Abstract
Thymosin beta 4 (Tβ4) is a low molecular weight protein present in all cells except erythrocytes. Although Tβ4 is the major monomeric actin-sequestering peptide in cells and can depolymerize F-actin, evidence is mounting to support the idea that it has multiple, seemingly diverse, cellular functions. In cornea, as in other tissues, Tβ4 promotes cell migration and wound healing, has anti-inflammatory properties, and suppresses apoptosis. In this review we discuss the current state of knowledge regarding the effects of Tβ4 in maintaining the healthy, functional cornea. The clinical implications of the use of Tβ4 as a wound healing and anti-inflammatory agent are discussed.
Corneal epithelial wounds: overview
The cornea consists of three types of cells, epithelial, stromal, and endothelial. It is avascular, transparent, and the most sensitive tissue in the body. The cornea functions as an optical lens that refracts light, but it also contributes to the rigid shell of the eyeball. In order to optimally refract light and ensure clear vision, the cornea must maintain optical transparency. This is accomplished in the stroma by a well-organized arrangement of collagen fibers into lamellae (CitationMeek et al 2003). The endothelium functions to preserve corneal transparency by regulating the level of hydration in the stroma. The corneal epithelium also contributes to the maintenance of corneal transparency (CitationVerkman 2003). Damage to the corneal epithelium caused by chemical, physical or microbial insults can lead to swelling of the stroma, the activation of stromal fibroblasts, and the infiltration of inflammatory cells, leading to the loss of corneal transparency and integrity. Timely resurfacing of the epithelium after injury is thus essential in preventing the loss of normal corneal function and loss of vision.
Figure 1 Tβ4 promotes corneal wound healing. By modulating the corneal inflammatory response and promoting re-epithelialization in response to injury, Tβ4 may be a new a therapeutic option for ophthalmologists treating corneal inflammatory and wound healing disorders.
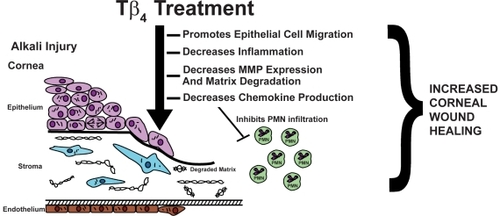
Following wounding, corneal epithelial healing occurs in several stages by a combination of three separate mechanisms, migration, mitosis, and differentiation (CitationGipson and Inatomi 1995). Through a highly organized chain of events, cells must migrate to cover the denuded stroma, and then proliferate and differentiate to restore the normal multi-layered epithelial cytoarchitecture (CitationZhao et al 2003). In most instances, corneal epithelial defects caused by a simple injury, such as a surface scrape or abrasion, are healed promptly. However, in individuals with certain clinical disorders, such as diabetic keratopathy, basement membrane dystrophies, neuropathies, and infections, corneal epithelial defects persist and do not respond to conventional treatment regimens (CitationSanchez-Thorin 1998). Not only is the rate of corneal re-epithelialization important after injury, the re-normalization of the epithelial cytoarchitecture and re-establishment of the epithelial barrier are paramount (CitationHuang et al 1990).
Table 1 Properties of thymosin beta 4 in the eye
In order to re-establish the epithelial barrier, cells migrate over the denuded surface of the cornea in a manner that is dependent both on the interaction of the cells with the underlying substrate via cell-extracellular matrix adhesions called hemidesmosomes, and on cell–cell adhesions called desmosomes (CitationSuzuki et al 2003). Regeneration of cell adhesions to reconstitute the epithelial barrier protects the cornea from infectious agents and also is important to prevent the persistence of corneal epithelial fragility that can lead to recurrent erosions (CitationMcCartney and Cantu-Crouch 1992).
The wound repair process is intricately linked to a complex inflammatory response that must be precisely regulated to ensure proper healing and optimal visual outcome. Infiltration of inflammatory cells into injured corneal tissue is a hallmark of wound repair (CitationKenyon 1985). Retardation of epithelial recovery by persistent inflammation, release of enzymatic products from resident corneal cells and infiltrating inflammatory cells, and stimulation by cytokines and chemokines, all contribute to poor re-epithelialization. With regard to the cornea, detailed information on cytokine expression and activity is emerging, and great interest centers around identifying which cytokines and chemokines are induced in the cornea by a particular stimulant or insult (CitationWakefield and Lloyd 1992; CitationCubitt et al 1993, Citation1995).
Although much progress has been made in understanding how the cornea heals, current pharmacological therapies that accelerate and/or promote epithelial healing are severely limited. Clinicians still mainly aim to provide the patient with an environment conducive to healing and rely on the eye’s innate ability to repair itself. Emerging evidence from our laboratory and others shows that thymosin beta 4 (Tβ4) is a novel wound healing agent that promotes corneal repair and decreases inflammation after injury. In this review, we focus on the studies that provide evidence for the potential clinical usage of Tβ4 as a corneal wound healing agent.
Properties of Tβ4
Tβ4 is a 43-amino acid, 4.9 kDa protein originally isolated from bovine thymus (CitationLow et al 1981; CitationGoodall et al 1983; CitationYu et al 1994). Tβ4 is the major monomeric actin-sequestering peptide in cells and can depolymerize F-actin. For example, it has been found to depolymerize cystic fibrosis-derived sputum actin in both a dose-dependent and a time-dependent manner (CitationRubin et al 2006). Tβ4 influences T cell differentiation (CitationHu et al 1981; CitationLow and Goldstein 1984). It has no signal sequence (CitationGondo et al 1987) but is found in serum (CitationNaylor et al 1984; CitationWeller et al 1988). Tβ4 does not appear to bind to either heparin or other extracellular matrix molecules, suggesting it can rapidly diffuse throughout tissue compartments.
Tβ4 is an ubiquitous polypeptide, highly conserved across species, and is found at concentrations of 1 × 10−5 to 5.6 × 10−1 M in a variety of tissues and cell types, yet, no receptors for the protein have been identified (CitationHannappel et al 1982; CitationHannappel and Leibold 1985; CitationHannappel and van Kampen 1987). A recent study suggests that internalization of exogenous Tβ4 is essential for its subsequent cellular functions (CitationHo et al 2007).
Previous work identified Tβ4 as an induced gene in endothelial cells undergoing differentiation in vitro (Grant et al 1995). Tβ4 is active for endothelial cell and keratinocyte migration in vitro and for angiogenesis in vivo (CitationMalinda et al 1997; CitationMalinda et al 1999). Tβ4 levels are highest in platelets and polymorphonuclear neutrophilic leukocytes (PMN), which are among the first formed elements and cells, respectively, to enter a wound and release their factors, some of which recruit additional cells to the wound site (CitationCassimeris et al 1992). Although the mechanism(s) of action of exogenous Tβ4 on wound repair remains unclear, the high levels of Tβ4 present in human wound fluid (13 μg/ml) suggest its importance in wound healing (CitationFrohm et al 1996). Tβ4 promotes cardiomyocyte migration, survival, and repair after experimental myocardial infarction, suggesting its potential as a new therapeutic target in the setting of acute myocardial damage (CitationBock-Marquette et al 2004). It is interesting to note that Tβ4 does not have any effect on cell proliferation (CitationLeeanansaksiri et al 2004; CitationHuang et al 2006; CitationSosne et al 2006).
Table 2 Thymosin β4: Some potential clinical ocular applications
In addition to its effects on wound healing, the anti-inflammatory properties of Tβ4 are beginning to be elucidated. CitationYoung et al (1999) reported that Tβ4 inhibited PMN chemotaxis to the bacterial chemoattractant, N-for-myl-methionyl-leucyl-phenylalanine (fMLP). Whether Tβ4 exerts its effects on PMN directly or indirectly is an intriguing question. Other studies showed that the activation-responsive expression of the lymph-specific form of Tβ4 may be one mechanism by which dendritic epidermal T cells, and possibly other intraepithelial lymphocytes, down-regulate local inflammation (CitationGirardi et al 2003). In a separate study, Tβ4 lowered circulating levels of inflammatory cytokines and intermediates following LPS administration in vivo (CitationBadamchian et al 2003). Additionally, Tβ4 levels rapidly disappeared in the blood following LPS administration or during septic shock, suggesting that it may be involved in early events leading to activation of the inflammatory cascade and ultimately the clinical sequelae of sepsis.
Tβ4 stimulates corneal epithelial cell migration and wound healing
Two major epithelial cell responses to wounding are cell migration to cover the wound bed, and cell proliferation to re-epithelialize the wound. We and others have shown that while not affecting cell proliferation, Tβ4 significantly enhances the migration of endothelial cells, keratinocytes, tumor cells, and corneal epithelial cells (CitationSosne et al 2001, 2007; CitationMoon et al 2006; CitationPhilp et al 2006). It has been proposed that Tβ4 may promote migration via its upregulation of zyxin expression, a protein that regulates the actin cytoskeleton (CitationMoon et al 2006). Tβ4 promoted full thickness dermal wound repair in normal, steroid-treated and diabetic animal models (CitationMalinda et al 1999; CitationPhilp et al 2003). These findings suggest that Tβ4 may be useful in promoting corneal wound healing in healthy individuals as well as in people with healing-impaired pathologies like diabetes.
Because diabetic retinopathy (DR) corneas have delayed wound healing (CitationKabosova et al 2003), we investigated the expression of Tβ4, a potent epithelial cell migration stimulator, in DR corneas. Human DR corneas were analyzed by gene microarray and quantitative RT-PCR and were found to express significantly less Tβ4 compared to normal corneas (CitationSaghizadeh et al 2006). We hypothesize that reduced expression of Tβ4 may contribute to delayed wound healing in DR corneas. These findings may have important clinical relevance in the treatment of complications stemming from diabetic keratopathy.
In addition to systemic diseases that manifest in the cornea such as diabetic keratopathy, other commonly seen corneal wounds can range from simple epithelial scrape wounds, recurrent erosions, persistent epitheliopathies post-refractive surgery, to severe inflammatory reactions such as seen in alkali injury. Tβ4 accelerated the re-epithelialization of corneal wounds in both heptanol debridement and alkali-burn animal models (CitationSosne et al 2001, Citation2002). Corneal alkali injury is marked by a massive PMN infiltration and inflammatory response characterized by over-expression of pro-inflammatory cytokines/chemokines and matrix-metalloproteinases (MMPs), much more extreme than that seen in the setting of scrape injury (CitationWagoner, 1997; Kenyon et al 1979; Foster et al 1982). The mouse alkali burn model mimics many aspects of the injury seen in humans by clinical slit-lamp and histopathological criteria. Slit-lamp observations of mouse eyes with alkali injury showed that 70% had readily apparent hyphema and total corneal opacification. In contrast 20% of alkali-injured eyes treated with Tβ4 exhibited the same characteristics. The overall anatomical integrity of the anterior segment in the Tβ4-treated eyes was markedly more normal in appearance, providing evidence that Tβ4 treatment improves corneal healing and clarity following alkali injury (CitationSosne et al 2005).
Corneal epithelial wounds cannot heal properly without the re-establishment of cell attachment to the underlying connective tissue ie, basement membrane. Hemidesmosomes, specialized adhesion junctions present along the basal cell membrane of the basal cells of stratified squamous epithelia, are involved in the adherence of the basal cells to the basement membrane (Susi et al 1967; CitationJones et al 1994). Heptanol or alkali-burned corneal epithelial cells treated with Tβ4 showed more hemidesmosomal adhesions to the basement membrane, and less widening of the space between epithelium and basement membrane. Laminin-5 is a major glycoprotein of the basement membrane underlying corneal epithelial cells. It plays a central role in both epithelial cell migration and in adhesion, and is associated with hemidesmosomes (CitationLarjava et al 1993). In cultured corneal epithelial cells and in mouse cornea wounded by scraping, topical Tβ4 treatment resulted in increased laminin-5 expression (CitationSosne et al 2002). This property of Tβ4 has important clinical implications, since it could be utilized in the treatment of recurrent erosions or other situations in which corneal epithelial cell adhesion to the basement membrane is impaired.
As an essential step in the cascade of cellular events culminating in wound healing, epithelial cells participate in extracellular matrix synthesis and remodeling (CitationLu et al 2001; CitationSantoro and Gaudino 2005). Human MMPs are a family of more than 20 zinc-dependent endopeptidases that upon activation degrade both matrix and non-matrix proteins as part of key rate-limiting steps in tissue repair and remodeling (Nagase et al 2006). Tβ4 treatment modulates MMP expression in several epithelial models (CitationSosne et al 2005Sosne et al 2007; CitationPhilp et al 2006). The majority of MMPs is secreted from the cell as non-catalytic zymogens (pro-MMPs) and is activated via removal of the inhibitory propeptides by proteinases such as thrombin, plasmin, trypsin, and other activated MMPs. Studies suggest that part of the wound healing activity of Tβ4 may be related to its ability to transiently increase MMP activity (MMP-1, -2, and -9) via its central actin-binding domain (CitationPhilp et al 2006).
Using an in vitro model of corneal epithelial cell scrape wound healing we showed that blocking MMP activity with specific or broad-spectrum inhibitors decreased Tβ4-mediated epithelial cell migration. This suggests that MMP catalytic activity is necessary for Tβ4 promotion of epithelial cell migration (Sosne et al 2007). Alterations in MMP expression are linked to improper corneal wound healing and the formation of persistent epithelial defects and chronic corneal ulcers (CitationFini et al 1996). Tβ4 differentially regulates the expression of MMPs in many tissues, and also regulates the MMP/TIMP balance in the cornea. Thus, it is plausible to hypothesize that Tβ4 may promote corneal repair and matrix remodeling following wounding by modulating MMP expression and activity.
Tβ4 modulates corneal inflammation
Many studies have contributed information regarding the roles of cytokine and chemokine expression and activity in the post-wound corneal inflammatory response in a wide array of clinical pathologies (CitationGillitzer and Goebeler 2001; CitationWilson et al 2003; CitationAgrawal and Tsai 2003; Stramer et al 2004). For example, in chemically injured corneal epithelial cells, the levels of pro-inflammatory cytokines and chemokines are upregulated (CitationPlanck et al 1997; CitationSotozono et al 1997; CitationSosne et al 2002), and dry eye stimulates the expression of tumour necrosis factor-alpha (TNF-α) in mice (CitationLuo et al 2004). Corneal epithelial monolayers infected with Pseudomonas aeruginosa demonstrated increased expression and secretion of IL-6, IL-8, and TNF-α (CitationZhang et al 2005). IL-6 has been associated with enhanced corneal epithelial cell migration (Wang et al 1994; CitationNakamura and Nishida 1999).
In the first ocular studies to provide evidence that Tβ4-treatment accelerates in vivo wound healing and modulates corneal cytokine production, we showed in the rat corneal epithelial debridement model that mRNA transcripts for IL-1β and IL-6 were increased in Tβ4-treated corneas after injury. Additionally, topical Tβ4 treatment after alkali injury down-regulated the expression of the potent PMN chemoattractants, MIP-2 and KC, in the murine cornea. We suggested that these cytokines may be involved in corneal inflammation and wound healing, and that the decreased corneal chemokine expression may be responsible for the observed decreased PMN infiltration.
TNF-alpha is a potent pro-inflammatory mediator that influences wound healing and apoptotic cell death (CitationZhang et al 2004). TNF-α stimulation activates transcription factors including nuclear factor kappa B (NFκB) (Baud and Karin 2001; CitationHanada and Yoshimura 2002; CitationRitchie et al 2004). NFκB dimers are maintained in an inactivated state in the cytoplasm by a family of inhibitory proteins, the IκBs. IκB can be phosphorylated following cellular stimulation, resulting in its degradation and release from the NFκB dimer. Activatied NFκB translocates to the nucleus where it binds to κB enhancer elements of a wide panel of target genes (CitationKarin and Ben-Neriah 2000). Because of its ability to regulate the expression of inflammatory genes, NFκB is believed to play a major role in the inflammatory process (CitationHayden and Ghosh 2004).
In cultured human corneal epithelial cells stimulated with TNF-α, Tβ4 treatment significantly decreased nuclear NFκB protein levels, NFκB activity, and p65 subunit phosphorylation. The nuclear translocation of NFκB was also inhibited. Although there are many inflammatory signaling pathways, we hypothesize that Tβ4 mediates NFκB inflammatory signaling pathways in the cornea based on our findings. These observations have important clinical implications for the potential role of Tβ4 as a corneal anti-inflammatory agent.
Tβ4 inhibits corneal epithelial cell apoptosis
Evidence that Tβ4 functions as an anti-apoptotic agent is mounting. HeLa cells that overexpressed Tβ 4 showed a higher growth rate and a lower percentage of basal apoptosis compared to control HeLa cells. In addition, the overexpressing cells were more resistant to paclitaxel-induced cell death (CitationOh et al 2006). The death of human corneal epithelial cells (HCET) induced by FasL or by hydrogen peroxide was significantly inhibited by Tβ4 treatment. In this study it was observed that the internalization of exogenous Tβ4 was essential for its anti-apoptotic activity in human corneal epithelial cells (CitationHo et al 2007). Resistance to FasL-induced apoptosis was also achieved in SW480 colon carcinoma cells that overexpressed Tβ4 (CitationHsiao et al 2006). Using an in vitro culture model, we demonstrated that Tβ4 suppressed ethanol-induced corneal epithelial cell apoptosis via the inhibition of caspases and suppression of bcl-2 release from mitochondria (CitationSosne et al 2004). In cultured corneal and conjunctival epithelium, Tβ4 did not protect cells from benzalkonium chloride-induced inhibition of proliferation, but apoptosis was significantly inhibited (CitationSosne et al 2006).
Regarding the mechanism by which Tβ4 exerts its anti-apoptotic activity, it has been suggested that the reduction of early cell death initiation signals, such as the phosphorylation of c-Jun, may prevent apoptosis of neurons (CitationChoi et al 2006). Additionally, it has been demonstrated that Tβ4 promotes survival of cardiomyocytes through the formation of a functional complex with PINCH and integrin-linked kinase, resulting in activation of the survival kinase Akt (CitationBock-Marquette et al 2004).
Future directions
The mounting evidence suggesting the use of Tβ4 as a novel corneal wound healing and anti-inflammatory agent presents an exciting new potential therapy for practicing ophthalmologists. The plethora of clinical entities related to retarded corneal wound healing present significant challenges for the practicing ophthalmologist. Tβ4, a ubiquitous, naturally occurring molecule, has been demonstrated to promote corneal re-epithelialization, reduce inflammation and inhibit apoptosis. From routine corneal injuries such as abrasions and recurrent erosions to more severe inflammatory-mediated pathologies, ophthalmologists need an agent that can quickly promote corneal healing and properly modulate the inflammatory response.
More basic science studies focused on the mechanisms of action of Tβ4 are needed to help guide future clinical applications. For example, it is interesting to speculate whether the anti-apoptotic properties of Tβ4 could help to preserve tissue after acute injury or for corneal transplantation. Additionally, Tβ4 may be useful as an adjuvant therapy to BAK-containing eye drops to prevent the adverse ocular surface side effects seen with many anti-glaucoma medications. Regarding the anti-inflammatory properties of Tβ4, a wide array of clinical entities such as inflammatory keratopathies, corneal graft rejection, infections, uveitis, and dry eye syndromes may be just a few examples of potential therapeutic treatments. In addition to the basic science studies, we believe that the published animal studies showing Tβ4’s efficacy in a variety of models of injury provide a solid basis for testing the efficacy and safety of Tβ4 in well controlled clinical trials and this is presently being planned (http://www.regenerx.com).
References
- AgrawalVBTsaiRJ2003Corneal epithelial wound healingIndian J Ophthalmol5151512701857
- BadamchianMFagarasanMODannerRL2003Thymosin beta(4) reduces lethality and down-regulates inflammatory mediators in endotoxin-induced septic shockInt Immunopharmacol312253312860178
- Bock-MarquetteASaxenaMWhiteMD2004Thymosin beta 4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repairNature4324667215565145
- CassimerisLSaferDNachmiasVT1992Thymosin beta 4 sequesters the majority of G-actin in resting human polymorphonuclear leukocytesJ Cell Biol1191261701447300
- ChoiSYKimDKEunB2006Anti-apoptotic function of thymosin-beta in developing chick spinal motoneuronsBiochem Biophys Res Commun346872816782066
- CubittCLTangQMonteiroCA1993IL-8 gene expression in cultures of human corneal epithelial cells and keratocytesInvest Ophthalmol Vis Sci3431992067691777
- CubittCLLauschRNOakesJE1995Differences in interleukin-6 gene expression between cultured human corneal epithelial cells and keratocytesInvest Ophthalmol Vis Sci3633067843904
- FiniMEParksWCRinehartWB1996Role of matrix metalloproteinases in failure to re-epithelialize after corneal injuryAm J Pathol14912873028863676
- FrohmMGunneHBergmanAC1996Biochemical and antibacterial analysis of human wound and blister fluidEur J Biochem23786928620898
- GillitzerRGoebelerM2001Chemokines in cutaneous wound healingJ Leukoc Biol695132111310836
- GipsonIKInatomiT1995Extracellular matrix and growth factors in corneal wound healingCurr Opin Ophthalmol631010150880
- GirardiMSherlingMAFillerRB2003Anti-inflammatory effects in the skin of thymosin-beta 4 splice-variantsImmunology1091712709011
- GondoHKudoJWhiteJW1987Differential expression of the human thymosin-beta 4 gene in lymphocytes, macrophages, and granulocytesJ Immunol139384083500230
- GoodallGJHempsteadJLMorganJI1983Production and characterization of antibodies to thymosin beta 4J Immunol13182156863931
- GrantDSRoseWYaenC1999Thymosin beta 4 enhances endothelial cell differentiation and angiogenesisAngiogenesis31253514517430
- HanadaTYoshimuraA2002Regulation of cytokine signaling and inflammationCytokine Growth Factor Rev134132112220554
- HannappelEXuGJMorganJ1982Thymosin beta 4: a ubiquitous peptide in rat and mouse tissuesProc Natl Acad Sci USA79217256954532
- HannappelELeiboldW1985Biosynthesis rates and content of thymosin beta 4 in cell linesArch Biochem Biophys240236412990345
- HannappelEvan KampenM1987Determination of thymosin beta 4 in human blood cells and serumJ Chromatogr397279853654821
- HaydenMSGhoshS2004Signaling to NF-kappaBGenes Dev18219522415371334
- HoJHChuangCHHoCY2007Internalization is essential for the antiapoptotic effects of exogenous thymosin beta-4 on human corneal epithelial cellsInvest Ophthalmol Vis Sci48273317197512
- HsiaoHLWangWSChenPM2006Overexpression of thymosin beta-4 renders SW480 colon carcinoma cells more resistant to apoptosis triggered by FasL and two topoisomerase II inhibitors via down-regulating Fas and upregulating Survivin expression, respectivelyCarcinogenesis279364416364925
- HuSKLowTLGoldsteinAL1981Modulation of terminal deoxynucleotidyl transferase activity by thymosinMol Cell Biochem4149587329413
- HuangAJTsengSCKenyonKR1990Alteration of epithelial paracellular permeability during corneal epithelial wound healingInvest Ophthalmol Vis Sci31429351690686
- HuangWQWangBHWangQR2006Thymosin beta4 and AcSDKP inhibit the proliferation of HL-60 cells and induce their differentiation and apoptosisCell Biol Int305142016677835
- JonesJCAsmuthJBakerSE1994Hemidesmosomes: extra-cellular matrix/intermediate filament connectorsExp Cell Res21311118020577
- KabosovaAKramerovAAAokiAM2003Human diabetic corneas preserve wound healing, basement membrane, integrin and MMP-10 differences from normal corneas in organ cultureExp Eye Res77211712873452
- KarinMBen-NeriahY2000Phosphorylation meets ubiquitination: the control of NF-[kappa]B activityAnnu Rev Immunol186216310837071
- KenyonKR1985Inflammatory mechanisms in corneal ulcerationTrans Am Ophthalmol Soc83610633914132
- LarjavaHSaloTHaapasalmiK1993Expression of integrins and basement membrane components by wound keratinocytesJ Clin Invest921425358376596
- LeeanansaksiriWDeSimoneSKHuffT2004Thymosin beta 4 and its N-terminal tetrapeptide, AcSDKP, inhibit proliferation, and induce dysplastic, non-apoptotic nuclei and degranulation of mast cellsChem Biodivers1109110017191900
- LindseyML2006Novel strategies to delineate matrix metalloproteinase (MMP)-substrate relationships and identify targets to block MMP activityMini Rev Med Chem61243817100635
- LowTLHuSKGoldsteinAL1981Complete amino acid sequence of bovine thymosin beta 4: a thymic hormone that induces terminal deoxynucleotidyl transferase activity in thymocyte populationsProc Natl Acad Sci USA78116266940133
- LowTLGoldsteinAL1984Thymosins: structure, function and therapeutic applicationsThymus627426087503
- LuLReinachPSKaoWW2001Corneal epithelial wound healingExp Biol Med (Maywood)2266536411444101
- LuoLLiDQDoshiA2004Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surfaceInvest Ophthalmol Vis Sci45429330115557435
- MalindaKMGoldsteinALKleinmanHK1997Thymosin beta 4 stimulates directional migration of human umbilical vein endothelial cellsFASEB J11474819194528
- MalindaKMSidhuGSManiH1999Thymosin beta 4 accelerates wound healingJ Invest Dermatol1133646810469335
- McCartneyMDCantu-CrouchD1992Rabbit corneal epithelial wound repair: tight junction reformationCurr Eye Res1115241559388
- MeekKMLeonardDWConnonCJ2003Transparency, swelling and scarring in the corneal stromaEye179273614631399
- MoonHSEven-RamSKleinmanHK2006Zyxin is upregulated in the nucleus by thymosin β4 in SiHa cellsExp Cell Res1734253116956606
- NakamuraMNishidaT1999Differential effects of epidermal growth factor and interleukin 6 on corneal epithelial cells and vascular endothelial cellsCornea18452810422859
- NaylorPHMcClureJESpangeloBL1984Immunochemical studies on thymosin: radioimmunoassay of thymosin beta 4Immunopharmacology79166715146
- OhSYSongJHGilJE2006ERK activation by thymosin-beta-4 (TB4) overexpression induces paclitaxel-resistanceExp Cell Res3121651716515784
- PhilpDBadamchianMScheremetaB2003Thymosin beta 4 and a synthetic peptide containing its actin-binding domain promote dermal wound repair in db/db diabetic mice and in aged miceWound Repair Regen11192412581423
- PhilpDNguyenMScheremetaB2004Thymosin β4 increases hair growth by activation of hair follicle stem cellsFASEB J18385714657002
- PhilpDScheremetaBSiblissK2006Thymosin beta4 promotes matrix metalloproteinase expression during wound repairJ Cell Physiol20819520016607611
- PlanckSRRichLFAnselJC1997Trauma and alkali burns induce distinct patterns of cytokine gene expression in the rat corneaOcul Immunol Inflamm5951009234373
- RitchieMHFillmoreRALauschRN2004A role for NF-kappa B binding motifs in the differential induction of chemokine gene expression in human corneal epithelial cellsInvest Ophthalmol Vis Sci45229930515223809
- RubinBKKaterAPGoldsteinAL2006Thymosin beta 4 sequesters actin in cystic fibrosis sputum and decreases sputum cohesivity in vitroChest13014334017099021
- SaghizadehMKramerovAATajbakhshJ2006Proteinase and growth factor alterations revealed by gene microarray analysis of human diabetic corneasInvest Ophthalmol Vis Sci4636041516186340
- Sanchez-ThorinJC1998The cornea in diabetes mellitusInt Ophthalmol Clin3819369604736
- SantoroMMGaudinoG2005Cellular and molecular facets of keratinocyte reepithelization during wound healingExp Cell Res3042748615707592
- SosneGChanCCThaiK2001Thymosin beta 4 promotes corneal wound healing and modulates inflammatory mediators in vivoExp Eye Res72605811311052
- SosneGSzliterEABarrettR2002Thymosin beta 4 promotes corneal wound healing and decreases inflammation in vivo following alkali injuryExp Eye Res74293911950239
- SosneGSiddiqiAKurpakus-WheaterM2004Thymosin beta-4 inhibits corneal epithelial cell apoptosis after ethanol exposure in vitroInvest Ophthalmol Vis Sci45109510015037574
- SosneGChristophersonPLBarrettRP2005Thymosin-beta 4 modulates corneal matrix metalloproteinase levels and polymorphonuclear cell infiltration after alkali injuryInvest Ophthalmol Vis Sci4623889515980226
- SosneGAlbeirutiARHollisB2006Thymosin beta4 inhibits benzalkonium chloride-mediated apoptosis in corneal and conjunctival epithelial cells in vitroExp Eye Res83502716630613
- QiuPKurpakus-WheaterMSosneG2007Matrix metalloproteinase activity is necessary for thymosin beta 4 promotion of epithelial cell migrationJ Cell Physiol2121657317348036
- SotozonoCHeJMatsumotoY1997Cytokine expression in the alkali-burned corneaCurr Eye Res1667069222084
- SuzukiKSaitoJYanaiR2003Cell-matrix and cell-cell interactions during corneal epithelial wound healingProg Retin Eye Res221133312604055
- VerkmanAS2003Role of aquaporin water channels in eye functionExp Eye Res761374312565800
- WagonerMD1997Chemical injuries of the eye: current concepts in pathophysiology and therapySurv Ophthalmol412753139104767
- WakefieldDLloydA1992The role of cytokines in the pathogenesis of inflammatory eye diseaseCytokine4151617154
- WellerFEMutchnickMGGoldsteinAL1988Enzyme immunoassay measurement of thymosin beta 4 in human serumJ Biol Response Mod79163286825
- WilsonSENettoMAmbrosioRJr2003Corneal cells: chatty in development, homeostasis, wound healing, and diseaseAm J Ophthalmol136530612967809
- YoungJDLawrenceAJMacLeanAG1999Thymosin beta 4 sulfoxide is an anti-inflammatory agent generated by monocytes in the presence of glucocorticoidsNat Med51424710581087
- YuFXLinSCMorrison-BogoradM1994Effects of thymosin beta 4 and thymosin beta 10 on actin structures in living cellsCell Motil Cytoskeleton2713258194107
- ZhangSLinZNYangCF2004Suppressed NF-kappaB and sustained JNK activation contribute to the sensitization effect of parthenolide to TNF-alpha-induced apoptosis in human cancer cellsCarcinogenesis252191915256485
- ZhangJWuXYYuFS2005Inflammatory responses of corneal epithelial cells to Pseudomonas aeruginosa infectionCurr Eye Res305273416020286
- ZhaoMSongBPuJ2003Direct visualization of a stratified epithelium reveals that wounds heal by unified sliding of cell sheetsFASEB J1739740612631579