Abstract
Purpose:
Compare patient adherence to glaucoma therapy with morning versus evening dosing schedules.
Methods:
Retrospective chart review of 41 consecutive patients who had used the Travatan™ Dosing Aid (Alcon, Fort Worth, TX). Patients had been nonrandomly assigned morning or evening dosing at initiation of usage. Dosing aid data was recorded and analyzed using paired student t-tests where appropriate. Adherence was defined as the dose being administered within 3 hours of the prescribed dosing time.
Results:
Records of 18 patients in the morning group and 23 in the evening group were reviewed. Average time of use was 51.06 ± 4.94 days for the morning group and 50.65 ± 5.38 days for the evening group (p = 0.80). Patients taking the morning dose were less likely to miss an entire day of dosing (days missed in morning group = 3.33 ± 1.33; evening group = 5.87 ± 1.52, p < 0.001). The overall adherence rate was not statistically different between groups (morning group = 82.72 ± 7.32%; evening group = 78.96 ± 6.12%, p = 0.08) although the morning group tended to be more adherent to therapy. Patients in the morning group were also more likely to take their drops later rather than miss the entire day’s dose.
Conclusions:
The rate of adherence for both the morning and evening dosing groups was better than previously reported. Dosing drops in the morning, rather than in the evening, may lead to fewer entirely missed days of glaucoma eye drop therapy.
Introduction
Primary open-angle glaucoma (POAG) is a leading cause of blindness worldwide (CitationQuigley 1996; CitationGoldberg 2000). Current therapy relies heavily on patient cooperation in self-administration of drops that decrease intraocular pressure (CitationTsai and Kanner 2005). It is widely accepted that patient compliance and adherence with prescribed medical regimens can often be suboptimal. Nonadherence to therapy can be secondary to patient failure to take medications, missing dosages, inability to instill drops, failure to persist with usage as well as many other factors (CitationSchartz 2005). Several studies have addressed the issue of adhering to prescribed dosing regimens and report nonadherence rates ranging from 24% to 59% (CitationGurwitz et al 1993; CitationPatel and Spaeth 1995; CitationRotchford and Murphy 1998; CitationTsai et al 2003; CitationSchwartz 2005). Further complicating this problem, many patients self-report adherence at much higher levels than studies suggest. This can lead to disconnect between what the physician might believe is happening and what the patient is actually practicing at home (CitationKass et al 1986).
There have been multiple studies attempting to quantify patient adherence with glaucoma medication use. Self report, dosing aids, and microchips attached to eye drop bottles have all been used (CitationKass et al 1984; CitationRivers 1992). Each technique has its own positive and negative attributes with self-reporting relying on patient memory/perception and electronic devices assumed to be fail-proof. Recently, a new electronic dosing aid was introduced which acts as both a dosing monitor as well as compliance aid (CitationBoden et al 2006). The Travatan™ Dosing Aid (TDA) (Alcon, Fort Worth, TX) is a portable electronic device specifically designed to assist patients in dosing travoprost (Travatan®, Alcon).
Each TDA is preprogrammed with patient information, preferred dosing time, and reminder prompts that can be specifically altered by the prescribing physician for certain times of the day depending on patient needs and daily habits. The device also records the number and times the drop administration lever is depressed as a patient instills travoprost. In this study we use the TDA device to investigate the effect of dosing time on patient adherence to glaucoma therapy.
Methods
This was a retrospective nonrandomized consecutive case series of patients placed on glaucoma monotherapy using the TDA. Institutional Review Board approval was obtained for this retrospective review. Forty-one total participants who had been given the TDA were identified and data was collected from each device for review. Demographic information regarding age and diagnosis was also collected.
Patients had been assigned to administer their once daily medication either in the morning (5–10 am) or the evening (6–11 pm) based on their own preference. The dosing regimen for each patient consisted solely of one drop of travoprost in each treated eye administered either in the morning (18 patients) or at night (23 patients).
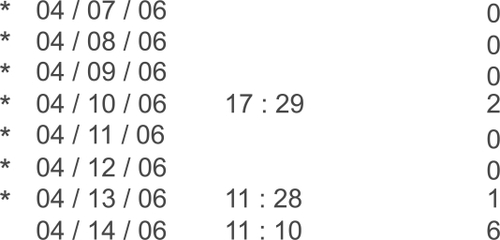
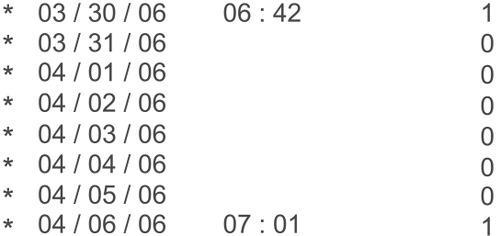
In this study, nonadherence as determined by the dosing device was defined in multiple ways. Days on which the patient completely failed to take the medication, failed to take the medication within the preprogrammed time frame (three hours before or after the designated dosing time), or over-medicated were considered nonadherent days. The TDA automatically marks a failure to take the medication as failure to adhere. Taking the medication outside the designated time frame is also noted as a nonadherence event and marked with a star on the printout (). Multiple dosing events on the same day could still be marked as adherent as long as one drop is taken at the designated time. Days missed are marked as a ‘zero’ on the TDA print out and are easily identifiable as dosing failures. The percentage of days in which the patient adhered to the regimen was recorded and compared between the two groups. Statistical analysis using paired t-tests was performed.
Figure 1 The Travatan™ Dosing Aid printout is divided in to three columns that include date, time, and number of lever depressions. In this case, the patient appears to have not dosed drops on most days as indicated by multiple ‘zeros’ in the right hand column. The star denotes nonadherence to programmed regimen.
Results
The mean age for each group, morning (66.94 ± 9.36) and evening (69.52 ± 8.72) was similar (p = 0.88). There were 16 POAG patients and two low-tension glaucoma (LTG) in the morning group and 17 POAG, three LTG, one congenital glaucoma, and two inflammatory glaucoma patients in the evening group. The duration of TDA usage was similar for the morning group (51.06 ± 4.94 days) and the evening group (50.65 ± 5.38 days). The differences were not statistically significant (p = 0.80).
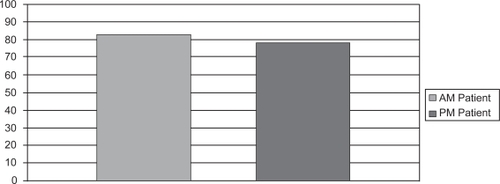
Patients who were taking the morning dose were less likely than the evening dosing patients to miss an entire day of dosing () and this difference was statistically significant (p < 0.001). The percent adherence, on the other hand, which included both dosing misses or an improper dosing event was higher for morning patients compared with evening patients (). However, this difference was not statistically significant between the two groups (p = 0.08).
Figure 2 Mean days missed for those dosing travoprost in the morning (3.33 ± 1.33) compared with evening (5.87 ± 1.52) were statistically significant (p < 0.001).
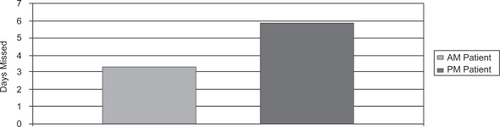
Figure 3 Mean percent adherence of morning (82.72 ± 7.32) and evening (78.96 ± 6.12) dosing groups were similar (p = 0.08).
Patients in the morning group were more likely to take their drops past the 3-hour dosing window preprogrammed into the TDA rather than miss a dose entirely. The majority (70%) of patients in the morning group who missed the 3-hour dosing window took their drops within 6 hours of the dosing period while the remainder did not to take their drops on that given day. Twenty-eight percent of patients in the morning group and 35% of patients in the evening group appeared to over-medicate on the day of or night prior to clinical examination (). Discussions regarding over-medicating with these patients revealed that most believed it would improve their results upon intraocular pressure (IOP) measurement. It is also possible that, in some of these patients, the lever was depressed multiple times during transport to clinic. None of the patients included in this review were 100% adherent to the prescribed regimen of drop therapy.
Discussion
The issue of poor adherence to prescribed medication regimens crosses into all fields of medicine. A meta-analysis of 569 studies across multiple specialties found an average nonadherence rate of 25% (CitationDiMatteo 2004). Chronic diseases that are asymptomatic in their early stages, such as diabetes and hypertension, often are accompanied by patient nonadherence to prescribed medication regimens (CitationDegli Esposti et al 2002a, 2002b; CitationHertz et al 2005). Glaucoma is a chronic disease similar to these, in that initially, the patient does not note any symptoms and is less likely to be concerned with taking medications if not properly motivated (CitationZimmerman and Zalta 1983; CitationWeinreb 1992; CitationTsai et al 2003). The majority of patients with glaucoma are managed initially with medical therapy, which has been proven to prevent or delay progression of nerve fiber layer loss and visual field deterioration (CitationKass et al 2002). This highlights the importance of studying patient compliance and adherence to better understand how to improve quality of care and decrease the chance of disease progression.
One unique problem with glaucoma therapy is the difficulty that some individuals have with instilling eye drops into their own eyes. There have been multiple reports on devices that assist the patient in instillation of drops as well as instruments which monitor and record compliance data. Assistive devices are particularly useful in patients who suffer with dexterity problems or arthritis. Several devices, such as the Easidrop® (Vidcom Marketing, London, UK), Owen-Mumford Autodrop® (Marietta, GA), and Opticare® (Cameron-Graham Ltd., Huddersfield, UK), have been designed to help patients with proper drop installation and have been met with varying degrees of success (CitationRivers 1992). The design of many glaucoma drop bottles, often slender and small, makes it harder for some elderly patients to grasp and squeeze in a controlled manner. The lever design of the TDA may allow for some patients to overcome dexterity and/or strength limitations that effect their ability to instill eye medications.
Electronic monitoring of eye drops is currently viewed as the most objective, albeit indirect, measure of a patient’s adherence to their medical regimen (CitationSchwartz 2005). Past attempts at electronic monitoring included the development of an eye drop medication monitor that electronically recorded the date and time of each drop administration over a six-week period (CitationKass et al 1984). An electronic time stamp was recorded when the cap was removed followed by inversion of the bottle. The electronic attachment, housed inside the bottle, was found to monitor accurately when tested against known medication regimens. The data collecting accuracy of the dosing device used in the current study has been validated, both through published studies and within our own clinical experience, and appears to be accurate and consistent in measuring drop installation when used correctly (CitationBoden et al 2006; CitationFriedman et al 2007).
A study by CitationLaster and colleagues (1996) investigated the use of an electronic medication alarm device (Prescript TimeCap™, Wheaton Medical Technologies, Inc., Millville, NJ) to enhance compliance in glaucoma patients. Thirteen patients, all on pilocarpine, were selected for the study and followed for a period of time while using the alarm device and when not using the device. They estimated compliance by the amount of pilocarpine used and by patient questionnaire. They found that, while using the alarm device, patients were more likely to take their medication as measured by amount of pilocarpine used. In addition, all participants reported that the alarm device helped them remember to take their medication and was easy to use. The investigators concluded that the alarm device was useful in improving compliance.
Another commonly cited reason for noncompliance is the difficulty of remembering to use drops at night prior to sleeping. Many of our patients have complained that they fall asleep or are too tired to place drops with the more common nighttime dosing regimen. Several of our patients work late shifts and find it difficult to place drops in during work hours. For these patients, we often recommend morning dosing in association with a commonly held ritual such as eating breakfast or brushing teeth. The current study was designed to identify any differences in compliance between patients who take their drops in the evening versus those who take drops in the morning.
Once concern about morning dosing is that the prostaglandin analogs may be less effective compared with evening dosing. CitationKonstas and colleagues (2006) studied this question and found that both morning and evening dosing of travoprost provided effective 24-hour IOP control, with evening dosing of travoprost demonstrating slightly greater daytime efficacy and a narrower range of 24-hour pressure while mean IOP was essentially the same in both groups. Although diurnal fluctuations do appear to be slightly less with evening dosing in a controlled study situation, this difference is irrelevant if a patient fails to take the medication as prescribed due to external factors such as lifestyle or working hours.
The electronic time stamp data in the current study demonstrates that patients using travoprost once daily in the morning tended to be more likely to remain adherent to prescribed medical regimen compared with a cohort of patients taking the same medication at night. While the difference in adherence was not statistically significant, it does highlight two important facts. First, patients appear to have an equal chance of being adherent to drop therapy regardless of time of administration. Second, it is important to address patient needs through discussion and written instructions to better plan therapeutic regimens to fit within individual daily routines (CitationKharod et al 2006). Those who took their drops in the morning were less likely to be nonadherent due to missing a drop while slightly more likely to be nonadherent due to taking the drop at a later time than preprogrammed in to the TDA. This issue could be remedied by altering dosing regimens even further based on real world dosing data.
Finally, the adherence rate in both groups in this study was near 80%. This value is higher than those reported in previous manuscripts dealing with adherence in glaucoma therapy. This may indicate that the use of the TDA improves adherence to medical therapy by fulfilling its intended role as both a dosing aid and adherence device regardless of dosing time. Further testing is needed in a prospective manner to investigate the long-term effect of dosing aids on patient eye drop administration habits and potential impact on therapeutic outcomes.
Disclosures
Both authors have received speaking honoraria from Alcon. No financial support was received for work covered in this manuscript.
References
- BodenCSitAWeinrebRN2006Accuracy of an electronic monitoring and reminder device for use with travoprost eye dropsJ Glaucoma15303416378015
- Degli EspostiESturaniADi MartinoM2002Long-term persistence with antihypertensive drugs in new patientsJ Hum Hypertens164394412037702
- Degli EspostiLDegli EspostiEValpianiG2002A retrospective, population based analysis of persistence with antihypertensive drug therapy in primary care practice in ItalyClin Ther2413475712240784
- DiMatteoMR2004Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of researchMed Care42200915076819
- FriedmanDSJampelHDCongdonNG2007The TRAVATAN Dosing Aid accurately records when drops are takenAm J Ophthalmol14369970117386285
- GoldbergI2000How common is glaucoma worldwide?WeinrebRNKitazawaYKrieglsteinGGlaucoma in the 21st CenturyLondonHarcourt Health Communications38
- GurwitzJHGlynnRJMonaneM1993Treatment for glaucoma: adherence for the elderlyAm J Public Health83711168484454
- HertzRPUngerANLustikMB2005Adherence with pharmacotherapy for type 2 diabetes: a retrospective cohort study of adults with employer-sponsored health insuranceClin Ther2710647316154485
- KassMAGordonMMeltzerDW1986Can ophthalmologists correctly identify patients defaulting from pilocarpine therapy?Am J Ophthalmol101524303706456
- KassMAHeuerDKHigginbothamEJ2002The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucomaArch Ophthalmol1207011312049574
- KassMAMeltzerDWGordonM1984A miniature compliance monitor for eyedrop medicationArch Ophthalmol102155046385936
- KharodBVJohnsonPBNestiHA2006Effect of written instructions on accuracy of self-reporting medication regimen in glaucoma patientsJ Glaucoma15244716778648
- KonstasAGMikropoulosDKaltsosK200624-hour intraocular pressure control obtained with evening-versus morning-dosed travoprost in primary open-angle glaucomaOphthalmology1134465016513458
- LasterSFMartinJLFlemingJB1996The effect of a medication alarm device on patient compliance with topical pilocarpineJ Am Optom Assoc6765488979657
- PatelSCSpaethGL1995Compliance in patients prescribed eyedrops for glaucomaOphthalmic Surg2623367651690
- QuigleyHA1996Number of people with glaucoma worldwide. 1996Br J Ophthalmol80389938695555
- RiversPH1992Compliance aids—do they work?Drugs Aging2103111596593
- RotchfordAPMurphyKM1998Compliance with timolol treatment in glaucomaEye1223469683946
- SchwartzGF2005Compliance and persistency in glaucoma follow-up treatmentCurr Opin Ophthalmol161142115744142
- TsaiJCKannerEM2005Current and emerging medical therapies for glaucomaExpert Opin Emerging Drugs1010918
- TsaiJCMcClureCARamosSE2003Compliance barriers in glaucoma: a systematic classificationJ Glaucoma12393814520147
- WeinrebRN1992Compliance with medical treatment of glaucomaJ Glaucoma11348
- ZimmermanTJZaltaAH1983Facilitating patient compliance in glaucoma therapySurv Ophthalmol28Suppl25286665702