Abstract
Introduction:
Primary open-angle glaucoma (POAG) is one of the leading causes of blindness. Activation of optic nerve head astrocytes (ONHA) and loss of trabecular meshwork cells (TMC) are pathognomonic for this neurodegenerative disease. Oxidative stress and elevated levels of transforming growth factor beta (TGFβ) play an important role in the pathogenesis of POAG. This study investigates the possible antiapoptotic and cytoprotective effects of minocycline on TMC and ONHA under oxidative stress and increased TGFβ levels.
Methods:
TMC and ONHA were treated with minocycline 1–150 μM. Possible toxic effects and IC50 were evaluated after 48 hours. Cell proliferation and viability were examined in order to assess the protective effects of minocycline on TMC and ONHA. Expression of Bcl-2, XIAP, and survivin, as well as their mRNA expression, were assessed by real time polymerase chain reaction (RT-PCR) and Western Blot analysis 48 hours after treatment with minocycline alone and additional incubation with TGFβ-2 or oxidative stress.
Results:
Minocycline 1–75 μM showed no toxic effects on TMC and ONHA. Under conditions of oxidative stress, both TMC and ONHA showed an increase in viability and an ability to proliferate when treated with minocycline 20–40 μM. RT-PCR and Western blotting yielded an overexpression of Bcl-2, XIAP, and survivin when TMC or ONHA were treated with minocycline 20–40 μM under conditions of oxidative stress and when additionally incubated with TGFβ-2.
Conclusion:
Minocycline up to 75 μM does not have toxic effects on TMC and ONHA. Treatment with minocycline 20–40 μM led to increased viability and proliferation under oxidative stress and TGFβ-2, as well as overexpression of Bcl-2, XIAP, and survivin. This protective pathway may help to prevent apoptotic cell death of TMC and ONHA and therefore be a promising approach to avoidance of progression of glaucomatous degeneration.
Introduction
Glaucoma is a chronic optic neuropathy characterized by retinal ganglion cell (RGC) loss, excavation of the optic nerve, and concomitant visual field defects.Citation1 It is the second leading cause of blindness, and almost 75 million people are affected worldwide.Citation2–Citation4 Elevated intraocular pressure (IOP) is the main risk factor for the developmentCitation5 and progressionCitation6 of glaucomatous damage, and most cases of glaucoma are associated with elevated IOP.Citation7,Citation8 Elevated IOP results from increased aqueous outflow resistance, and is due to several morphologic changes in the trabecular meshwork (TM). including trabecular cell loss.Citation6,Citation9,Citation10 Additional factors, such as nutritional statusCitation11 and retinal ischemia,Citation12,Citation13 have been implicated as pathognomonic, and there is growing evidence that oxidative stressCitation14–Citation16 and elevated levels of transforming growth factor beta (TGFβ)Citation17,Citation18 play a crucial role in the pathogenesis of glaucoma. It has also been shown in several animal models and in human glaucoma that both RGC and TM cells (TMC) die as a result of apoptosis.Citation16,Citation19–Citation24
Minocycline is a semisynthetic, second-generation tetracycline that crosses the blood–brain barrier effectively,Citation25 penetrates well into the eye,Citation26 and has had proven antimicrobial and anti-inflammatory efficacy for many years.Citation27,Citation28 In ophthalmology, minocycline has been suggested as a treatment for several palpebral disorders and age-related macular degeneration, due to its anti-inflammatory activities.Citation29 In addition to its antibiotic and anti-inflammatory activities, it has shown neuroprotective properties in experimental models of several neurodegenerative diseases, including multiple sclerosis, Parkinson’s disease, Huntington’s chorea, amyotropic lateral sclerosis, ischemic stroke, traumatic brain injury, and optic nerve transection.Citation30–Citation35
The neuroprotective effects of minocycline are considered to be anti-inflammatory and antiapoptotic. Recent reports have demonstrated the antioxidant properties and influence of minocycline on caspases and nitric oxide synthase activity.Citation36 Minocycline has an inhibiting effect on the release of apoptotic proteins, including cytochrome c and Smac/DIABLO, and induces antiapoptotic proteins, such as Bcl-2 or XIAP.Citation30,Citation37,Citation38 These effects have been demonstrated mainly for cells derived from the nervous system but also for non-neuronal cells, such as kidney epithelial cells.Citation38 A rodent study evaluating the effects of minocycline on RGC in experimental glaucoma revealed enhanced survival of RGC because of delayed apoptosisCitation39 but, so far, little is known about the possible protective and antiapoptotic effects of minocycline on the TM and neuroretinal cells of the human eye.
In this study, we investigated the effects of minocycline on primary human optic nerve head astrocytes (ONHA) and primary human TMC with regard to viability and cell death, and we evaluated whether toxic effects occur and, if so, at which concentrations of minocycline. In order to investigate the possible antiapoptotic effects of minocycline, the expression of Bcl-2, XIAP, survivin, as well as their mRNA expression, were determined when cells were treated with minocycline, under oxidative stress or elevated levels of TGFβ, to simulate glaucomatous conditions.
Methods
Human optic nerve head astrocytes
Primary cell cultures of human lamina cribrosa astrocytes were obtained from the Ludwig-Maximilian-University eye bank in Munich, Germany. Monolayer cultures were established from eyes of four human donors (aged 35, 53, 58, and 72 years, obtained 3–10 hours post mortem) without any history of eye diseases. The eyes were prepared and grown as previously described.Citation40–Citation42
Human trabecular meshwork cells
Cultures of human TMC were established from the same donors as for the ONHA according to protocols published previously.Citation40,Citation43,Citation44 TMC of passages 3–6 were seeded in 6-well plates and grown to a confluent monolayer. After seven days’ confluence, the wells were treated with minocycline as described below. Primary TMC and ONHA of passages 3–6 were used in the experiments. All procedures for gathering human tissue were undertaken with appropriate consent, complied with the Declaration of Helsinki, and were approved by the local ethics committee.
Cell culture treatment
For the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay and viability testing, TMC (passages 3–6) and ONHA (passages 3–6) were seeded in 6-well plates and exposed to minocycline concentrations of 1, 5, 7.5, 10, 15, 20, 40, 50, 75, 100, and 150 μM. To investigate the effects of minocycline on TMC and ONHA and on RNA and protein levels, minocycline concentrations of 20 and 40 μM were tested.
Before treatment, TMC and ONHA were kept for 24 hours in serum-free conditions. The cells were washed with phosphate-buffered saline (PBS) and incubated for 48 hours with minocycline concentrations soluted in DMEM/F12 medium for both TMC and ONHA cell lines. The tested substance concentrations were then removed by carefully rinsing the cells with serum-free medium three times. Thereafter, the cell proliferation assay, RNA isolation, and protein extraction was performed.
For testing the effects of minocycline under conditions of oxidative stress, cells were treated for 48 hours with the different minocycline concentrations and addition of 600 μM H2O2 for the last four hours. Thereafter the serum-free medium containing H2O2 and minocycline was removed by carefully rinsing the cells with serum-free medium three times. After another four hours of incubation with serum-free medium, cell proliferation assay, RNA isolation, and protein extraction was performed.
To test the effects of minocycline under treatment with TGFβ-2, the cells were first treated for 24 hours with the various concentrations of minocycline followed by addition of 1 ng/mL TGFβ-2. After another 24 hours of incubation with minocycline and TGFβ-2, RNA isolation and protein extraction was performed.
MTT assay
The MTT assay was used to determine the cell survival rate. This assay, as performed in our study, is a well established test for cell viability. It was performed as described in the literature by Mosmann,Citation45 with some modifications. The medium was removed, cells were washed with PBS, and 1000 μL/well MTT solution (1.5 mL MTT stock, 2 mg/mL in PBS, plus 28.5 mL DMEM) was added. TMC and ONHA were incubated at 37°C for one hour. The formazan crystals that formed were dissolved by the addition of dimethylsulfoxide (1000 μL/well). Absorption was measured by a scanning multiwell spectrophotometer at 550 nm (Molecular Probes, Garching, Germany). Results were expressed as the mean percentage of control proliferation. Experiments were performed in triplicate and repeated three times. TMC and ONHA of the same passage incubated with balanced saline without the addition of dyes served as the control.
Viability assay
Confluent TMC and ONHA were prepared and treated as described above. Cell viability was quantified based on a two-color fluorescence assay in which the nuclei of unviable cells appear red because of staining by the membrane-impermeable dye, propidium iodide (Sigma-Aldrich, Steinheim, Germany), while the nuclei of all cells were stained with the membranepermeable dye Hoechst 33342 (Intergen, Purchase, NY). Confluent cultures of TMC or ONHA growing on coverslips in 24-well tissue culture plates were exposed to the various concentrations of minocycline as described above for the MTT assay. To evaluate cell viability, the cells were washed in PBS and incubated with 2.0 μg/mL propidium iodide and 1.0 μg/mL Hoechst 33342 for 20 minutes at 37°C. Subsequently, the cells were analyzed with an epifluorescence microscope (Aristoplan, Leitz, Wetzlar, Germany). The labeled nuclei were then counted in fluorescence photomicrographs, and dead cells were expressed as a percentage of the total nuclei in the field. The data are based on counts from three experiments performed in duplicate wells, with 3–5 documented representative fields per well. TMC and ONHA of the same passage incubated with serum-free medium served as the control.
RNA isolation and real-time polymerase chain reaction
Total RNA was isolated from 10 cm Petri dishes by the guanidium thiocyanate-phenol-chloroform extraction method. Structural integrity of the RNA samples was confirmed by electrophoresis in 1% Tris-acetate-ethylenediamine tetraacetic acid agarose gels. The yield and purity were determined photometrically (BioPhotometer, Eppendorf, Germany). Real-time polymerase chain reaction (RT-PCR) enables quantitative detection of small amounts of mRNA. After the usual isolation of mRNA, this mRNA was transferred to cDNA via reverse transcriptase. This cDNA was then used for the specific PCR. Quantitation of Bcl-2, survivin, and XIAP mRNA was performed with specific primers (see ) with a LightCycler Instrument (LightCycler System, Roche Diagnostics, Mannheim, Germany). Primers and probes were found with the program ProbeFinder version 2.04. All primers and probes were designed to cross intron/exon boundaries, in order to avoid amplification of genomic DNA. All PCR products were sequenced to ensure product validity. Each 14 μL reaction volume contained 1 × FastStart DNA Master Hybridization Probes Mix (Roche Diagnostics), 4 mM MgCl2, 0.5 mM of each primer, 0.2 mM TaqMan probe, and 2 μL cDNA.
Table 1 Primer used for reverse transcription polymerase chain reaction
The amplification signals were detected in real time, which enabled accurate quantification of the amounts of the initial RNA template, because the system can select signals easily during the exponential amplification phase of PCR. The cDNA of cells either treated with minocycline alone (20 μM and 40 μM) or additionally treated with TGFβ-2 (1 ng/mL) or 600 μM H2O2 was amplified with specific primers for 40 cycles. Two oligonucleotides with differently-labeled fluorophores were hybridized to the amplified fragment during the annealing phase. When the two probes came into close proximity, fluorescence resonance energy transfer developed between the two fluorophores. The emitted fluorescence was then measured by the LightCycler instrument. Hybridization probes were displaced during the extension step. Depending on the initial concentration of target genes, the signal intensity increased in different cycles, and these cycles were used as the crossing point. The standard curve was made with three different probes of untreated TMC or ONHA. To normalize for differences in the amount of total RNA added to each reaction, 18S rRNA was simultaneously processed in the same sample as an internal control. The level of Bcl-2, survivin, and XIAP mRNA was determined as the relative ratio, which was calculated by dividing the level of Bcl-2, survivin, and XIAP mRNA by the level of the 18S rRNA housekeeping gene in the same samples. Ratios were expressed in a decimal format. All experiments were performed at least in triplicate and repeated three times.
Protein extraction and western blot analysis
TMC and ONHA grown on 35 mm tissue culture dishes were washed twice with ice-cold PBS, collected, and lysed in radioimmunoprecipitation assay cell lysis buffer. After centrifugation for 30 minutes at 19,000 × g in a microfuge (5810R, Eppendorf, Hamburg, Germany) in cold conditions, the supernatant was transferred to fresh tubes and stored at −70°C for future use. The protein content was measured by the bicinchoninic acid protein assay (Pierce, Rockford, IL). Denatured proteins (1e2 mg) were separated by electrophoresis under reducing conditions using a 5% sodium dodecyl sulfate-polyacrylamide stacking gel and a 12% sodium dodecyl sulfate-polyacrylamide separating gel, transferred with semidry blotting onto a polyvinyl difluoride membrane and probed with a mouse anti-Bcl-2 antibody, a mouse anti-survivin antibody, or a mouse anti-XIAP antibody, as described previously.Citation46 Chemiluminescence was detected with an imager (LAS-1000, RayTest) and generated light units. Exposure times were in the range 1–10 minutes. Quantification was performed on a computer (AIDA software, RayTest). All experiments were performed at least in triplicate and repeated three times in ONHA and TMC cultures from three donors. An even protein load in each lane was confirmed by staining of the polyvinyl difluoride membranes with Coomassie Brilliant Blue after the blotting procedure.
Statistics
All data were entered in an MS-Excel 2000 spreadsheet (Microsoft Corporation, Redmond, WA) and analyzed using SPSS 13.0 for Windows (SPSS Inc., Chicago, IL). All statistical tests were considered significant at P < 0.05. In detail, the statistical comparison between the different concentrations of minocycline (each measured in triplicate and performed three times) was performed using the Mann-Whitney test. For the MTT assay, quantitative results are presented as mean (± standard deviation, SD) units of absorbance. Ten individual samples per group were measured in triplicate and performed three times, and comparisons performed applying the Mann-Whitney test. For RT-PCR, the results are presented as mean ratios (± SD) of the investigated mRNA and 18S rRNA. Again, Mann-Whitney testing was applied, and all experiments were performed in triplicate and repeated three times. Western Blot analysis was performed analogous, experiments were performed at least in triplicate in ONHA and TMC cultures from three donors.
Results
Concentrations of minocycline in primary human TMC and ONHA
Minocycline concentrations of 1, 5, 7.5, 10, 15, 20, 40, 50, 75, 100, and 150 μM were chosen to investigate the possible toxic effects of minocycline on TMC and ONHA. With phase-contrast microscopy, no gross abnormalities could be detected in primary TMC and ONHA for minocycline at concentrations up to 75 μM. The numbers of cells counted in phase-contrast microscopy were comparable with the results of the MTT test (data not shown).
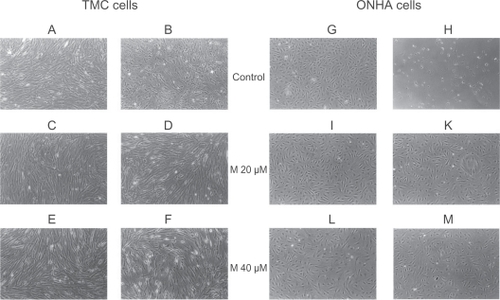
When TMC and ONHA were additionally treated with 600 μM H2O2, those cells treated with 20 μM and 40 μM minocycline did not show any signs of toxicity, cellular death, or other abnormalities. The control cells treated with 600 μM H2O2 alone showed pronounced morphologic signs of toxicity, including abnormal shape and appearance, cellular lysis, and destruction (). When ONHA and TMC were treated with 20 μM and 40 μM minocycline and additionally with TGFβ-2, the cells did not show any signs of toxicity, cellular death, or other abnormalities (data not shown). The concentration of minocycline that inhibited control cell growth by 50% (IC50) was determined from the dose-response curves (data not shown). For a 24-hour application, this concentration was approximately 75 μM (95% confidence interval [CI]: 65.5–84.5) for primary TMC and 100 μM for ONHA (95% CI: 88.8–111.2).
Figure 1 Phase-contrast microscopy of primary TMC (A–F) and ONHA cells (G–M). After treatment with minocycline 20 μM and 40 μM for both cell lines (TMC: C, E; ONHA: I, L), no morphologic changes could be detected. When cells were treated additionally with 600 μM H2O2, neither TMC nor ONHA pretreated with minocycline 20 μM and 40 μM (TMC: D, F; ONHA: K, M) showed any significant morphologic changes. In contrast, cells that were only treated with 600 μM H2O2 showed marked signs of destruction and cell death (TMC: B; ONHA: H). scale (10×)
MTT assay
Minocycline showed no significant toxic effects in the two investigated cell cultures (48 hours exposure) at concentrations between 1 μM and < 50 μM ( and ). All experiments were performed at least in triplicate and repeated three times. No significant decrease was detected in cellular viability for either primary TMC or ONHA, compared with the controls at concentrations <50 μM. Minocycline concentrations of 50 μM (P < 0.001) and higher for primary ONHA led to a dose-dependent reduction of viability. When ONHA cells were treated additionally with H2O2, cells pretreated with minocycline concentrations >15 μM (P = 0.02) up to 50 μM (P = 0.01) showed a significantly increased viability with a peak at 20 μM (P < 0.001) compared with the control. Only concentrations as high as 150 μM showed significantly decreased viability (P < 0.001, ).
Figure 2 Viability of primary ONHA cells after treatment with the investigated concentrations of minocycline (black curve, diamond) and additionally treated with 600 μM H2O2 (grey curve, triangles), measured by a colorimetric test (MTT). Tests were performed in triplicate and repeated three times. Untreated primary ONHA of the same passage served as the control. Results are expressed as the mean percentage of control cell survival. The data are the mean of results of three experiments, each performed in triplicate. Cells treated only with minocycline concentrations up to 40 μM showed no reduction in viability compared with the control, while 50 μM and higher showed a significantly reduced viability (P < 0.001). When cells were treated additionally with H2O2, those pretreated with minocycline > 15 μM (P = 0.02) up to 50 μM (P = 0.01) showed a significantly increased viability, with a peak at 20 μM (P < 0.001) compared with the control. Only concentrations as high as 150 μM showed significantly decreased viability (P < 0.001). Error bars, ± SD.
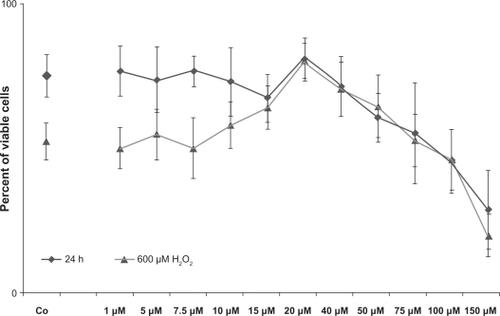
Figure 3 Viability of primary TMC after treatment with the investigated concentrations of minocycline (black curve, squares) and additionally treated with 600 μM H2O2 (grey curve, triangles), measured by MTT assay. TMC treated only with minocycline concentrations up to 40 μM showed no reduction in viability compared with the control. Minocycline 50 μM (P = 0.005) and higher for primary TMC led to a dose-dependent reduction of viability. When TMC cells were treated additionally with H2O2, those pretreated with minocycline >7.5 μM (P < 0.001) up to 50 μM (P = 0.002) showed significantly increased viability, with a peak at 40 μM (P < 0.001) compared with the control. Concentrations of 75 μM (P = 0.01) and higher showed a significantly decreased viability. Error bars, ± SD.
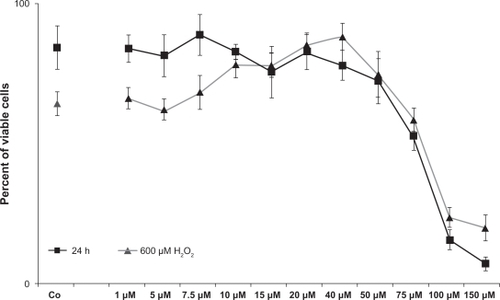
Minocycline concentrations of 50 μM (P = 0.005) and higher for primary TMC led to a dose-dependent reduction of viability (). When TMC cells were treated additionally with H2O2, the cells pretreated with minocycline concentrations of >7.5 μM (P < 0.001) up to 50 μM (P = 0.002) showed significantly increased viability, with a peak at 40 μM (P < 0.001) compared with the control. Concentrations of 75 μM (P = 0.01) and higher showed significantly decreased viability (). Minocycline concentrations of 100 μM and 150 μM for primary TMC and 150 μM for ONHA markedly reduced the proliferation rate to less than 20%. ONHA and TMC treated with minocycline and TGFβ-2 showed similar results to those of ONHA and TMC treated with minocycline only (data not shown).
Viability assay
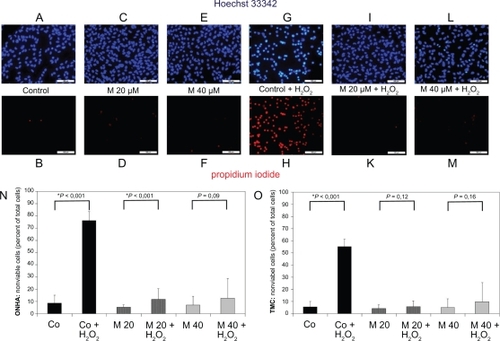
The viability of primary ONHA was tested by labeling the nuclei of nonviable cells with propidium iodide 48 hours after treatment of the cells. For ONHA, minocycline concentrations 1–75 μM did not show any significant effect on cell viability (data not shown). If primary ONHA were treated with minocycline concentrations for 48 hours and additionally treated with 600 μ M H2O2 for four hours, minocycline concentrations of 20 μM (P < 0.001) led to a significant increase in viability compared with the control treated with 600 μM H2O2 only (). By contrast, minocycline concentrations of 75 μM and higher induced a marked reduction of viable ONHA cells in a dose-dependent manner (). TMC () showed results similar to those of primary ONHA cells.
Figure 4 Primary ONHA and TMC were treated for 48 hours with various concentrations of minocycline only or additionally treated with 600 μM H2O2 for four hours. After exposure of the cells to the substance alone and in combination with oxidative stress, viability was determined by staining all nuclei with Hoechst 33342 and dead cells with propidium iodide. (A) Representative fluorescence photomicrograph of Hoechst 33342-stained, untreated ONHA as control. (B) Nonviable cells in the corresponding field. Fluorescence photomicrograph of ONHA treated with minocycline 20 μM (C) and 40 μM (E) for 48 hours and labeled with Hoechst 33342. Nonviable ONHA treated with minocycline 20 μM (D) and 40 μM (F) for 48 hours in the same field as in (C) and (E). Fluorescence photomicrograph of ONHA treated with 600 μM H2O2 only (G) and with minocycline 20 μM (I) and 40 μM (L) for 48 hours and additionally treated with 600 μM H2O2 and labelled with Hoechst 33342. Nonviable ONHA treated with 600 μM H2O2 only (H) and with minocycline 20 μM (K) and 40 μM (M) for 48 hours and additionally treated with 600 μM H2O2 in the same field as in (G, I, L). TMC yielded a similar result (data not shown). Quantification of effect of minocycline treatment alone and additional treatment with H2O2 on numbers of nonviable primary ONHA (N) and TMC (O). The percentage of dead cells was scored by counting at least 500 cells in fluorescence photomicrographs of representative fields. Data (mean±SD) are based on the sampling of 6–10 photomicrographs per condition in three independent experiments performed in duplicate. Comparison with controls is shown above the bars with significant differences marked by an asterisk.
Expression of Bcl-2, XIAP, and survivin mRNA in ONHA and TMC
ONHA and TMC from four donors were used to investigate mRNA expression of Bcl-2, XIAP, and survivin. Bcl-2, XIAP, and survivin mRNA expression were detectable in every sample. All detected mRNA levels of Bcl-2, XIAP, and survivin were normalized to those of 18S rRNA, and the values are expressed as the relative ratio of Bcl-2/18S, XIAP/18S, or survivin/18S.
Our findings indicate that treatment with minocycline leads to increased mRNA expression for both investigated cell lines for all three tested antiapoptotic proteins. In contrast, TGFβ-2 and H2O2 treatment decreased mRNA expression of Bcl-2, XIAP, and survivin in both ONHA and TMC (, , and ). Cells treated with minocycline and with TGFβ-2 or H2O2 seemed to compensate for the detected decrease of Bcl-2, XIAP, and survivin mRNA expression. Minocycline-treated cells reached mRNA levels of Bcl-2, XIAP, and survivin comparable with those of the untreated control, or even higher. In addition, cells pretreated with minocycline and then treated with TGFβ-2 or H2O2 showed significantly higher levels of Bcl-2, XIAP, and survivin mRNA in ONHA and TMC than cells treated only with TGFβ-2 or H2O2. These effects were found for both ONHA and TMC when cells were treated with minocycline concentrations of 20 μM and 40 μM (, , and ). When cells were treated with minocycline concentrations less than 10 μM, the described effects decreased and did not reach statistical significance (data not shown).
Figure 5 Bcl-2 mRNA expression of ONHA and TMC after treatment with minocycline 20 μM and 40 μM only or after additional treatment with 600 mM H2O2 or TGFβ-2, as investigated by quantitative RT-PCR. All tests were performed in triplicate and repeated three times. The mRNA levels were normalized to those of 18S rRNA and expressed as the ratio of Bcl-2 mRNA/18S rRNA. ONHA treated with minocycline concentrations showed a significant increase in Bcl-2 mRNA expression compared with the control in both cell lines. TGFβ-2 and H2O2 treatment decreased Bcl-2 expression. When ONHA cells were treated with minocycline 20 μM and 40 μM and TGFβ-2 or H2O2, Bcl-2 expression was increased compared with ONHA treated only with TGFβ-2 or H2O2. Similarly, TMC treated with minocycline 20 μM and TGFβ-2 or H2O2 showed an increased expression of Bcl-2 mRNA compared with TMC treated only with TGFβ-2 or H2O2. All differences between the two minocycline concentrations and the corresponding controls are statistically significant.
Key: x-axis, RR of Bcl-2 mRNA normalized to 18s rRNA expressed in decimal format; y-axis, tested concentrations of minocycline.
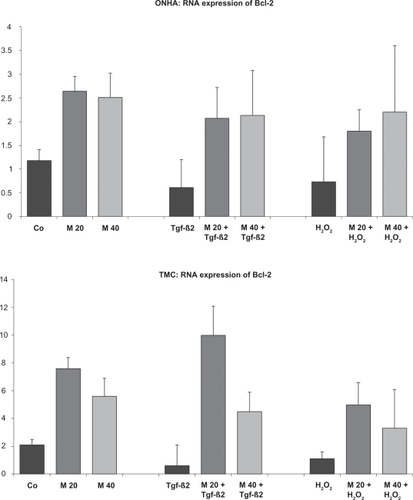
Figure 6 XIAP mRNA expression of ONHA and TMC after treatment with minocycline only and after additional treatment with H2O2 or TGFβ-2. ONHA treated with 40 minocycline μM showed a significant increase in XIAP mRNA expression compared with the control in both cell lines. TGFβ-2 and H2O2 treatment slightly decreased XIAP expression in ONHA and treatment with minocycline 20 μM and 40 μM and TGFβ-2 or H2O2 increased expression of XIAP compared with ONHA treated only with TGFβ-2 or H2O2. TMC treated with minocyline 20 μM TGFβ-2 or H2O2 showed an increased expression of XIAP mRNA compared with TMC treated only with TGFβ-2 or H2O2. All differences between the two minocycline concentrations and the corresponding controls are statistically significant.
Key: x-axis, RR of XIAP mRNA normalized to 18s rRNA expressed in decimal format; y-axis, tested concentrations of minocycline.
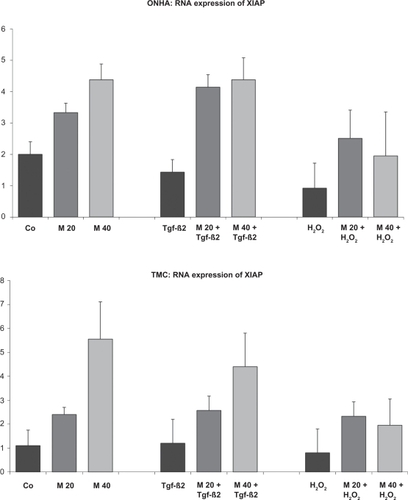
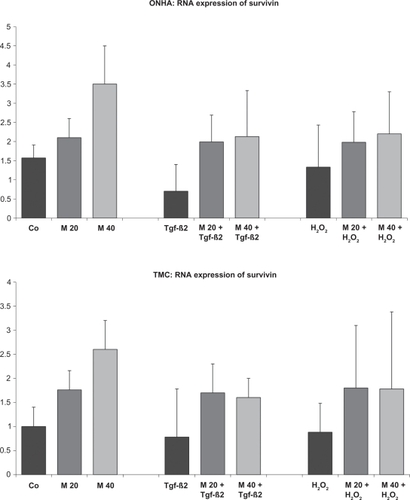
Figure 7 Survivin mRNA expression measured by quantitative RT-PCR. ONHA treated with minocycline 40 μM showed a significant increase in survivin mRNA expression compared with the control. TMC showed this increase for minocycline 20 μM and 40 μM. TGFβ-2 decreased survivin expression, and H2O2 treatment slightly decreased survivin expression for both cell lines. Treatment with minocycline 20 μM and 40 μM and additional treatment with TGFβ-2 increased expression of survivin compared with cells treated only with TGFβ-2. For cells treated additionally with H2O2, ONHA cells only showed a slight increase and TMC cells showed a more pronounced increase when pretreated with minocycline concentrations compared with cells treated with H2O2 only. All differences between the two minocycline concentrations and the corresponding controls are statistically significant except for the H2O2 group with ONHA.
Protein expression of Bcl-2, XIAP, and survivin in ONHA and TMC
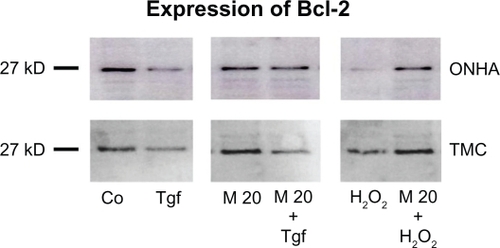
To verify that the increase in mRNA transcription of Bcl-2, XIAP, and survivin by minocycline translates into increased protein synthesis, whole cellular protein extracts of untreated control cells, cells treated with minocycline alone, and cells treated with minocycline and then with TGFβ-2 or 600 μM H2O2, were analyzed by Western blotting. When cells were treated with 600 μM H2O2, Bcl-2 expression decreased 10-fold in ONHA and twofold in TMC compared with the control (). TGFβ-2 treatment (1 ng/mL) decreased Bcl-2 expression by approximately 2.5-fold in ONHA and by twofold in TMC. Treatment with minocycline alone did not increase protein expression of Bcl-2 or XIAP in primary human ONHA or TMC. Compared with cells treated only with H2O2, cells pretreated with 20 μM minocycline and then with H2O2 showed a significantly lower decrease of Bcl-2 of approximately 1.5-fold in ONHA cells and a 1.25-fold increase in TMC and a significant increase of 7.5-fold in ONHA cells and twofold in TMC. When cells were treated with 20 μM minocycline and TGFβ-2, Bcl-2 expression increased by approximately twofold in ONHA cells and by 1.5-fold in TMC cells, compared with cells treated with TGFβ-2 only. The decrease of Bcl-2 compared with the untreated control was 1.5-fold in ONHA cells and two-fold in TMC cells, which was significantly lower than the decrease after TGFβ-2 treatment only ().
Figure 8 Effect of minocycline treatment on Bcl-2 protein expression. ONHA and TMC were treated with minocycline 20 μM only or additionally with TGFβ-2 (1 ng/mL) or 600 μM H2O2. Western blotting was used to analyze protein expression in control (Co) and treated cell extracts: TGFβ-2 (Tgf), 20 μM minocycline (M20), minocycline 20 μM and TGFβ-2 (M20+Tgf), 600 μM H2O2 (H2O2), and minocycline 20μM and 600 μM H2O2 (M20 + H2O2). Ten micrograms of protein was loaded per lane.
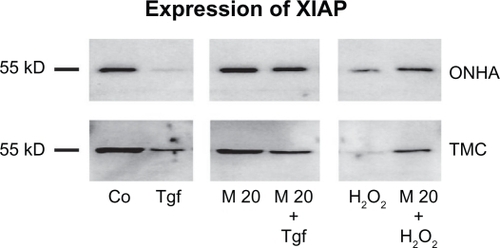
The XIAP expression decreased 2.5-fold in ONHA cells and fivefold in TMC cells treated with 600 μM H2O2 (). TGFβ-2 decreased XIAP expression 10-fold in ONHA cells and twofold in TMC cells, compared with the control. Cells pretreated with 20 μM minocycline and then with H2O2 showed a significantly lower decrease of XIAP expression of 1.5-fold in ONHA cells and 1.25-fold in TMC cells than those cells that were treated with H2O2 only (2.5×) (TMC 5×). The same effect could be detected for TGFβ-2 treatment, whereby ONHA and TMC cells pre-treated with minocycline 20 μM showed no decrease in XIAP expression compared with the control, but cells treated with TGFβ-2 only did show a 10-fold decrease in XIAP expression in ONHA cells and a twofold decrease in expression in TMC cells ().
Figure 9 Expression of XIAP. Western blot showing the protein expression of XIAP in control (Co) and treated cell extracts: TGFβ-2 (Tgf), minocycline 20 μM (M20), minocycline 20 μM and TGFβ-2 (M20+Tgf), 600 μM H2O2 (H2O2), and treatment with minocycline 20 μM and 600 μM H2O2 (M20 + H2O2). Ten micrograms of protein were loaded per lane.
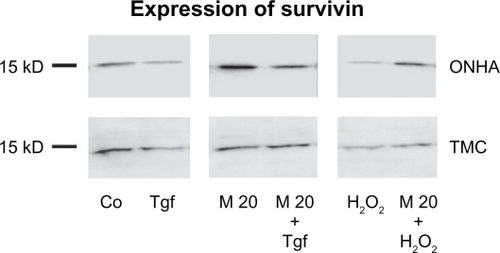
Survivin expression in ONHA decreased fivefold in ONHA cells and 2.5-fold in TMC cells treated with 600 μM H2O2 (). TGFβ-2 decreased XIAP expression by 2.5-fold in ONHA cells and by twofold in TMC compared with the control. When ONHA cells were treated with minocycline 20 μM, a 2.5-fold increase in survivin expression was detected in ONHA cells but not for TMC cells. ONHA cells pretreated with minocycline 20 μ M and then with H2O2 600 μM showed a slight increase of survivin expression of 1.1-fold in ONHA cells, while TMC cells showed a decrease of twofold compared with the control. By contrast, cells treated only with H2O2 showed a fivefold decrease in survivin expression in ONHA cells and a 2.5-fold decrease in TMC cells. ONHA cells pretreated with minocycline 20 μM and then treated with TGFβ-2 showed a 1.5-fold increase in survivin expression compared with the control, which was a 3.5-fold higher survivin expression compared with cells treated with TGFβ-2 only. TMC cells pretreated with minocycline 20 μM showed a lower decrease of survivin expression of 1.25-fold than cells treated with TGFβ-2 only (twofold, see ).
Figure 10 Expression of survivin. Western blot showing the protein expression of survivin in control (Co) and treated cell extracts: TGFβ-2 (Tgf), minocycline 20μM (M20), minocycline 20 μM and TGFβ-2 (M20+Tgf), 600 μM H2O2 (H2O2), and minocycline 20 μM and 600 μ M H2O2 (M20 + H2O2). Ten micrograms of protein were loaded per lane.
Discussion
Glaucomatous loss of visual field is mainly due to RGC death and neuronal degeneration, and there is evidence that these cells mainly die as a result of apoptosis.Citation19–Citation22 IOP elevation and visual field damage have been shown to be proportional to oxidative damage in the human TM.Citation16 Cell loss, altered functionality, and a decrease in the outflow facility of the TM have been suggested as a result of oxidative damage in the TM.Citation47,Citation48 In addition, a significantly decreased TM cellularity, especially in the filtering area of the TM, is found in patients suffering from glaucoma, compared with age-matched nonglaucomatous subjects.Citation9 Recent data suggest that these structural changes in the TM and a decrease in TMC, which leads to elevated IOP, are also triggered by apoptosis,Citation10,Citation23,Citation24 so antiapoptotic and neuroprotective agents could support and complement the proven but incomplete therapeutic effect of decreasing IOP. Our findings demonstrate that minocycline effectively protects primary human TMC and ONHA in vitro against oxidative stress and induces a marked reduction in cellular death. We were able to show that this protection is associated with the induction of three antiapoptotic proteins, ie, Bcl-2, XIAP, and survivin. These three proteins are known inhibitors of major pathways of apoptosis.
Apoptosis is a mechanism that is genetically controlled by the dying cell, resulting in the activation of tumor suppressor proteinsCitation49 and apoptosis-initiating caspases.Citation50 One mechanism in the cell death program is upregulation of the proapoptotic Bax protein. This increase in Bax expression effects a change in the permeability of mitochondrial membranes. That leads to the release of cytochrome c and the activation of caspases, which finally proteolyze cellular components.Citation51 Bcl-2 is one of the key genes known to downregulate Bax and p53, and therefore has the potency to inhibit this apoptotic pathway.Citation52,Citation53 Therefore, pharmacologic upregulation of Bcl-2 is a highly interesting approach to inhibition of apoptosis.
XIAP and survivin are members of a group of proteins called inhibitors of apoptosis (IAPs).Citation54–Citation56 XIAP inhibits apoptosis by directly blocking the activity of the terminal effector cell death proteases, caspases 3, 7, and 9. It has been demonstrated in vivo that overexpression of XIAP protects injured neuronal cells and photoreceptors from further compromise.Citation57,Citation58 Survivin interacts with p21Citation59 and thus suppresses Fas-mediated cell death.Citation60 Its antiapoptotic effects seem to result from its ability to block caspases 3 and 7. In addition, survivin prevents cells from responding to apoptotic stimuli.Citation61 Bcl-2, XIAP, and survivin, all show antiapoptotic effects. Because minocycline upregulates RNA levels of Bcl-2, XIAP, and survivin in ONHA and TMC, it has high antiapoptotic potency on those cell lines.
There is evidence that both oxidative stress and elevated levels of TGFβ-2 support the development and progression of glaucoma.Citation16–Citation18,Citation62,Citation63 For this reason, the present study investigated TGFβ-2, the isoform of TGFβ that is expressed most in human eyes. Recent studies have shown that elevated levels of TGFβ, especially TGFβ-2, are found in glaucomatous eyes and seem to have a major influence on the pathogenesis of glaucoma.Citation17,Citation18 So another important finding of our study is that TGFβ-2 downregulates both mRNA levels and protein expression of Bcl-2, XIAP, and survivin in primary human ONHA and TMC. In contrast, minocycline protects both cell lines against TGFβ-2-induced downregulation of these three antiapoptotic proteins and returns levels back to normal or even increases them. We were also able to show that minocycline protects ONHA and TMC against oxidative stress, which is another important pathologic trigger for glaucoma.
Because of its antibiotic and anti-inflammatory actions, minocycline is widely used, and has had proven safety as an oral antibiotic for many years.Citation27,Citation28 Because minocycline crosses the blood–brain barrier effectivelyCitation25 and penetrates well into the eye,Citation26 the optic nerve and ocular cells are potentially susceptible to oral treatment. Due to the very good bioavailability of minocycline, the tested concentration of 20 μM would correspond to an estimated dose of approximately 750 mg/day in adult humans, which is undoubtedly a high dosage. On the other hand, previous reports have shown that daily tetracycline doses even higher than 2000 mg are still relatively safe in healthy pregnant women.Citation27 Therefore, it might be worthwhile to investigate the antiapoptotic and cytoprotective effects of minocycline in glaucoma treatment further.
Glaucoma is a chronic disease, and the long-term utilization of antibiotics is known to be associated with side effects.Citation64,Citation65 Minocycline seems to be safe even for long-term high-dose treatmentCitation66 but, in the end, the relatively high dosages needed to achieve the cytoprotective effects described here might limit its application as long term medication. On the other hand, there are situations where acute cell stress is predominant, ie, in cases of acute glaucoma or in end-stage glaucoma patients needing glaucoma-filtering surgery, where the risk of “wipeout” (loss of the central visual field) is feared.Citation67,Citation68 In such cases, minocycline might offer a new option to protect affected eyes from further damage.
Our results demonstrate the cytoprotective effects of minocycline on primary human ONHA and TMC. These in vitro data cannot be automatically extrapolated to an in vivo situation. Even high doses of minocycline are tolerated well in vivoCitation27,Citation66 but it is not clear if this cytoprotective action of minocycline is effective in the long term and whether it is actually possible to reach therapeutic concentrations inside the eye. Further investigations are therefore needed on these points. Certainly further basic and clinical studies are needed, but the present study makes an early contribution towards shedding light on the potential protective effects of minocycline and to find possible new treatment strategies for glaucoma.
Acknowledgements
The authors thank Katja Obholzer for providing excellent technical assistance.
Disclosure
The authors do not have any commercial interest in any of the materials used in this study.
References
- Van BuskirkEMCioffiGAGlaucomatous optic neuropathyAm J Ophthalmol19921134474521558122
- World Health OrganizationMagnitude and causes of visual impairment. Fact Sheet Number 282: World Health Organization2004 Available at: http://www.who.int/mediacentre/factsheets/fs282/en/. Accessed May 12, 2010.
- QuigleyHAGreenWRThe histology of human glaucoma cupping and optic nerve damage: Clinicopathologic correlation in 21 eyesOphthalmology19798618031830553256
- GoldbergLDClinical guidelines for the treatment of glaucomaManag Care200211162415357535
- KassMAHeuerDKHigginbothamEJThe Ocular Hypertension Treatment Study: A randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucomaArch Ophthalmol200212070113 discussion 829–830.12049574
- HeijlALeskeMCBengtssonBHymanLHusseinMReduction of intraocular pressure and glaucoma progression: Results from the Early Manifest Glaucoma TrialArch Ophthalmol20021201268127912365904
- KrakauCEIntraocular pressure elevation – cause or effect in chronic glaucoma?Ophthalmologica19811821411477267002
- BonomiLMarchiniGMarraffaMMorbioRThe relationship between intraocular pressure and glaucoma in a defined population. Data from the Egna-Neumarkt Glaucoma StudyOphthalmologica2001215343811125267
- AlvaradoJMurphyCJusterRTrabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normalsOphthalmology1984915645796462622
- RohenJWLutjen-DrecollEFlugelCMeyerMGriersonIUltrastructure of the trabecular meshwork in untreated cases of primary open-angle glaucoma (POAG)Exp Eye Res1993566836928595810
- VeachJFunctional dichotomy: Glutathione and vitamin E in homeostasis relevant to primary open-angle glaucomaBr J Nutr20049180982915182385
- FindlORainerGDallingerSAssessment of optic disk blood flow in patients with open-angle glaucomaAm J Ophthalmol200013058959611078837
- ButtZO’BrienCMcKillopGAspinallPAllanPColor Doppler imaging in untreated high- and normal-pressure open-angle glaucomaInvest Ophthalmol Vis Sci1997386906969071223
- FerreiraSMLernerSFBrunziniREvelsonPALlesuySFOxidative stress markers in aqueous humor of glaucoma patientsAm J Ophthalmol2004137626914700645
- LevinLAClarkJAJohnsLKEffect of lipid peroxidation inhibition on retinal ganglion cell deathInvest Ophthalmol Vis Sci199637274427498977490
- SaccaSCPascottoACamicionePCaprisPIzzottiAOxidative DNA damage in the human trabecular meshwork: Clinical correlation in patients with primary open-angle glaucomaArch Ophthalmol200512345846315824217
- FleenorDLShepardARHellbergPEJacobsonNPangIHClarkAFTGFbeta2-induced changes in human trabecular meshwork: Implications for intraocular pressureInvest Ophthalmol Vis Sci20064722623416384967
- MinSHLeeTIChungYSKimHKTransforming growth factor-beta levels in human aqueous humor of glaucomatous, diabetic and uveitic eyesKorean J Ophthalmol20062016216517004630
- NickellsRWApoptosis of retinal ganglion cells in glaucoma: An update of the molecular pathways involved in cell deathSurv Ophthalmol199943Suppl 1S151S16110416758
- Garcia-ValenzuelaEShareefSWalshJSharmaSCProgrammed cell death of retinal ganglion cells during experimental glaucomaExp Eye Res19956133447556468
- KerriganLAZackDJQuigleyHASmithSDPeaseMETUNEL-positive ganglion cells in human primary open-angle glaucomaArch Ophthalmol1997115103110359258226
- QuigleyHANickellsRWKerriganLAPeaseMEThibaultDJZackDJRetinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosisInvest Ophthalmol Vis Sci1995367747867706025
- CaoYWeiHPfafflMDaBLiZApoptosis of human trabecular meshwork cells induced by transforming growth factor-beta2 in vitroJ Huazhong Univ Sci Technolog Med Sci20042487899415165125
- ZiangirovaGGAntonovaOVLipid peroxidation in the pathogenesis of primary open-angle glaucomaVestn Oftalmol20031195455 Russian.12934509
- AronsonALPharmacotherapeutics of the newer tetracyclinesJ Am Vet Med Assoc1980176106110687216873
- PoirierRHEllisonACOcular penetration of orally administered minocyclineAnn Ophthalmol19791118591861556140
- KleinNCCunhaBATetracyclinesMed Clin North Am1995797898017791423
- RyanMEGreenwaldRAGolubLMPotential of tetracyclines to modify cartilage breakdown in osteoarthritisCurr Opin Rheumatol199682382478796985
- WirostkoEWirostkoWJWirostkoBMAge-related macular degeneration is an inflammatory disease possibly treatable with minocyclineActa Ophthalmol Scand20048224324415043554
- ChenMOnaVOLiMMinocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington diseaseNat Med2000679780110888929
- YrjanheikkiJKeinanenRPellikkaMHokfeltTKoistinahoJTetracyclines inhibit microglial activation and are neuroprotective in global brain ischemiaProc Natl Acad Sci U S A19989515769157749861045
- ZhuSStavrovskayaIGDrozdaMMinocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in miceNature2002417747811986668
- Sanchez MejiaROOnaVOLiMFriedlanderRMMinocycline reduces traumatic brain injury-mediated caspase-1 activation, tissue damage, and neurological dysfunctionNeurosurgery20014813939 discussion 1399–1401.11383749
- PopovicNSchubartAGoetzBDZhangSCLiningtonCDuncanIDInhibition of autoimmune encephalomyelitis by a tetracyclineAnn Neurol20025121522311835378
- DuYMaZLinSMinocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson’s diseaseProc Natl Acad Sci U S A200198146691467411724929
- KrausRLPasiecznyRLariosa-WillinghamKTurnerMSJiangATraugerJWAntioxidant properties of minocycline: Neuroprotection in an oxidative stress assay and direct radical-scavenging activityJ Neurochem20059481982716033424
- TikkaTFiebichBLGoldsteinsGKeinanenRKoistinahoJMinocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microgliaJ Neurosci2001212580258811306611
- WangJWeiQWangCYHillWDHessDCDongZMinocycline up-regulates Bcl-2 and protects against cell death in mitochondriaJ Biol Chem2004279199481995415004018
- Levkovitch-VerbinHKalev-LandoyMHabot-WilnerZMelamedSMinocycline delays death of retinal ganglion cells in experimental glaucoma and after optic nerve transectionArch Ophthalmol200612452052616606878
- KerntMKampikAIntraocular caspofungin: In vitro safety profile for human ocular cellsMycoses2192010 [Epub ahead of print]
- KerntMLieglRGRuepingJSorafenib protects human optic nerve head astrocytes from light-induced overexpression of vascular endothelial growth factor, platelet-derived growth factor, and placenta growth factorGrowth Factors2192010 [Epub ahead of print]
- KerntMNeubauerASUlbigMWKampikAWelge-LussenUIn vitro safety of intravitreal moxifloxacin for endophthalmitis treatmentJ Cataract Refract Surg20083448048818299076
- SiegnerAMayCAWelge-LussenUWBloemendalHLutjen-DrecollEAlpha B-crystallin in the primate ciliary muscle and trabecular meshworkEur J Cell Biol1996711651698905293
- Welge-LussenUEichhornMBloemendalHLutjen-DrecollEClassification of human scleral spur cells in monolayer cultureEur J Cell Biol19987578849523158
- MosmannTRapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assaysJ Immunol Methods19836555636606682
- Welge-LussenUMayCAEichhornMBloemendalHLutjen-DrecollEAlpha B-crystallin in the trabecular meshwork is inducible by transforming growth factor-betaInvest Ophthalmol Vis Sci1999402235224110476788
- KahnMGGiblinFJEpsteinDLGlutathione in calf trabecular meshwork and its relation to aqueous humor outflow facilityInvest Ophthalmol Vis Sci198324128312876885312
- De La PazMAEpsteinDLEffect of age on superoxide dismutase activity of human trabecular meshworkInvest Ophthalmol Vis Sci199637184918538759353
- LevineAJp53, the cellular gatekeeper for growth and divisionCell1997883233319039259
- McKinnonSJLehmanDMKerrigan-BaumrindLACaspase activation and amyloid precursor protein cleavage in rat ocular hypertensionInvest Ophthalmol Vis Sci2002431077108711923249
- MiyashitaTReedJCTumor suppressor p53 is a direct transcriptional activator of the human bax geneCell1995802932997834749
- MiyashitaTHarigaiMHanadaMReedJCIdentification of a p53-dependent negative response element in the bcl-2 geneCancer Res199454313131358205530
- HockenberyDMThe bcl-2 oncogene and apoptosisSemin Immunol199244134201286168
- DeverauxQLTakahashiRSalvesenGSReedJCX-linked IAP is a direct inhibitor of cell-death proteasesNature19973883003049230442
- HolcikMGibsonHKornelukRGXIAP: Apoptotic brake and promising therapeutic targetApoptosis2001625326111445667
- SasakiTLopesMBHankinsGRHelmGAExpression of survivin, an inhibitor of apoptosis protein, in tumors of the nervous systemActa Neuropathol (Berl)200210410510912070671
- McKinnonSJLehmanDMTahzibNGBaculoviral IAP repeat-containing-4 protects optic nerve axons in a rat glaucoma modelMol Ther2002578078712027563
- PetrinDBakerACouplandSGStructural and functional protection of photoreceptors from MNU-induced retinal degeneration by the X-linked inhibitor of apoptosisInvest Ophthalmol Vis Sci2003442757276312766084
- SuzukiAItoTKawanoHSurvivin initiates procaspase 3/p21 complex formation as a result of interaction with Cdk4 to resist Fas-mediated cell deathOncogene2000191346135310713676
- SuzukiAHayashidaMItoTSurvivin initiates cell cycle entry by the competitive interaction with Cdk4/p16(INK4a) and Cdk2/cyclin E complex activationOncogene2000193225323410918579
- ShinSSungBJChoYSAn anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and -7Biochemistry2001401117112311170436
- NeufeldAHNitric oxide: A potential mediator of retinal ganglion cell damage in glaucomaSurv Ophthalmol199943Suppl 1S129S13510416755
- KumarDMAgarwalNOxidative stress in glaucoma: A burden of evidenceJ Glaucoma20071633434317438430
- BjornbergARoupeGSusceptibility to infections during long-term treatment with tetracyclines in acne vulgarisDermatologica19721453343374265960
- MargolisDJAntibiotics, acne, and upper respiratory tract infectionsLDI Issue Brief2006111416708431
- GouldenVGlassDCunliffeWJSafety of long-term high-dose minocycline in the treatment of acneBr J Dermatol19961346936958733373
- TopouzisFTranosPKoskosasARisk of sudden visual loss following filtration surgery in end-stage glaucomaAm J Ophthalmol200514066166616226517
- AgarALiSAgarwalNCoroneoMTHillMARetinal ganglion cell line apoptosis induced by hydrostatic pressureBrain Res2006108619120016638612