Abstract
Background:
We analyzed the effects of a docosahexaenoic acid (DHA) supplementation in patients affected with late onset Stargardt disease (STGD).
Methods:
DHA (840 mg/day) was given to 20 STGD patients for six months. A complete ophthalmologic examination, including best-corrected visual acuity (BCVA) and multifocal electroretinogram (mfERG), was performed at inclusion day 0 (D0) and at month 6 (M6).
Results:
Overall, no statistical differences have been observed at M6 vs D0 as regards BCVA and mfERG (P > 0.05). Mild Improvement of BCVA and improvement of mfERG was noted in seven/40 eyes of four/20 patients. In the first patient, the peak of the a wave increased from 66 nV/deg2 to 75.4 nV/deg2 in the right eye (RE) and 24.5 nV/deg2 to 49.1 nV/deg2 in the left eye (LE). The peak of the b wave improved from 122 nV/deg2 to 157 nV/deg2 in the RE, and 102 nV/deg2 to 149 nV/deg2 in the LE. In the second patient peaks of the a and b waves respectively increased from 11.8 nV/deg2 to 72.1 nV/deg2 and 53 nV/deg2 to 185 nV/deg2 in the RE. In the third patient the peak of the a wave increased from 37 nV/deg2 to 43 nV/deg2 in the RE, and from 31 nV/deg2 to 45 nV/deg2 in the LE; the peak of the b wave improved from 70 nV/deg2 to 89 nV/deg2 in the RE, and from 101 nV/deg2 to 108 nV/deg2 in the LE. In the fourth patient, the peak of the a wave increased from 39 nV/deg2 to 42 nV/deg2 in the RE, and from 40 nV/deg2 to 43 nV/deg2 in the LE; the peak of the b wave improved from 86 nV/deg2 to 94 nV/deg2 in the RE, and from 87 nV/deg2 to 107 nV/deg2 in the LE.
Conclusion:
DHA seems to influence some functional parameters in patients affected with STGD. However, no short-term benefit should be expected from DHA supplementation.
Background
Stargardt disease (STGD), described by Karl Stargardt in 1909,Citation1–Citation3 and fundus flavimaculatus (FFM), a STGD-like phenotype described by Franceschetti in 1965Citation4 are variants of the same hereditary disease that affects the retinal pigment epithelium (RPE) and photoreceptor layer, both linked with the ABCA4 gene.Citation5–Citation7 When characterized by a juvenile onset (first two decades), a rapidly progressive course, and a poor visual outcome, the disease is usually termed STGD. The term FFM is favored when the disease begins at the end of the second decade or within the third decade, and has a slowly progressive course.Citation8–Citation9 The general course of STGD and FFM is a progressive central atrophy, resulting in a central vision loss (≤20/200). However, to date, the rapidity of visual loss remains unpredictable.Citation10–Citation11
The retina has a high concentration of omega-3, and particularly docosahexaenoic acid (DHA). The high concentration of DHA in the photoreceptors cells (>50% of the lipids of the external membrane) suggests its major role in the maintenance of the structure of these cells. Moreover, since photoreceptor outer segments require a constant supply of these omega-3 fatty acids owing to their continuous renewal, diets rich in DHA may improve retinal function and slow-down the progressive photoreceptor degeneration. Many studies demonstrated that DHA has a protective role in the retina,Citation12–Citation17 increased mitochondrial activity, increased RPE acid lipase activity, antioxidative, antiproliferative, and antiapoptotic effects.Citation18
For these reasons, in this study, we decided to analyze the effects of DHA supplementation in patients affected with late onset STGD, an inherited macular dystrophy characterized by progressive photoreceptor degeneration. The current study (NAT-3 study) is part of a clinical trial on nutritional prevention treatment, that we have been performing at our Department to study the preventive effects of DHA on progression of age-related macular degeneration (Nutritional AMD Treatment, NAT-1 and NAT-2 studies) and other macular diseases.
Methods
This study was designed as a prospective interventional pilot study. Patients diagnosed with late onset STGD (reported onset at >18 years old) were prospectively included. Informed consent was obtained according to a Paris XII University Institutional Review Board – approved protocol. Criteria for inclusion were: 1) age >18 years old; 2) evidence of hypo-autofluorescence from areas of macular atrophy, associated or not with retinal flecks; 3) presence of hyper-autofluorescent retinal flecks, associated or not with areas of macular atrophy, 4) diagnosis of dark choroid on fluorescein angiography (FA). This study was performed in agreement with the Declaration of Helsinki and French legislation, and was approved by our local ethics committee. In this trial DHA (840 mg/day) was given to all patients for six months. A complete ophthalmologic examination including best-corrected visual acuity (BCVA), measured at 4 m with standard Early Treatment Diabetic Retinopathy Study (ETDRS) charts, fundus examination, autofluorescent frames (AF), FA, optical coherence tomography (OCT 3; Humphrey Zeiss, San Leandro, CA), and multifocal electroretinogram (mfERG), was performed at inclusion day 0 (D0) and at month 6 (M6). Molecular biology analysis of ELOVL4 gene was also performed in order to establish predicting factors of efficacy of DHA treatment. In addition, a complete profile of fatty acids in serum (S) and in red blood cell membranes (RBCM) performed by gas chromatography was recorded at D0 and M6.
Statistical calculations were performed using Epinfo 3.3 software package (CDC, Atlanta, GA). The Mann–Whitney/Wilcoxon two-sample test was used to compare main ophthalmologic findings, and fatty acids in S and in RBCM, at D0 and M6. The chosen level of statistical significance was P < 0.05.
Results
Twenty unrelated patients (nine women, eleven men) with clinically definite late onset STGD were included (). Mean age of patients was 45 ± 15 years old (range 24–72). Neither side effects nor dropouts were observed in all patients. Overall, for the included patients, no statistical differences have been observed at M6 vs D0 as regards BCVA and mfERG (P > 0.05). Four out of the 20 patients showed a mild improvement of visual acuity (three eyes) and mfERG recordings (seven eyes). In the first patient (case three, a 66 years old man), BCVA improved from 20/25 to 20/20 in the right eye (RE) and from 20/32 to 20/20 in the left eye (LE); the peak of the a wave increased from 66 nV/deg2 to 75.4 nV/deg2 in the RE, and from 24.5 nV/deg2 to 49.1 nV/deg2 in the LE (). The peak of the b wave improved from 122 nV/deg2 to 157 nV/deg2 in the RE, and from 102 nV/deg2 to 149 nV/deg2 in the LE (). In the second patient (case five, a 72 years old woman), BCVA improved from 20/25 to 20/20 in the RE; the peak of the a wave increased from 11.8 nV/deg2 to 72.1 nV/deg2 in the RE, and the peak of the b wave improved from 53 nV/deg2 to 185 nV/deg2 in the RE (). The LE was affected with choroidal neovascularization (CNV), and the patient was treated twice by photodynamic therapy. No improvement was observed on BCVA and the foveal function. Nevertheless, it is notable that the four external rings slightly improved (). In the third patient (case seven, a 24 years old woman), BCVA was 20/20 in both eyes at baseline, and remained unchanged throughout the six month period; the peak of the a wave increased from 37 nV/deg2 to 43 nV/deg2 in the RE, and from 31 nV/deg2 to 45 nV/deg2 in the LE (). The peak of the b wave improved from 70 nV/deg2 to 89 nV/deg2 in the RE, and from 101 nV/deg2 to 108 nV/deg2 in the LE (). In the fourth patient (case 11, a 25 years old man), BCVA was 20/32 in both eyes at baseline, and remained unchanged throughout the six-month period; the peak of the a wave increased from 39 nV/deg2 to 42 nV/deg2 in the RE, and from 40 nV/deg2 to 43 nV/deg2 in the LE (). The peak of the b wave improved from 86 nV/deg2 to 94 nV/deg2 in the RE, and from 87 nV/deg2 to 107 nV/deg2 in the LE ().
Figure 1 mfERG showing the peaks of a and b waves for case three. In the RE, the peak of the a wave increased from 66 nV/deg2 (top left panel) to 75.4 nV/deg2 (top right panel), and, in the LE, from 24.5 nV/deg2 (bottom left panel) to 49.1 nV/deg2 (bottom right panel), after six months. The peak of the b wave improved from 122 nV/deg2 (top left panel) to 157 nV/deg2 (top right panel) RE, and from 102 nV/deg2 (bottom left panel) to 149 nV/deg2 (bottom right panel) LE, after six months.
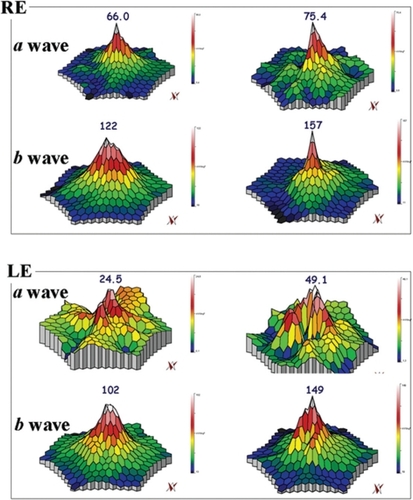
Figure 2 mfERG showing the peaks of a and b waves for case five. In the RE the peaks of the a and b waves respectively increased from 11.8 nV/deg2 (top right panel) to 72.1 nV/deg2 (top left panel) and from 53 nV/deg2 (top right panel) to 185 nV/deg2 (top left panel), after six months. The LE was affected with choroidal neovascularization: it is notable that the four external ring were improved when comparing before (bottom right panel) and after treatment (bottom left panel).
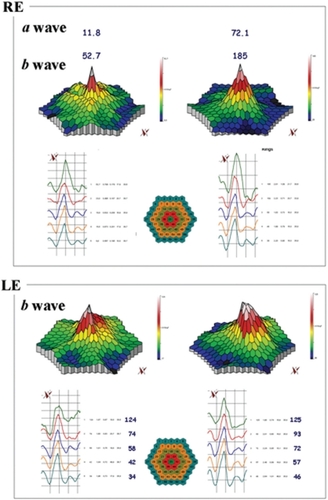
Figure 3 Fundus autofluorescent frames (upper left and right panels) and mfERG for case seven. The peak of the a wave increased from 37 nV/deg2 to 43 nV/deg2 in the RE, and from 31 nV/deg2 to 45 nV/deg2 in the LE. The peak of the b wave improved from 70 nV/deg2 (middle left panel) to 89 nV/deg2 (bottom left panel) in the RE, and from 101 nV/deg2 (middle right panel) to 108 nV/deg2 (bottom right panel) in the LE.
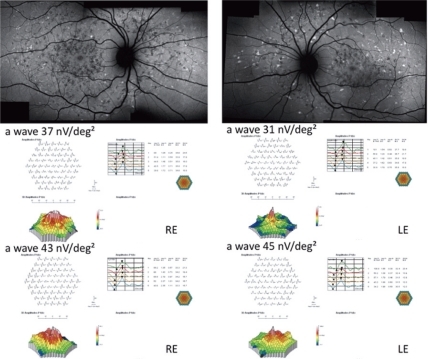
Figure 4 Fundus autofluorescent frames (upper left and right panels) and mfERG for case 11. The peak of the a wave increased from 39 nV/deg2 to 42 nV/deg2 in the RE, and from 40 nV/deg2 to 43 nV/deg2 in the LE. The peak of the b wave improved from 86 nV/deg2 (middle left panel) to 94 nV/deg2 (middle left panel) in the RE, and from 87 nV/deg2 (middle right panel) to 107 nV/deg2 (bottom right panel) in the LE.
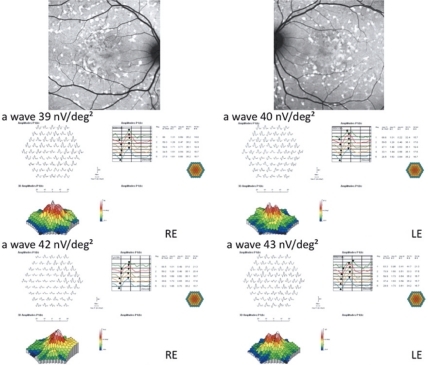
Table 1 Patients demographics and BCVA changes from baseline to the six month FU
During this short period of time, no progression was observed in the size of the central atrophy. Moreover, FA revealed no progression to CNV in any of the 20 patients within the six month period. Molecular biology analysis of ELOVL4 gene which was performed to establish predicting factors of efficacy of DHA treatment, revealed the absence of ELOVL4 gene mutations in all patients. Five patients were explored for their plasma lipids before and after DHA supplementation. These patients showed significant changes, with a 106% increase in S DHA (1.5% at D0 vs up to 2.7% at M6, P = 0.013), and: a 37% increase in RBCM DHA (4.0% at D0 up to 5.2 at M6, P = 0.035). Other fatty acids were unchanged in S or RBCM.
There was no evidence of systemic illness or toxic effects attributable to DHA during the course of this study.
Discussion
MacDonald et alCitation19 recently demonstrated the efficacy of DHA in a 15 years old girl affected with autosomal dominant STGD macular dystrophy associated with the ELOVL4 gene mutation. ELOVL4 is homologous to a fatty acid elongase presumably involved in the biosynthesis of DHA. An animal study previously demonstrated that the dietary level of n-3 fatty acids not only affects the level of DHA, but also the levels of very-long-chain fatty acids in rod outer segments (ROS) membranes.Citation20 This young patient was given a dietary supplementation of DHA (20 mg/kg body weight/day) on two occasions, and a clear improvement was observed on visual function both subjectively (visual acuity, VF-14) and objectively (mfERG amplitudes). No conclusion can be drawn from one single case but this case report provides some hope for patients affected with STGD macular dystrophy associated with ELOVL4 mutations and a larger and longer study is warranted.
Hubbard et alCitation21 investigated the influence of dietary factors on the phenotypic severity in a family with autosomal dominant STGD associated with ELOVL4 mutation. Red blood cell membrane and adipose tissue lipids were analyzed as an indication of short-term and long-term dietary fatty acid intake. The authors found a significant inverse relationship between phenotypic severity and levels of eicosapentaenoic acid and docosahexaenoic acid. These results indicate that the phenotypic diversity may be related to differences in dietary fat intake as reflected by adipose and red blood cell lipids.
In our series, molecular biology analysis of ELOVL4 gene, which was performed in order to establish predicting factors of efficacy of DHA treatment, revealed the absence of ELOVL4 gene mutations in all the 20 patients. Despite this absence of ELOVL4 gene mutations (excluding that the direct disease-causing mechanism might be the ELOVL4 gene involvement in fatty acid elongation in the retina), four out of 20 patients had an improvement of visual acuity and improvement of mfERG.
DHA is known to have specific retinal effects such as increased mitochondrial activity, increased RPE acid lipase activity, antioxidative, antiproliferative, and antiapoptotic effects.Citation18 DHA has also shown to inhibit eicosanoid synthesis from arachidonic acid and cytokine production form macrophages.Citation22 This antiinflammatory effect could play a beneficial role in the retina as the pathway of inflammation is suggested to be involved in the occurrence of several retinal diseases. Moreover, since photoreceptor outer segments require a constant supply of these omega-3 fatty acids due to their continuous renewal (DHA represents the main component of the lipids of the external membrane of the photoreceptors cells, suggesting a major role in the maintenance of the structure of these cells), diets rich in DHA may improve retinal function and slow-down the progressive photoreceptor degeneration.
Of note, in our series, patients who had regular DHA intake during six months, and that were explored for their plasma lipids before and after DHA supplementation, displayed a strong and significant increase in serum and erythrocyte membrane DHA. Therefore, it may be that, DHA supplementation, mainly due to antiinflammatory, antioxidative and antiapoptotic properties,Citation18 could have a protective effect in some cases of STGD whatever the genotype.
Mild improvement of BCVA and/or improvement of mfERG were noted in four/20 cases. Moreover, during this short period of time, no progression was observed either in the size of the central atrophy, or to CNV, in any of the 20 patients. There was no evidence of systemic illness or a toxic effect that was attributable to DHA during the course of this study.
Our study is flawed by several shortcomings: the sample size was small, with no control group, and relatively short follow-up. No major improvement was observed in this series, given that only a mild effect was recorded, even on the four improved cases, we could not rule out normal inter-examination variations of mfERG. Therefore, larger series and analysis of both ABCA4 and ELOVL4 genes is needed.
Conclusion
DHA seems to influence some functional parameters in patients affected with STGD however, no short-term benefit should be expected from DHA supplementation.
Acknowledgements
Giuseppe Querques and Eric H Souied were involved in the conception and design of the study; Giuseppe Querques, Pascale Benlian, Bernard Chanu, Nicolas Leveziel and Eric H Souied were involved in the analysis of the data; Giuseppe Querques, Gabriel Coscas, Gisele Soubrane and Eric H Souied, were involved in the preparation of the manuscript. The authors report no conflicts of interest in this work.
References
- StargardtKUeber familiare progressive degeneration in der makulagegend des Auges. Albrecht V GraefesArch Ophthalmol190971534550
- CibisGWMoreyMKarrisDJDominantly inherited macular dystrophy with flecks (Stargardt)Arch Ophthalmol1980981017857425904
- MerlinSLandauJAbnormal findings in relatives of patients with juvenile hereditary macular degeneration (Stargardt’s disease)Ophthalmologica1970161115432144
- FranceschettiAFrancoisJFundus flavimaculatusArch Ophthalmol196525505530
- AllikmetsRSinghNSunHA photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophyNat Genet19971532362469054934
- CremersFPvan de PolDJvan DrielMAutosomal recessive retinitis pigmentosa and cone-rod dystrophy caused by splice site mutations in the Stargardt’s disease gene ABCRHum Mol Genet1998733553629466990
- RozetJMGerberSSouiedESpectrum of ABCR gene mutations in autosomal recessive macular dystrophiesEur J Hum Genet1998632912959781034
- AabergTMStargardt’s disease and fundus flavimaculatus: evaluation of morphologic progression and intrafamilial co-existenceTrans Am Ophthalmol Soc1980844534873590477
- LoisNHolderGEBunceCFitzkeFWBirdACPhenotypic subtypes of Stargardt macular dystrophy-fundus flavimaculatusArch Ophthalmol2001119335936911231769
- ErgunEHermannBWirtitschMAssessment of central visual function in Stargardts’ disease/fundus flavimaculatus with ultrahigh-resolution optical coherence tomographyInvest Ophthalmol Vis Sci200546131031615623790
- HargitaiJZernantJSomfaiGMCorrelation of clinical and genetic findings in Hungarian patients with Stargardt diseaseInvest Ophthalmol Vis Sci2005464402440816303926
- KaganVEShvedovaAANovikovKNParticipation of phospholipases in the “repair” of photoreceptor membranes subjected to peroxidationBiofizika197823227984306262
- TermanABrunkUTLipofuscin: mechanisms of formation and increase with ageAPMIS19981062265769531959
- WolmanMOxidation of lipids and membranes I: in vitro formation of peroxidative lipid polymersJ Supramol Struct197538091152469
- TappelALLipid peroxidation damage to cell componentsFed Proc1973328187044352451
- FeeneyLBermanEROxygen toxicity: membrane damage by free radicalsInvest Ophthalmol1976151078992824221
- KeysSABoleyEZimmermanWFA model membrane system to investigate antioxidants in bovine rod outer segmentsExp Eye Res1997643313219196382
- RotsteinNPPolitiLEGermanOLGirottiRProtective effect of docosahexaenoic acid on oxidative stress-induced apoptosis of retina photoreceptorsInvest Ophthalmol Vis Sci20034452252225912714668
- MacDonaldIMHebertMYauRJEffect of docosahexaenoic acid supplementation on retinal function in a patient with autosomal dominant Stargardt-like retinal dystrophyBr J Ophthalmol200488230530614736799
- XiZPWangJYEffect of dietary n-3 fatty acids on the composition of long- and very-long-chain polyenoic fatty acid in rat retinaJ Nutr Sci Vitaminol200349321021312953800
- HubbardAFAskewEWSinghNLeppertMBernsteinPSAssociation of adipose and red blood cell lipids with severity of dominant Stargardt macular dystrophy (STGD3) secondary to an ELOVL4 mutationArch Ophthalmol2006124225726316476896
- JamesMJGibsonRAClelandLGDietary polyunsaturated fatty acids and inflammatory mediator productionAm J Clin Nutr2000711 Suppl343S348S10617994