Abstract
Aims:
To evaluate the age-related variations of ocular parameters in Korean subjects.
Methods:
We recruited 314 normal subjects who visited the department of Ophthalmology between January 2007 and October 2007. Refraction, axial length, corneal curvature, white-to-white distance, anterior chamber depth, corneal endothelial cell density, and retinal nerve fiber layer (RNFL) thickness were measured using auto-refractive keratometer, intraocular lens master, noncontact specular microscope, and optical coherence tomography.
Result:
In correlation analysis, from 19 to 82 years, hyperopic shift showed a strong positive statistical correlation with age (r = 0.553, P < 0.001). Corneal curvatures increased (r = 0.221, P < 0.001), while axial length (r = −0.506, P < 0.001), anterior chamber depth (r = −0.491, P < 0.001) and white-to-white distance (r = −0.205, P < 0.001) decreased with age. Also, corneal endothelial cell density was lower in older patients than in younger patients (r = −0.409, P < 0.001). Compared to younger patients, RNFL thickness was lower in the older patients as well, in all quadrants (superior, r = −0.283, P < 0.001; inferior, r = −0.230, P < 0.001; nasal, r = 0.025, P = 0.676; and temporal, r = −0.393, P < 0.001). According to multiple regression analysis, out of the six parameters measured, only hyperopic shift, anterior chamber depth and corneal endothelial cell density (P < 0.05) had statistically significant correlation with age.
Conclusion:
Some of the ocular parameters changed with aging. Hyperopic shift, shallowing anterior chamber depth, and reduction of corneal endothelial cell density were only definitely related to age.
Introduction
All organs go through various changes due to the aging process, and the ocular structures are no different.Citation1 With the exception of the lens, most ocular structures continue developing until around age 14 years, but after age 15, axial length, corneal diameter, and corneal curvature remain more or less the same.Citation2 According to a study about ocular development, axial length is 18 mm at birth, increasing rapidly to 23 mm till age 3 years, after which the growth slows down to about 0.1 mm per year to reach the adult stage at age 14.Citation3–Citation5
Laule et al have reported a negative correlation between age and corneal endothelial cell density,Citation6 and in Kanamori et al, retinal nerve fiber layer (RNFL) thickness was found to decrease with age as well.Citation7 According to many studies on the relationship between refractory errors and aging, most refractory errors at birth are hyperopic, and a switch to myopia occurs with time, initially presenting at around ages 7 and 8 years, and rapidly increasing in incidence between ages 9 and 11 years,Citation8 with the degree and incidence of myopia continuing to increase with age until physical maturity in early 20’s;Citation9 after the age of 40 years, a conversion back to hyperopia takes place, thus increasing its incidence.Citation10
Although a number of previous studies have investigated the various changes in ocular parameters in normal eyes, either they have not examined every parameter and age at the same time, or have failed to consider all parameters involved. However, various factors contribute to the changes that are seen at any particular age, and they affect each other. As yet there has not been a study showing the changes in ocular parameter according to age, hence the decision to investigate the correlation between age and each parameter, along with different factors that may affect those correlations, and analyze the results.
Materials and methods
In this prospective study conducted between January, 1 to December, 31 2007, we measured axial length, corneal curvature, white-to-white distance, corneal endothelial cell density, and RNFL thickness in 314 subjects who visited the Department of Ophthalmology for a routine check-up or spectacle correction. Inclusion criteria were as follows: absence of systemic illness, intraocular pressure of 21 mmHg or less (measured by Goldmann applanation tonometer), and normal findings on slit lamp examination and fundus examination. Excluded were those with pre-existing oculopathy, corneal diseases like as pannus, pterygium, pinguecula, retinal diseases, a history of previous eye surgery, and use of contact lenses. Refractory error was measured repeatedly three times using an auto-refractive keratometer (RK-F1 Full Auto Ref-Keratometer; Cannon Inc, USA), and the mean value of spherical equivalent was employed. Axial length, corneal curvature, and white-to-white distance (horizontal limbus to limbus distance) were measured using IOL Master (Carl Zeiss, Meditec, Dublin, CA); we excluded from the data any signal noise ratio measurements equal to or less than 100.
For the measurement of corneal endothelial cell density, we used a noncontact specular microscope (Noncon ROBO PACHY SP-9000, Konan Medical Inc, Hyogo, Japan). After taking an automatically focused photo of central cornea, we selected 60 endothelial cells and measured the density in cells/mm2 – the average of two measurements was used for analysis; photos were taken again when cell margins were unclear, or the focus was suboptimal. Stratus Optical Coherence Tomography (Stratus OCT; Carl Zeiss) was used to measure RNFL thickness. In order to hold the macula in place, and limit ocular movements, we examined the eyes in an internal fixation mode. Measurements were analyzed in each of the superior, inferior, nasal, and temporal quadrants, and were included in the study if the signal strength was equal to or more than 6.
Age-related differences in ocular parameters were analyzed using statistical software PASW 17.0 (SPSS, Inc., Chicago, IL, USA). For each parameter, linear regression analysis and Pearson correlation were used, and in order to obtain results corrected for the possible effects of other parameters, multiple regression analysis was performed. P-values of less than 0.05 were considered statistically significant.
Results
The mean age of the subjects was 48.95 years (19 to 82 years), and 169 males and 145 females included. The exact number of the subjects in different age groups is listed in .
Table 1 Demographic data on normal subjects between the mean for each of the six age groups and each of ocular parameters
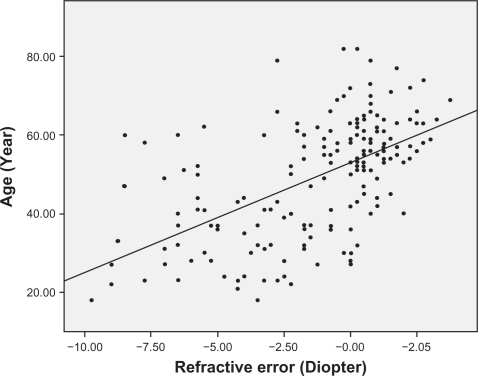
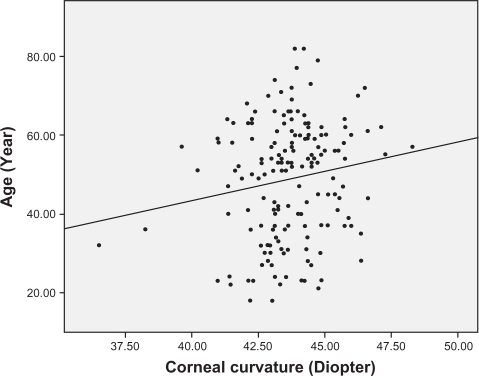
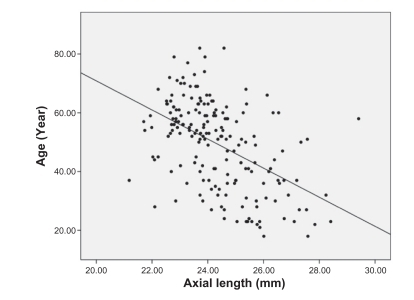
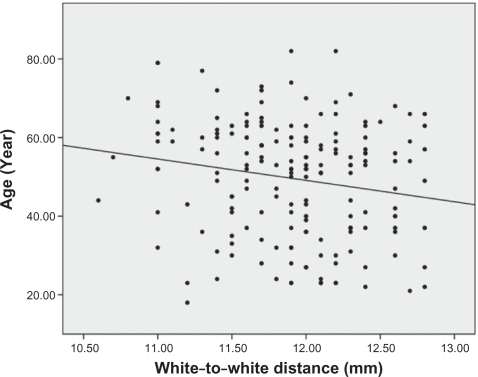
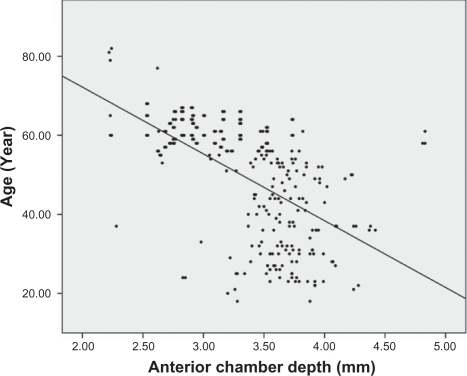
Analysis of the correlations between age and other ocular parameters were below. The mean spherical equivalent of refractive error was −1.38 diopters, and there was a strong positive correlation between hyperopic shift and age (r = 0.553, P < 0.001) (). Corneal curvature (keratometric value) increased with age (r = 0.221, P < 0.001) (), but axial length (r = −0.506, P < 0.001) (), white-to-white distance (r = −0.205, P = 0.001) (), corneal endothelial cell density (r = −0.409, P < 0.001) (), and anterior chamber depth () (r = −0.491, P < 0.001) decreased with age. RNFL thickness was observed to decrease with age in all four quadrants: superior (r = −0.238, P < 0.001), nasal (r = −0.025, P = 0.676), inferior (r = −0.230, P < 0.001), and temporal (r = −0.393, P < 0.001). and show the values between the mean for each of the six age groups and each of ocular parameters.
Figure 5 Corneal endothelial cell density was lower in older patients than in younger patients (r = −0.409, P < 0.001).
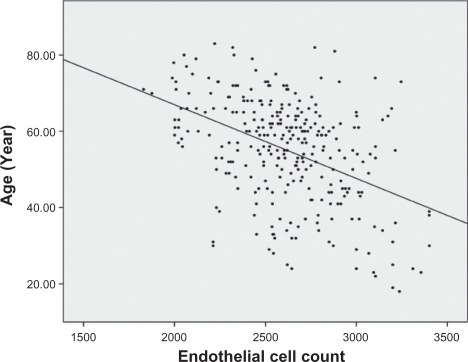
Table 2 Difference in ocular parameters (Mean value) by the mean for each of the six age groups
Table 3 Average of retinal nerve fiber layer (RNFL) thickness in each quadrant (μm) (Mean value) between the mean for each of the six age groups
Analysis of the correlations between the mean for each of the six age groups and each of the ocular parameters determined that all ocular parameters except RNFL thickness in the nasal quadrant had statistically significant correlations with age; subsequent multiple regression analysis found, however, that the changes in just three parameters were statistically significant – the incidence of hyperopia, anterior chamber depth, and reduction of corneal endothelial cell density (P < 0.05) ().
Table 4 Multiple linear regression analysis of the relationship between ocular parameters with age
Discussion
In human eyes, axial length, refractory power of the cornea and lens, and anterior chamber depth are interrelated determinants of optical function. Changes in each of ocular parameters accompany not only various refractory errors but also cataract, glaucoma, retinal disease, and other oculopathies. Therefore, looking into the normal reference values of ocular parameters can provide a basis for recognition of abnormalities and an understanding of the pathological states of various diseases.
Eyes originate from three germ layers: surface ectoderm (including the neural crest), neural ectoderm, and periocular mesenchyme; while corneal epithelium and the lens develop from surface ectoderm; and the retina, the epitheilum of the iris and the ciliary body all arise from neural ectoderm. From the periocular mesenchyme originate the corneal stroma and endothelium, sclera, ciliary muscle, and trabeculae. Among the structures mentioned above, the cornea, iris, lens, and ciliary body all form the anterior segment, and the vitreous, choroid, retina, and optic discs make up the posterior segment. Generally, differentiation of the posterior segment occurs faster at the beginning, but at later stages, the anterior segment tends to develop more rapidly than the posterior.Citation11–Citation12 With the exception of the macula which completes its development six months post-partum, and lens, which continues to change throughout one’s life, each of the ocular structures reach functional maturity prenatally and change size after birth.Citation2
Our study aimed to look at age-related differences in ocular parameters in normal adults that have completed functional and structural maturation. The degree of refractory errors is related to various ocular parameters such as corneal curvature, anterior chamber depth, lens thickness, axial length, and the acquisition and maintenance of normal vision requires a balance between them.Citation13–Citation16 In this study, we were able to observe refractive errors becoming hyperopic with aging; as mentioned above, this may have many causes, but axial length and lens status have been thought to be the main cause. In the study by Grosvenor, axial length showed a decreasing trend after age 20 years, and more specifically, subjects older than 50 years had axial length that was 0.6 mm shorter on average than the subjects aged from 20 to 29 years.Citation14 In our results, the hyperopic tendency in older subjects was statistically significant (P < 0.05) even after axial length and corneal curvature were taken into account. Our analysis of the relationship between aging and axial length revealed a negative correlation (P < 0.001) as shown in many previous studies, but it was not statistically significant (P = 0.700) by multiple linear regression analysis.
White-to-white distance was shorter in older subjects than in younger ones, and the negative correlation with age was statistically significant; this may be due to differences in the size of the eyeball; because axial length can be used as a measure of the eyeball size, the age-related reduction in the axial length may account for shorter white-to-white distance in the older subjects. In addition, clinically speaking, features such as pannus, pterygium, pinguecula, which may affect white-to-white distance, are common in the older population. In this present study, we made efforts to exclude them through anterior segment examination with slit lamp by an expert clinician before registration of subjects, prospectively.
Hayashi et al reported that the degree of both horizontal and vertical corneal curvatures increases with age.Citation15 Also, Kim et al suggested that the degree of anterior corneal curvature starts increasing at 25 years, and more rapidly between the ages of 45 and 54 years.Citation16 In our study, we concluded from correlation analysis that older people have a higher corneal curvature, and this implies that they have reduced corneal curvature diameter and higher corneal refractory power. However, in multiple linear regression analysis, after adjusting for the effects of other parameters, changes in corneal curvature did not have a statistically significant correlation with age (P = 0.418).
The corneal endothelium consists of a single layer of cells, which vary from 4 to 6 μm in thickness and span 20 μm in width, and have a stable and metabolically efficient hexagonal shape. In 1975, Laing et al succeeded in capturing corneal endothelial cells using a specular microscope, and reported in a subsequent study that the corneal endothelial cell count in humans is negatively correlated with age.Citation17 The average corneal endothelial cell density in the Korean population is about 3025/mm2 and has negative correlation with age.Citation18 In the studies mentioned above, the negative correlation between the corneal endothelial cell density and age was very strong (P < 0.001).
In conditions such as glaucoma, changes in RNFL thickness have been observed, and many researchers have attempted to measure the thickness. In the past, histopathological methods were employed to experimentally measure the retinal thickness, and while the advancement of ultrasound technology allowed relatively accurate measurements, resolution was suboptimal. These days, researchers frequently use the recently introduced optical coherence tomography (OCT) to measure retinal thickness as it offers superb resolution and allows tomography. There have been numerous studies around the world on the relationship between aging and RNFL thickness.Citation19 Varma et al used the OCT to measure RNFL thickness in normal Latin American subjects, and reported a higher thickness in young people,Citation20 and Jonas et al in a histological study, reported reduction of 4000–5000 retinal ganglion cells per year of aging.Citation21 In addition, Kang et al reported thinning of macula with aging.Citation22
In this study, although the older subjects had lower RNFL thickness compared to the younger subjects in all four quadrants – superior, inferior, nasal, and temporal – the reduction in the nasal quadrant had no statistical relevance. We found that the superior and inferior quadrants had clearly higher RNFL thickness than the nasal and temporal regions, and according to Hee et al, this is likely due to the path of retinal arcuate fibers which pass superiorly and inferiorly to the fovea.Citation23 Also, on average, the nasal quadrant had a higher RNFL thickness than the temporal quadrant. In our multiple regression analysis, after adjusting for the effects of other parameters, changes in RNFL thickness of all quadrants did not have a statistically significant correlation with age (P > 0.05). This result may be due to not adjusting for the factors associated with aging process (such as ocular media opacity).Citation24–Citation26
Through this study, we aimed to explore the effects of aging on various ocular parameters in a Korean subjects with normal eyes, and we found differences in the parameters between the younger and older groups. Although the results from this study do not differ significantly from previous findings, accounting for the effects of the parameters on each other led to a conclusion that is unique with respect to previous studies: ocular parameters do not change because of one specific cause, but because of the effects they have on each other. In univariate analysis, many parameters correlated with aging, this shorter ocular parameters in older patients could be related to either a cohort effect (with older generations having shorter axial length because of poorer nutrition, general health, or other unknown factors) or to an actual reduction with increasing age. However, in multiple regression analysis, only the hyperopic change of refractive error, anterior chamber depth, and reduction of corneal endothelial cell density were definitely related to age. We think that a cohort effect is a plausible explanation of this result. While we can assume that aging led to changes in the ocular parameters, differences in nutrition, living conditions, and body size of the subjects may have contributed to the variations as well. After growing-up, we think that eyeball may maintain its shape and volume although aging. Corneal curvature, axial length, and limbus to limbus distance don’t change with age, but subjective environmental situations may affect the difference of eyeball size. However, hyperopic change cannot be avoided, probably as a result of lens changes related to aging.
Because of the nature of this study, which was consisted of patients of different age groups during the same time period, we cannot claim that the ocular parameters definitely change with age. Also, the number of subjects was limited, and because we only included those who visited the hospital, our results may differ from that of a population-based study. Therefore, in order to address the aforementioned problems, we think that a prospective cohort study involving a representative sample similar in age and regional distribution is required.
Disclosure
The authors report no conflicts of interest in this work
References
- Ferrer-BlascoTGonzalez-MeijomeJMMontes-MicoRAge-related change in the human visual system and prevalence of refractive conditions in patients attending an eye clinicJ Catarct Refract Surg200834424432
- LeeDYAhnCSStatistical study of the ocular dimensions on refractive errorJ Korean Ophthalmol Soc19943511371146
- SorsbyALearyGAA longitudinal study of refraction and its components during growthRep Ser Med Res Counc (G.B.)1969300141
- ZadnikKMannyREYuJAOcular component data in school children as a function of age and genderOptom Vis Sci20038022623612637834
- SawSMChuaWHGazzardGKohDTanDTStoneRAEye growth changes in myopic children in SingaporeBr J Ophthalmol2005891489149416234459
- LauleACableMKHoffmanCEHannaCEndothelial cell population changes of human cornea during lifeArch Ophthalmol19789620312035718491
- KanamoriAEscanoMFTEnoANEvaluation of the effect of aging on retinal nerve fiber layer thickness measured by Optical Coherence TomographyOphthalmologica200321727327812792133
- KimSDHongSGRefraction of school ageJ Korean Ophthalmol Soc197314341345
- JorgeJAlmeidaJBParafitaMARefractive, biometric and topographic changes among Portuguese university science students: a 3-year longitudinal studyOphthalmic Physiol Opt20072728729417470242
- GudmundsdottirEJonassonFJonssonVfor the Iceland-Japan Co-Working Study Groups“With the rule” astigmatism is not the rule in the elderly. Reykjavik Eye Study: a population based study of refraction and visual acuity in citizens of Reykjavik 50 years and olderActa Ophthalmol Scand20007864264611167223
- GouldDBSmithRSJohnSWAnterior segment development relevant to glaucomaInt J Dev Biol20044810152915558492
- LarsenJSThe sagittal growth of the eye. III: Ultrasonic measurement of the posterior segment (axial length of the vitreous) from birth to pubertyActa Opthalmol197149873886
- TroiloDNeonatal eye growth and emmetropization-a literature reviewEye (Lond)19926Pt 21541601624037
- GrosvenorTReduction in axial length with age: An emmetropizing mechanism for the adult eye?Am J Optom Physiol Opt1987646576633688185
- HayashiKHayashiHHayashiFTopographic analysis of the change in corneal shape due to agingCornea1995145275328536468
- KimSDLeeDSKimJDStudy of the corneal refractive power and axial length of the adult korean eyeballJ Korean Ophthalmol Soc19903113651369
- LaingRASandstromMMBerrospiARLeibowitzHMChange in the corneal endothelium as a function of ageExp Eye Res197622587594776638
- JeongBWParkCKHanHJEndothelial cell density change in the normal Korean cornea during the lifeJ Korean Ophthalmol Soc198829503508
- FunakiSShirakashiMFunakiHYaoedaKAbeHRelationship between age and the thickness of the retinal nerve fiber layer in normal subjectsJpn J Opthalmol1999431805
- VarmaRBazzazSLaiMOptical tomography-measured retinal nerve fiber layer thickness in normal latinosInvest Ophthalmol Vis Sci2003443369337312882783
- JonasJBSchmitdtAMMuller-BerghJAHuman optic nerve fiber count and optic disc sizeInvest Ophthalmol Vis Sci199233201281582806
- KangJHKimSASongWGMacular thickness change with age in normal subjects measured by optical coherence tomographyJ Korean Ophthalmol Soc200445592598
- HeeMRPuliafitoCADukerJSTopography of diabetic macular edema with optical coherence tomographyOphthalmology1998105360709479300
- El-AshryMAppaswamySDeokuleSPagliariniSThe effect of phacoemulsification cataract surgery on the measurement of retinal nerve fiber layer thickness using optical coherence tomographyCurr Eye Res20063140941316714232
- SaviniGZaniniMBarboniPInfluence of pupil size and cataract on retinal nerve fiber layer thickness measurements by Stratus OCTJ Glaucoma20061533634016865012
- YoumDJKimJMParkKHChoiCYThe effect of soft contact lenses during the measurement of retinal nerve fiber layer thickness using optical coherence tomographyCurr Eye Res200934788319172474