Abstract
Pathological changes in striate (B17, V1) and extrastriate (B18, V2) visual cortex were studied in variant Creutzfeldt-Jakob disease (vCJD). No differences in densities of vacuoles or surviving neurons were observed in B17 and B18 but densities of glial cell nuclei and deposits of prion protein (PrP) were greater in B18. PrP deposit densities in B17 and B18 were positively correlated. Diffuse deposit density in B17 was negatively correlated with the density of surviving neurons in B18. The vacuoles either exhibited a density peak in laminae II/III and V/VI or were more uniformly distributed across the laminae. Diffuse deposits were most frequent in laminae II/III and florid deposits more generally distributed. In B18, the surviving neurons were more consistently bimodally distributed and the glial cell nuclei most abundant in laminae V/VI than in B17. Hence, both striate and extrastriate visual cortex is affected by the pathology of vCJD, the pathological changes being most severe in B18. Neuronal degeneration in B18 appears to be associated with diffuse PrP deposit formation in B17. These data suggest that the short cortico-cortical connections between B17 and B18 and the pathways to subcortical visual areas are compromised in vCJD.
Introduction
Visual signs and symptoms are commonly observed in a significant number of patients with Creutzfeldt-Jakob disease (CJD). These include poor visual acuity, visual field defects, eye movement problems, nystagmus, and visual hallucinations (CitationPurvin et al 1989; CitationAguglia et al 1991; CitationGrant et al 1993; CitationSatish-Chandra et al 1996; CitationDe Seze et al 1998; CitationKropp et al 1999; CitationLueck et al 2000; CitationArmstrong 2006). Visual symptoms, such as hemianopias, may be the presenting feature or may develop at some stage during the disease.
The most obvious pathological signs in the brains of patients with CJD observed post-mortem are the presence of vacuolation (‘spongiform change’), neuronal loss, astrocytosis, and the presence of activated microglia (CitationMasters and Richardson 1978; CitationMizutani 1981; Sasaki et al 1981). In addition, there are deposits of a protease resistant form of prion protein (PrPsc), an abnormal structural conformation of normal PrP (PrPc). PrPsc is deposited in the extracellular space in the form of discrete protein aggregates or plaques (CitationWatanabe and Duchen 1993). These pathological changes are often evident within the visual cortex of patients with CJD and most consistently in the striate cortex (B17, V1) (CitationArmstrong 2003) and may provide the substrate for the observed visual symptoms.
A previous study of cases of sporadic CJD (sCJD), the commonest form of the disease, demonstrated that B17 was one of the most severely affected areas of the cerebral cortex (CitationArmstrong 2003). Vacuolation was most frequent in lamina III, PrP deposition in laminae II/III, while reactive glial cells were most abundant in laminae V/VI. It was concluded that pathological changes in B17 in sCJD could affect the transmission of visual information from B17 to other cortical and subcortical visual areas and could be a factor in the development of many of the visual symptoms seen in sCJD (CitationArmstrong 2003). Variant CJD (vCJD) is a new subtype of the disease first described in the UK in 1996 and believed to be the human form of bovine spongiform encephalopathy (BSE) (CitationWill et al 1996). There are distinct differences between vCJD and the sporadic form of the disease; vCJD having an earlier age at onset, longer duration of disease, a psychiatric presentation, and extensive deposition of florid-type PrP deposits in the brain (CitationIronside 2000). There may also be differences in the visual signs and symptoms in the two forms of CJD; diplopia, supranuclear palsies, visual hallucinations, and cortical blindness being particularly common in vCJD while saccadic and smooth pursuit movements are more common in sCJD (CitationLueck 2000).
To explain the development of visual symptoms in CJD and the differences between subtypes of the disease will require pathological studies of the different visual areas. Hence, the present study compared the densities of the vacuoles, surviving neurons, PrP deposits, and glial cell nuclei in striate and extrastriate visual cortex in eleven cases of vCJD. The specific objectives were: 1) to quantify the pathological features, 2) to study the correlations between the densities of the pathological changes both within and between visual areas, and 3) to determine the laminar distribution of the pathological changes.
Materials and methods
Cases
Eleven cases (details in ) of vCJD were obtained from the CJD Surveillance Unit, Western General Hospital, Edinburgh, UK and studied at the Brain Bank, Department of Neuropathology, Institute of Psychiatry, King’s College London, UK. The principles embodied in the 1975 Helsinki declaration were followed with respect to experiments involving material of human origin. All cases fulfilled the criteria for the pathological diagnosis of vCJD (CitationIronside et al 2000). None of the cases had any of the known mutations of the PrP gene or family history of prion disease, and there was no evidence of the common types of iatrogenic aetiology. The pattern of PrP deposition typical of vCJD was observed in all cases with florid-type plaques in the cerebral cortex, cerebellum, basal ganglia, thalamus, and brain stem.
Table 1 General clinical and pathological features of variant Creutzfeldt-Jakob disease (vCJD) cases studied
Histological methods
A block of the calcarine sulcus was taken at a distance of 2.5 cm from the occipital pole. Tissue was fixed in 10% phosphate buffered formal-saline and embedded in paraffin wax. Coronal 7 μm sections were stained with hematoxylin and eosin (H/E) while adjacent sections were immunostained against PrP using the monoclonal antibody 12F10 (dilution 1:250) which binds to a region of human PrP downstream of the neurotoxic domain adjacent to helix region 2: residues 142–160 (CitationKrasemann et al 1996) (kindly provided by Prof. G. Hunsmann, The German Primate Centre, Gottingen, Germany). Immunoreactivity was enhanced by formic acid (98% for 5 minutes) and autoclaving (121 °C for 10 minutes) pretreatment. Sections were treated with Dako Biotinylated Rabbit anti-Mouse (RAM) (dilution 1:100) and Dako ABComplex HRP kit for 45 minutes (Amersham, UK). Diaminobenzidine tetrahydrochloride was used as the chromogen. Immunostained sections were counterstained with haematoxylin for 1 minute to reveal the neuronal cell bodies and glial cell nuclei (CitationArmstrong 1996a).
Lesion densities
Identification of visual areas was based on Brodmann’s cortical map. Area B17 has well-defined boundaries although the detailed architecture is not uniform throughout its length. B17 is typical of granular cortex in which the laminar pattern is partially obscured because of the high density of granule cells present. It can be identified by the presence of the Band of Gennari and by the cell poor sublamina IVB (CitationClarke and Miklossy 1990). Area B18 occupies a ‘horseshoe’ shaped region surrounding B17 and has a clearer six-layered structure in which layer III is broader than IV, the latter with a high density of small pyramidal cells (CitationClarke and Miklossy 1990). In addition, B18 can be identified by the large pyramidal cells in the deeper parts of lamina III (CitationClarke and Miklossy 1990). The boundary between B18 and B19 is more difficult to detect as microscopic changes occur more gradually. Hence to delimitate area B18, the position of B19 was determined approximately by comparison of the morphology of the occipital cortex with Brodmann’s cortical maps (CitationClarke and Miklossy 1990).
The densities of the vacuoles, surviving neurons, glial cell nuclei, and the degree of PrP deposition were determined in areas B17 and B18 of each case. In each area, strips of tissue (3200 to 48090 μm in length) were sampled using 50 × 250 μm contiguous sample fields. A micrometer eyepiece with 100 horizontal divisions was used as the sample field. The sample fields were located in the upper and lower cortex approximately within laminae III and V; the short edge of the sample field was aligned with guidelines marked on the slide and located parallel to the pia mater. All vacuoles greater than 5 μm in diameter were counted in each sample field. Neurons were identified as cells containing at least some stained cytoplasm in combination with larger shape and non-spherical outline (CitationArmstrong 1996a). Small spherical or asymmetrical nuclei without cytoplasm but with the presence of a thicker nuclear membrane and more heterogeneous chromatin were regarded as glial cells. Two morphological types of PrPsc deposit are present in vCJD (CitationArmstrong et al 2003), viz., florid deposits which consist of a small dense core of PrPsc and are strongly stained with antibodies raised to PrP and the more lightly stained irregularly shaped ‘diffuse deposits’. Differences in the densities of each histological feature in B17 and B18 were tested using Students ‘t’ test. The correlations between the densities of the histological features both within and between visual areas were tested using Pearson’s correlation coefficient (‘r’) (CitationArmstrong et al 1990).
Laminar distribution of pathological changes
The laminar distribution of the pathological changes across the cortex was studied in B17 and B18 of each case using methods based on those of CitationDuyckaerts and colleagues (1986). Five traverses from the pia mater to white matter were located at random within each visual area. Lesions were counted manually in 50 × 250 μm sample fields, the larger dimension of the field being located parallel with the surface of the pia mater. The eye piece micrometer was moved down each traverse one step at a time using histological features to correctly position the field. Depending on the degree of occipital lobe atrophy, between 20 and 35 sample fields were necessary to sample each traverse. Counts from the five traverses were added together to study the laminar distribution of lesions within each area.
Variations in lesion density with distance below the pia mater were analyzed using a curve fitting procedure. For each area, a linear, quadratic, cubic, and quartic polynomial was fitted successively to the data. At each stage, the goodness of fit of the curve to the data was tested using correlation methods and analysis of variance. A more complex curve was accepted as a better fit to the data if it resulted in a significant increase in Pearson’s correlation coefficient (‘r’) and a significant reduction in the residual sums of squares compared with the preceding curves (CitationSnedecor and Cochran 1980). Where a significant relationship between density and distance from the pia mater was established, the lamina in which the maximum density of each pathological feature occurred was determined. The boundary between laminae I and II and the location of lamina IV were determined from the tissue sections. The location of the II/III and V/VI boundaries is more difficult to establish especially in B17 (CitationArmstrong 1996b). Hence, the approximate location of these boundaries was estimated based on the laminar profiles of the visual areas given in CitationBrodal (1981) and CitationClarke and Miklossy (1990).
Results
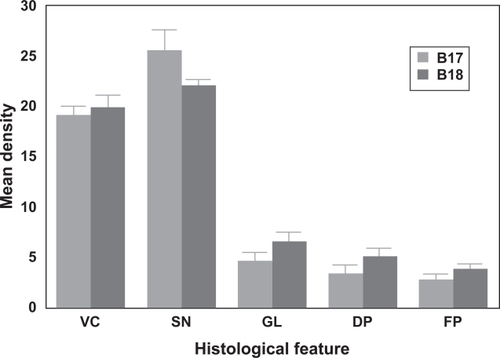
The mean density of each histological feature in areas B17 and B18 is shown in . There were no significant differences in the density of the vacuolation (t = 0.59, P > 0.05) or surviving neurons (t = 1.86, P > 0.05) between visual areas. However, the densities of the glial cell nuclei (t = 2.88, P < 0.02) and the diffuse (t = 2.71, P < 0.050 and florid (t = 5.97. P < 0.001) PrP deposits were significantly greater in B18 than B17.
Figure 1 Mean densities (per 50 × 250 μm sample field) of the vacuolation (VC), surviving neurons (SN), glial cell (GL) nuclei, diffuse prion protein (PrP) deposition (DP), and florid PrP (FP) deposition in visual areas B17 and B18 of 11 cases of variant Creutzfeldt-Jakob disease (vCJD).
Correlations between the densities of the histological features both within and between areas are shown in . Within each visual area, the only features that were mutually correlated were the densities of the diffuse and florid PrP deposits (B17 r = 0.80, P < 0.01; B18 r = 0.74, P < 0.01). Several correlations, however, were observed between the densities of features in B17 with those in B18. The densities of the diffuse deposits (r = 0.73, P < 0.05), florid deposits (r = 0.84, P < 0.01), and the glial cell nuclei (r = 0.72, P < 0.05) in B17 were positively correlated with their corresponding densities in B18. In addition, there was a negative correlation between the density of the vacuolation in B17 and the diffuse deposits in B18 (r = −0.68, P < 0.05) and between the diffuse deposits in B17 and the density of the surviving neurons in B18 (r = −0.62, P < 0.05).
Table 2 Correlation matrix (Pearson’s ‘r’) between the densities of pathological features in areas B17 and B18 of the visual cortex in patients with variant Creutzfeldt-Jakob disease (vCJD), (Correlation significant at * P < 0.05, ** P < 0.01)
Examples of the changes in the density of pathological features across the cortex in area B17 of a single case (Case E) are shown in . Changes in vacuolation were fitted by a fourth-order polynomial (r = 0.75, P < 0.01); suggesting a bimodal distribution, density reaching a maximum in the upper and lower cortical laminae. By contrast, the densities of the florid PrP deposits did not vary significantly with distance below the pia mater in this case. A summary of the laminar distributions exhibited by each histological feature in all vCJD cases studied is shown in . There were no significant differences between the laminar distributions of the vacuoles in B17 and B18 (χ2 = 1.09, P > 0.05). The vacuoles exhibited either a bimodal distribution with peaks of density in laminae II/III or V/VI or were present in all laminae with the exception of lamina I and a region of lamina VI immediately adjacent to the white matter. The laminar distribution of the surviving neurons varied significantly between visual areas. In B17, the surviving neurons were more evenly distributed across the cortex and were less often bimodally distributed than in B18. In B18 of three cases, neurons were uniformly distributed across the cortex. In addition, the glial cell nuclei were more consistently distributed in laminae V/VI in B18. The laminar distributions of the diffuse and florid PrP deposits were similar in both visual areas, the diffuse deposits being more frequently distributed in laminae II/III while the florid deposits were more generally distributed.
Figure 2 Laminar distribution of the vacuolation and florid PrP deposits in area B17 in a case of variant Creutzfeldt-Jakob disease (vCJD).
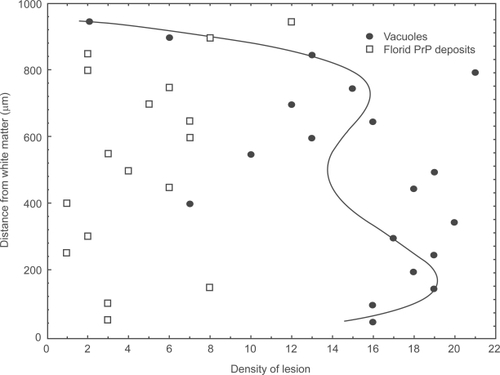
Table 3 The laminar distributions shown by the pathological features in areas B17 and B18 in cases of variant Creutzfeldt-Jakob disease (vCJD). Data are the number of cases (out of eleven) exhibiting a particular type of laminar distribution
Discussion
Similarities and differences were observed in the pathology of striate and extrastriate visual cortex in vCJD. The densities of the vacuoles and surviving neurons were similar in both visual areas but there were greater numbers of PrP deposits and glial cell nuclei in B18. Hence, both striate and extrastriate visual cortex is affected in vCJD with the pathological changes being more severe in B18. A similar pattern of pathology is seen in patients with Alzheimer’s disease (AD) in which B18 is more severely affected than B17 (CitationArmstrong et al 1990).
The laminar distributions of the vacuolation and PrP deposits were similar in B17 and B18 but there were differences in the distributions of the surviving neurons and glial cell nuclei. In B17, surviving neurons exhibited either a bimodal distribution or were distributed more evenly across the cortex while in B18, neurons were bimodally distributed in 8 cases and uniformly distributed in 3 cases. In normal brain, surviving neurons are often bimodally distributed with peaks of density in laminae II/III and V/VI. Lack of these peaks in individual cases suggests specific losses of neurons in these laminae. In addition, glial cell nuclei were distributed more consistently in laminae V/VI in area B18 while distributions in B17 were more variable. Since reactive gliosis is a response to neuronal loss, this result suggests that neurons are lost more consistently in the lower laminae in B18 while the pattern of neuronal loss in B17 is more variable.
Significant correlations were observed between the densities of pathological features both within and between visual areas. The density of the diffuse PrP deposits in B17 was negatively correlated with the density of surviving neurons in B18. These results suggest either that cell losses in B18 may have resulted in the formation of PrP deposits in B17 or that PrP deposition in B17 may have been responsible for the cell losses in B18. Areas B17 and B18 are reciprocally connected via the short cortico-cortical projections (CitationDeLacoste and White 1993). The feedforward cortico-cortical projections originate in lamina I–III in B17 and terminate in lamina IV of B18, while the feedback connections originate in laminae V and VI and a lesser extent in lamina III in B18 and terminate in lamina I in B17. In some cases, the diffuse deposits in B17 were distributed in laminae II/III while the surviving neurons had a bimodal distribution in B18 with peaks in the upper and lower laminae. Hence, the bimodal distribution of the surviving neurons in B18 could reflect in part neuronal losses in lamina IV associated with the development of diffuse PrP deposits in B17 and the loss of the feedforward cortico-cortical projections. These results provide further support for the hypothesis that the pathology associated with prion disease spreads from one cortical area to another (CitationArmstrong et al 2001).
Visual information arrives in the striate cortex from the lateral geniculate nucleus (LGN) terminating in lamina IVa and IVb (CitationSinger 1979) and is then conveyed to laminae II and III and to a lesser extent lamina V. The output from B17 to B18 is mainly via the large pyramidal neurons in laminae II/III which constitute the feedforward cortico-cortical projections (CitationDeLacoste and White 1993). In addition, there is a connection from B17 originating in lamina IVb to the medial temporal area (MT or V5) which is crucial for the analysis of motion in the visual field (CitationZeki and Shipp 1988). V5 also receives a projection from the deep part of lamina III originating in B18 (CitationShip and Zeki 1989). In B17, there was a significant laminar degeneration associated with a diffuse or bimodal distribution of the vacuolation. In addition, the diffuse PrP deposits were present mainly in laminae II/III while the florid deposits were more generally distributed down the cortex. These pathological changes are likely to affect visual processing within the upper laminae in B17 and subsequently, the transfer of information to B18. In addition, the pathological changes in the lower laminae could affect the neurons providing the output to subcortical visual areas, eg, from lamina VI to the dorsal and ventral LGN and from lamina V to the superior colliculus and the pretectal area (CitationParnavelas and McDonald 1983). A similar distribution of the pathological changes is present in B18 with the exception that neuronal losses and the glial cell reaction are more marked in the lower laminae. These results suggest that the short cortico-cortical pathways connecting B17 and B18 and the output from these areas to subcortical visual areas are likely to be compromised in vCJD. By contrast, the long cortico-cortical pathway from B17 to V5 is less likely to be affected although there is some evidence that the projection from B18 to this area may be compromised.
Pathological changes in striate and extrastriate visual cortex may contribute to several of the visual problems identified in patients with vCJD and explain some of the differences between vCJD and sCJD (CitationArmstrong 2003). For example, homonymous visual field defects appear to be more characteristic of sCJD than vCJD (CitationVargas et al 1995) and could be attributable to the more localised and less diffuse pathological changes in B17 in sCJD (CitationArmstrong 2003). Supranuclear palsies, by contrast, appear to be more characteristic of vCJD (CitationLueck 2000; CitationLueck et al 2000) and could be a consequence of the more extensive pathology in area B17 affecting the corticofugal fibres from B17 to the superior colliculus and pretectal areas. Cell losses affecting the output pathways from B17 to the subcortical visual areas could be a contributory factor in the development of oculomotor signs in vCJD. Similarly, visual hallucinations (CitationSatish-Chandra et al 1996) and cortical blindness appear to be more common in vCJD and could reflect the more diffuse and extensive pathology in B17, B18, and associated visual areas in vCJD.
Acknowledgements
The assistance of the CJD Surveillance Unit in providing cases for this study and the Brain Bank, Institute of Psychiatry, King’s College London for preparing the tissue sections is gratefully acknowledged.
References
- AgugliaVGambarelliDFarnarierG1991Different susceptibilites of the geniculate and extrageniculate visual pathways to human Creutzfeldt-Jakob diseaseElectroenceph Clin Neurophysiol784134231712277
- ArmstrongRA1996aCorrelations between the morphology of diffuse and primitive β-amyloid (Aβ) deposits and the frequency of associated cells in Down’s syndromeNeuropath Appl Neurobiol22527530
- ArmstrongRA1996bVisual field defects in Alzheimer’s disease patients may reflect differential pathology in the primary visual cortexOpt Vis Sci73677682
- ArmstrongRA2003Pathological changes in the primary visual cortex (area V1) in sporadic Creutzfeldt-Jakob disease (sCJD)Opt Vis Sci80298304
- ArmstrongRA2006Creutzfeldt-Jakob disease and visionClin Exp Optom8931116430434
- ArmstrongRANochlinDSumiSM1990Neuropathological changes in the visual cortex in Alzheimer’s diseaseNeurosci Res Commun6163171
- ArmstrongRALantosPLCairnsNJ2001The spatial patterns of prion protein deposits in patients with sporadic Creutzfeldt-Jakob disease: Comparison with β-amyloid deposits in Alzheimer’s diseaseNeurosci Lett298535611154834
- ArmstrongRALantosPLIronsideJW2003Differences in the density and spatial distribution of florid and diffuse plaque plaques in variant Creutzfeldt-Jakob diseaseClin Neuropathol2220921414531544
- BrodalA1981Neurological anatomyOxfordOxford Univ Pr
- ClarkeSMiklossyJ1990Occipital cortex in man: organization of callosal connections, related myelo- and cytoarchitecture and putative boundaries of functional visual areasJ Comp Neurol2981882142212102
- De LacosteMWhiteCLIII1993The role of cortical connectivity in Alzheimer’s disease pathogenesis: a review and model systemNeurobiol Aging141168450928
- De SezeJHacheJCVermerschP1998Neurophysiologic visual impairmentsNeurology519629679781513
- DuyckaertsCHauwJJBastenaireF1986Laminar distribution of neocortical senile plaques in senile dementia of the Alzheimer typeActa Neuropathol702492563766125
- GrantMPCohenMPetersenRB1993Abnormal eye-movements in Creutzfeldt-Jakob diseaseAnn Neurol341921978338343
- IronsideJW2000Pathology of variant Creutzfeldt-Jakob diseaseArch Virol Supp16143151
- IronsideJWHeadMWBellJE2000Laboratory diagnosis of variant Creutzfeldt-Jakob diseaseHistopathol3719
- KrasemannSGroschupMHHarmeyerS1996Generation of monoclonal antibodies against human prion proteins in PrPO/O miceMole Med2725734
- KroppSSchultz-SchaefferWJFinkenstaedtM1999The Heidenhain variant of Creutzfeldt-Jakob diseaseArch Neurol5655619923761
- LueckCJ2000Ocular features of CJDOptician2202327
- LueckCJMcIlwaineGGZeidlerM2000Creutzfeldt-Jakob disease and the eye. II. Ophthalmic and neuro-ophthalmic featuresEye1429130111026988
- MastersCLRichardsonEP1978Subacute spongiform encephalopathy (Creutzfeldt-Jakob disease): the nature and progression of spongiform changeBrain101333344352478
- MizutaniT1981Neuropathology of Creutzfeldt-Jakob disease in Japan: with special reference to the panencephalopathic typeActa Pathol Jpn319039227032195
- ParnavelasJGMcDonaldJK1983The cerebral cortexEmsonPCChemical neuroanatomyNew YorkRaven Press337358
- PurvinVBonninJGoodmanJ1989Palinopsia as a presenting manifestation of Creutzfeldt-Jakob diseaseNeuro-Ophthalmol9242246
- SasakiAHiratoJNakajatoY1993Immunohistochemical study of microglia in the Creutzfeldt-Jakob diseased brainActa Neuropathol863373448256583
- Satish-ChandraPSharmaPChatterjiS1996Psychiatric manifestations of Creutzfeldt-Jakob disease: Probable neuropathological correlatesNeurology India444346
- ShippSZekiS1989The organization of connections between areas V5 and V2 in macaque monkey visual cortexEur J Neurosci133335412106143
- SingerW1979Central-core control of visual cortex functionsSchmittFOWordenFGThe Neurosciences: 4th Study ProgramCambridgeMIT Pr
- SnedecorGWCochranWG1980Statistical methods7th EdIowa State Univ PrAmes, Iowa, USA
- VargasMEKupersmithMJSavinoPJ1995Homonymous field defect as the first manifestation of Creutzfeldt-Jakob diseaseAm J Ophthalmol1194975047709975
- WatanabeRDuchenLW1993Cerebral amyloid in human prion diseaseNeuropathol Appl Neurobiol192532608355811
- WillRIronsideJWZeidlerM1996A new variant of Creutzfeldt-Jakob disease in the United KingdomLancet3479219258598754
- ZekiSMShippS1988The functional logic of cortical connectionsNature3353113173047584