are known regulators of chondrocyte differentiation. They function through a negative feedback loop: PTHrP prevents chondrocyte hypertrophy, thus reducing levels of IHH which in turn, stimulates PTHrP expression.Citation83–Citation85 IHH is expressed at the prehypertrophic-hypertrophic boundary. Since PTHrP, ADAMTS-7, and ADAMTS-12 all function as negative regulators of chondrogenesis, these molecules have the potential to function within the same regulatory pathway. Data from real-time PCR, immunoflourescent cell staining, and immunoblotting assays all show that this is indeed the case: ADAMTS-7 and -12 are highly induced downstream targets of PTHrP.Citation78 These results have also been reproduced in vivo in PTHrP knockout mice, which exhibit reduced ADAMTS-7 and -12 expression.Citation78 Further experimental data involving fetal mouse metatarsals has not only confirmed the role of ADAMTS-7 as a downstream mediator of PTHrP signaling, but has also confirmed that ADAMTS-7 is crucial for PTHrP-mediated inihibition of chondrocyte hypertrophy.Citation78 The inhibition of chondrocyte hypertrophy, mineralization, and bone length by PTHrP is largely abolished by the addition of ADAMTS-7 antibody. Similar results have also been obtained in a micromass cell model with ADAMTS-12.Citation78 In addition, ADAMTS-12 can also enhance the expression of PTHrP, suggesting that ADAMTS-12 and PTHrP form a positive feedback regulatory loop in the course of chondrogenesis.Citation82
As discussed above, GEP has been implicated in development, tissue regeneration, tumorigenesis, and inflammation. Our recent data demonstrates that GEP stimulates chondrocyte differentiation in mesenchymal stem cells in vitro and endochondral ossification ex vivo. GEP knockdown mice display dwarfism and striking skeletal defects. In addition, GEP activates chondrogenesis through Erk1/2 signaling, with JunB transcription factor being one of the key downstream molecules (Feng et al, unpublished data). Given that GEP enhances chondrocyte differentiation and bone growth, and that ADAMTS-7 associates with and converts GEP,Citation78 it may be suggested that ADAMTS-7 inhibits chondrogensis by inhibiting the chondroinductive function of GEP. Indeed, both in vitro chondrogenic differentiation assays and ex vivo metatarsal culture experiments indicate this is the case ().Citation78,Citation82
Figure 3 A proposed model for explaining ADAMTS-7-mediated inhibition of chondrogenesis. ADAMTS-7, a direct target of PTHrP, inhibits chondrogenesis by associating with GEP growth factor and inactivating its chondroinductive activity.
Interestingly, although ADAMTS-7 and ADAMTS-12 may negatively regulate chondrocyte differentiation, they can also exert a stimulatory effect on chondrocyte proliferation, a feature that they share with PTHrP.Citation78,Citation82 Given these two effects, it remains to be determined how ADAMTS-7 and ADAMTS-12 affect cartilage development and endochondral bone formation in vivo.
Role in atherosclerosis
Interestingly, ADAMTS-7 and its ability to interact with COMP, have also been implicated in the pathogenesis of vascular disease processes including atherosclerosis, restenosis after coronary angioplasty, and late failure of vein grafting. These processes all feature media-to-intima migration of vascular smooth muscle cells (VSMCs), which results in thickening of the vessel’s intimal layer.Citation86–Citation88 This migratory process requires the protease-mediated degradation and remodeling of ECM, which forms a barrier to VSMC migration.Citation89 MMPs such as MMP-2, MMP-9, and MT1-MMP have been implicated in this process.Citation90,Citation91 COMP, which is a component of vascular ECM and has been found in atherosclerotic lesions, is thought to be involved in the migration of VSMCs as well.Citation92
The interplay between ADAMTS-7 and COMP has been examined in a recent study involving a model of balloon-injured rat carotid arteries. ADAMTS-7, which is localized in VSMCs, shows significantly increased levels in response to neointimal vessel injury.Citation28 In addition, ADAMTS-7 in VSMCs is also induced by proinflammatory cytokines, such as TNF-α and IL-1β.Citation28 This result, similar to the one seen in chondrocytes, suggests that the role of ADAMTS-7 as a mediator of inflammation may be maintained across different tissue types. Of note, the anti-inflammatory signaling molecule TGF-β has been found to downregulate ADAMTS-7.Citation28 Additionally, ADAMTS-7 is induced by TNF-pathway transcription factors NF-κB and AP-1, further solidifying its role in this regulatory cascade.Citation28
The supportive role of ADAMTS-7 in VSMC migration is established by data showing that VSMCs infected with ADAMTS-7 adenovirus exhibit significantly greater migration activity.Citation28 This result is also seen in vivo, where injured rat vessel walls exposed to ADAMTS-7 adenovirus show significantly greater neointima formation. Knockdown of ADAMTS-7 via perivascularly applied ADAMTS-7 siRNA results in significantly reduced neointima area, thus creating the potential that future therapeutic approaches could be developed using this strategy.Citation28
Following vessel injury, levels of full-length COMP are decreased, while levels of COMP fragment are increased.Citation28 Previous in vitro and in vivo assays have already established that ADAMTS-7 binds and cleaves COMP in chondrocytes and similar data has shown that this is also true in damaged vessels. VSMCs infected with ADAMTS-7 adenovirus display increased levels of COMP fragment. Furthermore, infection with COMP adenovirus resulted in decreased ADAMTS-7-mediated VSMC migration and neointima formation, both in vitro and in vivo.Citation28 These data strongly suggest that the cleavage of COMP by ADAMTS-7 is a key event which is required for the migration of VSMCs and the pathogenesis of atherosclerotic disease.
Role in cancer
Many members of the ADAMTS family are dysregulated in a variety of tumors. For example, ADAMTS-6 and -18 have been linked to breast cancer and expression of ADAMTS-8 and -15 are predictors of survival;Citation4,Citation93 ADAMTS-19 may play a role in osteosarcoma;Citation94 ADAMTS-20 is dysregulated in breast and colon cancer;Citation95 and ADAMTS-4 and -5 are associated with glioblastoma.Citation96,Citation97 This is unsurprising since ADAMTSs belong to the family of MMPs, which are thought to play a key role in tumor growth, invasion, and metastasis.Citation98–Citation103 Data concerning the potential role of ADAMTS-7 and -12 in malignancy is just beginning to emerge. One study detected ADAMTS-7 in the urine of patients with prostate, brain, bladder, breast, and liver carcinomas.Citation3 Further analysis has found that ADAMTS-7 is present in the urine of breast, bladder, and prostate carcinoma patients, but not in control urine, suggesting that ADAMTS-7 may play a role in growth and invasion of these tumors.Citation3
Another study involving Madin–Darby canine kidney (MDCK) cells has found that overexpression of ADAMTS-12 confers protection from a tumorigenic phenotype that is generated in the presence of hematocyte growth factor.Citation6 Further analysis has found that this effect is mediated by inhibition of the Ras-MAPK signaling pathway, and that such inhibition involves the thrombospondin domains of ADAMTS-12.Citation6 The antitumor property of ADAMTS-12 can also be observed in vivo, as tumors induced by injecting immunodeficient SCID (severe combined immunodeficient) mice with A549 cells are markedly growth deficient when the injected cells are overexpressing ADAMTS-12, in comparison to control cells.Citation6 Overall, the data suggest that ADAMTS-12 exerts a significant antitumor effect; a finding that may pave the way for the development of future therapy.
Other roles
Genetic analysis has also provided evidence that ADAMTS-7 and ADAMTS-12 may be involved in other diseases. Gene mapping data has found several single-nucleotide polymorphisms (SNPs) in the ADAMTS-7 gene which are linked to the gene for keratoconus with cataract, suggesting an association with this disease.Citation104 However, none of these mutations are considered pathogenic, as they were also found in control samples.
Several variants of ADAMTS-12 are linked to bronchial hyper-responsiveness and asthma. In one study, the SNPs of ADAMTS-12 were found to be significantly different between cases and controls.Citation105
Summary and perspectives
Although ADAMTS-7 and -12 are both known to play a role in the pathogenesis of arthritis, recent evidence has emerged to implicate these two molecules in a host of other biological and disease processes. Indeed, the potential roles of ADAMTS-7 and -12 in the pathogenesis of today’s most common and costly diseases, including arthritis, atherosclerosis, and cancer, highlight the importance of future study (). Of particular note, elucidating the functional pathways involving these molecules in one disease may lead to open avenues of discovery in the understanding of other disorders. For example, the binding and cleavage of COMP, a feature that is crucial to the role of ADAMTS-7 and -12 in the progression of arthritis, may also help to explain the pathogenesis of atherosclerotic disease. Learning the full relationship between ADAMTS-7 and -12 and their binding partners, such as COMP and GEP, holds the promise of helping us to better understand the pathogenesis of, as well as develop effective therapies for some of today’s most common and costly diseases.
Table 2 Role of ADAMTS-7 and -12 in biological and disease processes
Acknowledgments
C J Liu is grateful to his gifted collaborators who made the explorations in his laboratory possible. We apologize to the scientists who made contributions to the field, but have not been cited due to space limitations. Studies in the authors’ laboratory were aided by National Institutes of Health research grants AR050620, AR053210, and AG029388 and a grant from the Arthritis National Research Foundation. The authors report no conflicts of interest in this work.
References
- KunoKMatsushimaKADAMTS-1 protein anchors at the extracellular matrix through the thrombospondin type I motifs and its spacing regionJ Biol Chem19982732213912139179593739
- TangBLADAMTS: a novel family of extracellular matrix proteasesInt J Biochem Cell Biol2001331334411167130
- RoyRLouisGLoughlinKRTumor-specific urinary matrix metalloproteinase fingerprinting: identification of high molecular weight urinary matrix metalloproteinase speciesClin Cancer Res200814206610661718927302
- PorterSClarkIMKevorkianLEdwardsDRThe ADAMTS metalloproteinasesBiochem J2005386Pt 1152715554875
- LevyGGNicholsWCLianECMutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpuraNature2001413685548849411586351
- LlamazaresMObayaAJMoncada-PazosAThe ADAMTS12 metalloproteinase exhibits anti-tumorigenic properties through modulation of the Ras-dependent ERK signalling pathwayJ Cell Sci2007120Pt 203544355217895370
- HovingaJAStudtJDAlberioLLammleBvon Willebrand factor-cleaving protease (ADAMTS-13) activity determination in the diagnosis of thrombotic microangiopathies: the Swiss experienceSemin Hematol2004411758214727262
- ShenkmanBThe role of ADAMT-13 in platelet adhesion in flow: methods for diagnosis of thrombotic thrombocytopenic purpuraPathophysiol Haemost Thromb2006351–29810216855353
- MoakeJLvon Willebrand factor, ADAMTS-13, and thrombotic thrombocytopenic purpuraSemin Hematol2004411414
- ColigeASieronALLiSWHuman Ehlers-Danlos syndrome type VII C and bovine dermatosparaxis are caused by mutations in the procollagen I N-proteinase geneAm J Hum Genet199965230831710417273
- ColigeANuytinckLHausserINovel types of mutation responsible for the dermatosparactic type of Ehlers-Danlos syndrome (Type VIIC) and common polymorphisms in the ADAMTS2 geneJ Invest Dermatol2004123465666315373769
- ShindoTKuriharaHKunoKADAMTS-1: a metalloproteinase-disintegrin essential for normal growth, fertility, and organ morphology and functionJ Clin Invest2000105101345135210811842
- BasileDPFredrichKChelladuraiBLeonardECParrishARRenal ischemia reperfusion inhibits VEGF expression and induces ADAMTS-1, a novel VEGF inhibitorAm J Physiol Renal Physiol20082944F928F93618272597
- NakamuraASakaiYOhataCKomurasakiTExpression and significance of a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-1 in an animal model of renal interstitial fibrosis induced by unilateral ureteral obstructionExp Toxicol Pathol20075911717583485
- Jonsson-RylanderACNilssonTFritsche-DanielsonRRole of ADAMTS-1 in atherosclerosis: remodeling of carotid artery, immunohistochemistry, and proteolysis of versicanArterioscler Thromb Vasc Biol200525118018515539621
- SabatineMSPloughmanLSimonsenKLAssociation between ADAMTS1 matrix metalloproteinase gene variation, coronary heart disease, and benefit of statin therapyArterioscler Thromb Vasc Biol200828356256718174457
- WagsaterDBjorkHZhuCADAMTS-4 and -8 are inflammatory regulated enzymes expressed in macrophage-rich areas of human atherosclerotic plaquesAtherosclerosis2008196251452217606262
- Moriguchi-GotoSYamashitaATamuraNADAMTS-13 attenuates thrombus formation on type I collagen surface and disrupted plaques under flow conditionsAtherosclerosis2009203240941618801485
- SandyJDVerscharenCAnalysis of aggrecan in human knee cartilage and synovial fluid indicates that aggrecanase (ADAMTS) activity is responsible for the catabolic turnover and loss of whole aggrecan whereas other protease activity is required for C-terminal processing in vivoBiochem J2001358Pt 361562611535123
- AbbaszadeILiuRQYangFCloning and characterization of ADAMTS11, an aggrecanase from the ADAMTS familyJ Biol Chem199927433234432345010438522
- LohmanderLSNeamePJSandyJDThe structure of aggrecan fragments in human synovial fluid. Evidence that aggrecanase mediates cartilage degradation in inflammatory joint disease, joint injury, and osteoarthritisArthritis Rheum1993369121412228216415
- MalfaitAMLiuRQIjiriKKomiyaSTortorellaMDInhibition of ADAM-TS4 and ADAM-TS5 prevents aggrecan degradation in osteoarthritic cartilageJ Biol Chem200227725222012220811956193
- Collins-RacieLAFlanneryCRZengWADAMTS-8 exhibits aggrecanase activity and is expressed in human articular cartilageMatrix Biol200423421923015296936
- GlassonSSAskewRSheppardBDeletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritisNature2005434703364464815800624
- KunoKOkadaYKawashimaHADAMTS-1 cleaves a cartilage proteoglycan, aggrecanFEBS Lett2000478324124510930576
- StantonHRogersonFMEastCJADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitroNature2005434703364865215800625
- TortorellaMDBurnTCPrattaMAPurification and cloning of aggrecanase-1: a member of the ADAMTS family of proteinsScience199928454201664166610356395
- WangLZhengJBaiXADAMTS-7 mediates vascular smooth muscle cell migration and neointima formation in balloon-injured rat arteriesCirc Res2009104568869819168437
- LiuCJThe role of ADAMTS-7 and ADAMTS-12 in the pathogenesis of arthritisNat Clin Pract Rheumatol200951384519098927
- BurragePSMixKSBrinckerhoffCEMatrix metalloproteinases: role in arthritisFront Biosci20061152954316146751
- ClarkIMParkerAEMetalloproteinases: their role in arthritis and potential as therapeutic targetsExpert Opin Ther Targets200371193412556200
- FosangAJRogersonFMEastCJStantonHADAMTS-5: the story so farEur Cell Mater200815112618247274
- JonesGCRileyGPADAMTS proteinases: a multi-domain, multifunctional family with roles in extracellular matrix turnover and arthritisArthritis Res Ther20057416016915987500
- MurphyGLeeMHWhat are the roles of metalloproteinases in cartilage and bone damage?Ann Rheum Dis200564Suppl 4iv44iv4716239386
- MurphyGNagaseHReappraising metalloproteinases in rheumatoid arthritis and osteoarthritis: destruction or repair?Nat Clin Pract Rheumatol20084312813518253109
- RowanADLitherlandGJHuiWMilnerJMMetalloproteases as potential therapeutic targets in arthritis treatmentExpert Opin Ther Targets200812111818076366
- LiuCJKongWIlalovKADAMTS-7: a metalloproteinase that directly binds to and degrades cartilage oligomeric matrix proteinFASEB J200620798899016585064
- KevorkianLYoungDADarrahCExpression profiling of metalloproteinases and their inhibitors in cartilageArthritis Rheum200450113114114730609
- BevittDJMohamedJCatterallJBExpression of ADAMTS metalloproteinases in the retinal pigment epithelium derived cell line ARPE-19: transcriptional regulation by TNFalphaBiochim Biophys Acta200316261–3839112697333
- CrossAKHaddockGStockCJADAMTS-1 and -4 are up-regulated following transient middle cerebral artery occlusion in the rat and their expression is modulated by TNF in cultured astrocytesBrain Res200610881193016630594
- VorosGMaquoiECollenDLijnenHRDifferential expression of plasminogen activator inhibitor-1, tumor necrosis factor-alpha, TNF-alpha converting enzyme and ADAMTS family members in murine fat territoriesBiochim Biophys Acta200316251364212527424
- TagoeCEMarjanovicNParkJYAnnexin-1 mediates TNF-alpha-stimulated matrix metalloproteinase secretion from rheumatoid arthritis synovial fibroblastsJ Immunol200818142813282018684973
- YunHJYooWHHanMKLeeYRKimJSLeeSIEpigallocatechin- 3-gallate suppresses TNF-alpha -induced production of MMP-1 and -3 in rheumatoid arthritis synovial fibroblastsRheumatol Int2008291232918496696
- LuanYKongLHowellDRInhibition of ADAMTS-7 and ADAMTS-12 degradation of cartilage oligomeric matrix protein by alpha-2-macroglobulinOsteoarthritis Cartilage200816111413142018485748
- CalSArguellesJMFernandezPLLopez-OtinCIdentification, characterization, and intracellular processing of ADAM-TS12, a novel human disintegrin with a complex structural organization involving multiple thrombospondin-1 repeatsJ Biol Chem200127621179321794011279086
- HedbomEAntonssonPHjerpeACartilage matrix proteins. An acidic oligomeric protein (COMP) detected only in cartilageJ Biol Chem19922679613261361556121
- BriggsMDHoffmanSMKingLMPseudoachondroplasia and multiple epiphyseal dysplasia due to mutations in the cartilage oligomeric matrix protein geneNat Genet19951033303367670472
- BriggsMDMortierGRColeWGDiverse mutations in the gene for cartilage oligomeric matrix protein in the pseudoachondroplasia-multiple epiphyseal dysplasia disease spectrumAm J Hum Genet19986223113199463320
- CohnDHBriggsMDKingLMMutations in the cartilage oligomeric matrix protein (COMP) gene in pseudoachondroplasia and multiple epiphyseal dysplasiaAnn N Y Acad Sci19967851881948702126
- HechtJTNelsonLDCrowderEMutations in exon 17B of cartilage oligomeric matrix protein (COMP) cause pseudoachondroplasiaNat Genet19951033253297670471
- DiCesarePHauserNLehmanDPasumartiSPaulssonMCartilage oligomeric matrix protein (COMP) is an abundant component of tendonFEBS Lett199435422372407957930
- ChenFHThomasAOHechtJTGoldringMBLawlerJCartilage oligomeric matrix protein/thrombospondin 5 supports chondrocyte attachment through interaction with integrinsJ Biol Chem200528038326553266116051604
- ChanILiuLHamadaTSethuramanGMcGrathJAThe molecular basis of lipoid proteinosis: mutations in extracellular matrix protein 1Exp Dermatol2007161188189017927570
- Di CesarePEChenFSMoergelinMMatrix-matrix interaction of cartilage oligomeric matrix protein and fibronectinMatrix Biol200221546147012225811
- ManssonBCareyDAliniMCartilage and bone metabolism in rheumatoid arthritis. Differences between rapid and slow progression of disease identified by serum markers of cartilage metabolismJ Clin Invest199595107110777533784
- RosenbergKOlssonHMorgelinMHeinegardDCartilage oligomeric matrix protein shows high affinity zinc-dependent interaction with triple helical collagenJ Biol Chem199827320397204039685393
- NeidhartMHauserNPaulssonMDiCesarePEMichelBAHauselmannHJSmall fragments of cartilage oligomeric matrix protein in synovial fluid and serum as markers for cartilage degradationBr J Rheumatol19973611115111609402858
- SaxneTHeinegardDCartilage oligomeric matrix protein: a novel marker of cartilage turnover detectable in synovial fluid and bloodBr J Rheumatol19923195835911381980
- ManssonBCareyDAliniMCartilage and bone metabolism in rheumatoid arthritis. Differences between rapid and slow progression of disease identified by serum markers of cartilage metabolismJ Clin Invest1995953107110777533784
- KrausVBHuebnerJLFinkCUrea as a passive transport marker for arthritis biomarker studiesArthritis Rheum200246242042711840444
- MisumiKVilimVHatazoeTSerum level of cartilage oligomeric matrix protein (COMP) in equine osteoarthritisEquine Vet J200234660260812358001
- NeidhartMElevated serum prolactin or elevated prolactin/cortisol ratio are associated with autoimmune processes in systemic lupus erythematosus and other connective tissue diseasesJ Rheumatol19962334764818832986
- LohmanderLSIonescuMJugessurHPooleARChanges in joint cartilage aggrecan after knee injury and in osteoarthritisArthritis Rheum199942353454410088777
- PeterssonIFBoegardTSvenssonBHeinegardDSaxneTChanges in cartilage and bone metabolism identified by serum markers in early osteoarthritis of the knee jointBr J Rheumatol199837146509487250
- GanuVGoldbergRPeppardJInhibition of interleukin-1alpha-induced cartilage oligomeric matrix protein degradation in bovine articular cartilage by matrix metalloproteinase inhibitors: potential role for matrix metalloproteinases in the generation of cartilage oligomeric matrix protein fragments in arthritic synovial fluidArthritis Rheum19984112214321519870871
- StrackeJOFosangAJLastKMatrix metalloproteinases 19 and 20 cleave aggrecan and cartilage oligomeric matrix protein (COMP)FEBS Lett20004781–2525610922468
- DickinsonSCVankemmelbekeMNButtleDJRosenbergKHeinegardDHollanderAPCleavage of cartilage oligomeric matrix protein (thrombospondin-5) by matrix metalloproteinases and a disintegrin and metalloproteinase with thrombospondin motifsMatrix Biol200322326727812853037
- LiuCJKongWXuKADAMTS-12 associates with and degrades cartilage oligomeric matrix proteinJ Biol Chem200628123158001580816611630
- Martel-PelletierJWelschDJPelletierJPMetalloproteases and inhibitors in arthritic diseasesBest Pract Res Clin Rheumatol200115580582911812023
- XuKZhangYIlalovKCartilage oligomeric matrix protein associates with granulin-epithelin precursor (GEP) and potentiates GEP-stimulated chondrocyte proliferationJ Biol Chem200728215113471135517307734
- AnakweOOGertonGLAcrosome biogenesis begins during meiosis: evidence from the synthesis and distribution of an acrosomal glycoprotein, acrogranin, during guinea pig spermatogenesisBiol Reprod19904223173281692485
- OngCHBatemanAProgranulin (granulin-epithelin precursor, PC-cell derived growth factor, acrogranin) in proliferation and tumorigenesisHistol Histopathol20031841275128812973694
- WrightWESassoonDALinVKMyogenin, a factor regulating myogenesis, has a domain homologous to MyoDCell19895646076172537150
- ZhouJGaoGCrabbJWSerreroGPurification of an autocrine growth factor homologous with mouse epithelin precursor from a highly tumorigenic cell lineJ Biol Chem19932681510863108698496151
- DavidsonBAlejandroEFlorenesVAGranulin-epithelin precursor is a novel prognostic marker in epithelial ovarian carcinomaCancer2004100102139214715139056
- LuRSerreroGInhibition of PC cell-derived growth factor (PCDGF, epithelin/granulin precursor) expression by antisense PCDGF cDNA transfection inhibits tumorigenicity of the human breast carcinoma cell line MDA-MB-468Proc Natl Acad Sci U S A20009783993399810760271
- Zanocco-MaraniTBatemanARomanoGValentinisBHeZHBasergaRBiological activities and signaling pathways of the granulin/epithelin precursorCancer Res199959205331534010537317
- BaiXHWangDWKongLADAMTS-7, a direct target of PTHrP, adversely regulates endochondral bone growth by associating with and inactivating GEP growth factorMol Cell Biol200929154201421919487464
- ZhuJNathanCJinWConversion of proepithelin to epithelins: roles of SLPI and elastase in host defense and wound repairCell2002111686787812526812
- HongSJKangKWPurification of granulin-like polypeptide from the blood-sucking leech, Hirudo nipponiaProtein Expr Purif199916234034610419830
- DiCesarePEMorgelinMMannKPaulssonMCartilage oligomeric matrix protein and thrombospondin 1. Purification from articular cartilage, electron microscopic structure, and chondrocyte bindingEur J Biochem199422339279378055970
- BaiXHWangDWLuanYYuXPLiuCJRegulation of chondrocyte differentiation by ADAMTS-12 metalloproteinase depends on its enzymatic activityCell Mol Life Sci200966466768019151918
- KronenbergHMPTHrP and skeletal developmentAnn N Y Acad Sci2006106811316831900
- BurdanFSzumiloJKorobowiczAMorphology and physiology of the epiphyseal growth plateFolia Histochem Cytobiol200947151619419931
- KronenbergHMChungUThe parathyroid hormone-related protein and Indian hedgehog feedback loop in the growth plateNovartis Found Symp2001232144152 discussion 147–15211277077
- NewbyACZaltsmanABMolecular mechanisms in intimal hyperplasiaJ Pathol2000190330030910685064
- DaviesMGHagenPOPathobiology of intimal hyperplasiaBr J Surg1994819125412697953384
- RudijantoAThe role of vascular smooth muscle cells on the pathogenesis of atherosclerosisActa Med Indones2007392869317933075
- HuJVan den SteenPESangQXOpdenakkerGMatrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseasesNat Rev Drug Discov20076648049817541420
- SluijterJPde KleijnDPPasterkampGVascular remodeling and protease inhibition – bench to bedsideCardiovasc Res200669359560316387286
- NewbyACMatrix metalloproteinases regulate migration, proliferation, and death of vascular smooth muscle cells by degrading matrix and non-matrix substratesCardiovasc Res200669361462416266693
- RiessenRFenchelMChenHAxelDIKarschKRLawlerJCartilage oligomeric matrix protein (thrombospondin-5) is expressed by human vascular smooth muscle cellsArterioscler Thromb Vasc Biol2001211475411145932
- PorterSSpanPNSweepFCADAMTS8 and ADAMTS15 expression predicts survival in human breast carcinomaInt J Cancer200611851241124716152618
- CalSObayaAJLlamazaresMGarabayaCQuesadaVLopez-OtinCCloning, expression analysis, and structural characterization of seven novel human ADAMTSs, a family of metalloproteinases with disintegrin and thrombospondin-1 domainsGene20022831–2496211867212
- LlamazaresMCalSQuesadaVLopez-OtinCIdentification and characterization of ADAMTS-20 defines a novel subfamily of metalloproteinases-disintegrins with multiple thrombospondin-1 repeats and a unique GON domainJ Biol Chem200327815133821338912562771
- Held-FeindtJParedesEBBlomerUMatrix-degrading proteases ADAMTS4 and ADAMTS5 (disintegrins and metalloproteinases with thrombospondin motifs 4 and 5) are expressed in human glioblastomasInt J Cancer20061181556116003758
- NakadaMMiyamoriHKitaDHuman glioblastomas overexpress ADAMTS-5 that degrades brevicanActa Neuropathol2005110323924616133547
- DeryuginaEIQuigleyJPMatrix metalloproteinases and tumor metastasisCancer Metastasis Rev200625193416680569
- FingletonBMatrix metalloproteinases: roles in cancer and metastasisFront Biosci20061147949116146745
- JodeleSBlavierLYoonJMDeClerckYAModifying the soil to affect the seed: role of stromal-derived matrix metalloproteinases in cancer progressionCancer Metastasis Rev2006251354316680570
- NoelAJostMMaquoiEMatrix metalloproteinases at cancer tumor-host interfaceSemin Cell Dev Biol2008191526017625931
- OrlichenkoLSRadiskyDCMatrix metalloproteinases stimulate epithelial-mesenchymal transition during tumor developmentClin Exp Metastasis200825659360018286378
- RydlovaMHolubecLJrLudvikovaMJrBiological activity and clinical implications of the matrix metalloproteinasesAnticancer Res2008282B1389139718505085
- DashDPSilvestriGHughesAEFine mapping of the keratoconus with cataract locus on chromosome 15q and candidate gene analysisMol Vis20061249950516735990
- KurzTHoffjanSHayesMGFine mapping and positional candidate studies on chromosome 5p13 identify multiple asthma susceptibility lociJ Allergy Clin Immunol2006118239640216890764
- VazquezFHastingsGOrtegaMAMETH-1, a human ortholog of ADAMTS-1, and METH-2 are members of a new family of proteins with angio-inhibitory activityJ Biol Chem199927433233492335710438512
- MittazLRicardoSMartinezGNeonatal calyceal dilation and renal fibrosis resulting from loss of Adamts-1 in mouse kidney is due to a developmental dysgenesisNephrol Dial Transplant200520241942315615810
- LindTMcKieNWendelMRaceySNBirchMAThe hyalectan degrading ADAMTS-1 enzyme is expressed by osteoblasts and up-regulated at regions of new bone formationBone200536340841715777654
- RehnAPBirchMAKarlstromEWendelMLindTADAMTS-1 increases the three-dimensional growth of osteoblasts through type I collagen processingBone200741223123817560840
- BrownHMDunningKRRobkerRLPritchardMRussellDLRequirement for ADAMTS-1 in extracellular matrix remodeling during ovarian folliculogenesis and lymphangiogenesisDev Biol2006300269970917097630
- ShozuMMinamiNYokoyamaHADAMTS-1 is involved in normal follicular development, ovulatory process and organization of the medullary vascular network in the ovaryJ Mol Endocrinol200535234335516216914
- MittazLRussellDLWilsonTAdamts-1 is essential for the development and function of the urogenital systemBiol Reprod20047041096110514668204
- RocksNPaulissenGQuesada-CalvoFADAMTS-1 metalloproteinase promotes tumor development through the induction of a stromal reaction in vivoCancer Res200868229541955019010931
- MajumdarMKAskewRSchellingSDouble-knockout of ADAMTS-4 and ADAMTS-5 in mice results in physiologically normal animals and prevents the progression of osteoarthritisArthritis Rheum200756113670367417968948
- CheungKSHashimotoKYamadaNRoachHIExpression of ADAMTS-4 by chondrocytes in the surface zone of human osteoarthritic cartilage is regulated by epigenetic DNA de-methylationRheumatol Int200929552553418941754
- SongRHTortorellaMDMalfaitAMAggrecan degradation in human articular cartilage explants is mediated by both ADAMTS-4 and ADAMTS-5Arthritis Rheum200756257558517265492
- GlassonSSAskewRSheppardBCharacterization of and osteoarthritis susceptibility in ADAMTS-4-knockout miceArthritis Rheum20045082547255815334469
- CorpsANJonesGCHarrallRLCurryVAHazlemanBLRileyGPThe regulation of aggrecanase ADAMTS-4 expression in human Achilles tendon and tendon-derived cellsMatrix Biol200827539340118387286
- TsuzakiMGuytonGGarrettWIL-1 beta induces COX2, MMP-1, -3 and -13, ADAMTS-4, IL-1 beta and IL-6 in human tendon cellsJ Orthop Res200321225626412568957
- DunnJRReedJEdu PlessisDGExpression of ADAMTS-8, a secreted protease with antiangiogenic properties, is downregulated in brain tumoursBr J Cancer20069481186119316570050
- DemircanKHirohataSNishidaKADAMTS-9 is synergistically induced by interleukin-1beta and tumor necrosis factor alpha in OUMS-27 chondrosarcoma cells and in human chondrocytesArthritis Rheum20055251451146015880812
- LiZNardiMALiYSC-terminal ADAMTS-18 fragment induces oxidative platelet fragmentation, dissolves platelet aggregates, and protects against carotid artery occlusion and cerebral strokeBlood2009113246051606019218546