Abstract
The goal of treatment for open-angle glaucoma or ocular hypertension is to improve quality of life through reduction of intraocular pressure (IOP) to preserve visual function. Prostaglandins, as a newer class of ocular hypotensive agents, have been shown to be effective in IOP reduction by the primary mechanism of action of increase the uveoscleral outflow. Bimatoprost is a member this class, but different from the other members by having an ethyl amide group rather than an isopropyl ester at the C-1 carbon of the alpha chain. Bimatoprost used once daily has been shown to be more effect in IOP reduction than other classes of topical ocular hypotensive agents including beta-blockers, carbonic anhydrase inhibitors, and alpha agonists. Comparing with other topical prostaglandins, bimatoprost may be slightly more effective in IOP reduction, but the clinical significance is uncertain. The commonly reported adverse events associated with bimatoprost are localized to the eye and include conjunctival hyperemia, changes in the pigmentation of the periocular skin and iris, and eyelash darkening and growth. It is currently approved by the Food and Drug Administration (FDA) and the European Commission (EC) for first-line therapy for the reduction of elevated IOP in patients with open-angle glaucoma or ocular hypertension.
Introduction
Open-angle glaucoma is estimated to affect more than 2 million individuals in the United States according to a meta-analysis of recent population-based studies in the United States, Australia, and Europe (CitationEye Diseases Research Prevalence Group 2004). Owing to the rapid aging of the US population, this number is expected to increase to more than 3 million by 2020 (CitationEye Diseases Research Prevalence Group 2004). The definition of glaucoma has changed considerably over the past several decades. The disease is no longer defined as elevated intraocular pressure (IOP) but rather a disorder consisting of characteristic optic nerve head and visual field abnormalities (CitationAnderson 1989). Major risk factors for the development of glaucomatous optic nerve damage include the level of IOP (CitationHollows and Graham 1966; CitationDavid et al 1977; CitationSommer 1989; CitationSommer et al 1991), increasing age (CitationArmaly et al 1980), black race (CitationTielsch et al 1991), positive finding for the condition in the family history (CitationKolker 1972), and thin central corneal thickness (CitationGordon et al 2002). However, IOP remains the only risk factor readily amenable to therapy. Therefore, almost all currently used strategies for the treatment of glaucoma are aimed at lowering or preventing a rise in IOP (CitationLaw and Caprioli in press).
The goal of glaucoma treatment is to improve quality of life through reduction of IOP to preserve visual function. In the process of IOP reduction, an ideal medication should have a schedule that is simple to follow, be least interrupting with a patient’s life, highly tolerable and affordable. However, medical treatment of glaucoma has associated side effects, complications, and costs (CitationLaw and Caprioli in press). Prostaglandins (PGs), as a newer class of IOP-reducing agents, have gained tremendous popularity in management of glaucoma. This review is to examine the position of bimatoprost, a member of the PGs, in glaucoma medical management.
Prostaglandins in general
Prostaglandins (PGs) are biologically active derivatives of arachidonic acid with diverse local responses that are tissue dependent. Arachidonic acid is bound to phospholipids in the membranes of most mammalian cells. The release of arachidonic acid is catalyzed by the enzyme phospholipase A2, and arachidonic acids are then converted into PGs by cyclooxygenase and PG synthetase.
PGs are the most potent ocular hypotensive agents yet discovered. Multiple prostanoid receptors (eg, DP, EP1 to EP4, FP, IP, and TP) have been identified based on studies using molecular biologic, second-messenger, radio-ligand binding, and functional techniques (CitationColeman et al 1994). In particular, the FP receptor mediates little or no nociceptive response, plays little or no role in regulation of vessel tone and capillary permeability, and is responsible for little or no smooth muscle contraction of the bronchioles. PGF2α became the PG prototype in the development of PGs for glaucoma treatment. In a human study of 18 nonglaucomatous volunteers, IOP was significantly reduced through a 24-hour period after administration of PGF2α (CitationGiuffre 1985). However, it was associated with a high incidence of adverse effects including marked conjunctival hyperemia, ocular irritation, foreign-body sensation, and headache. As a result of intense effort to modify the chemical structure of PGF2α to develop a compound that could reduce IOP while minimizing the incidence of side effects, four agents in this class of topical glaucoma drops are currently commercially available. They include latanoprost, unoprostone, travoprost, and bimatoprost.
The primary mechanism of action of PGs is believed to reduce IOP by increasing uveoscleral outflow. This is in contrast to other classes of antiglaucoma medications, which act by increasing aqueous humor outflow via the trabecular meshwork or by inhibiting aqueous production. Although the exact mechanisms are still not entirely clear, it appears that PGs facilitates the uveoscleral pathway in the ciliary muscle by reducing the extracellular matrix in the spaces between and within the ciliary muscle fibers (CitationToris et al 1997; CitationSchachtschabel et al 2000). Activation of FP and EP2 receptors stimulates several intracellular second messenger mechanisms (CitationLindsey et al 1994; CitationZhan et al 1998), which are thought to activate the biosynthesis of matrix metalloproteinases (MMPs), a family of neutral proteinases that have a lytic effect on extracellular matrix molecules.
Bimatoprost
Bimatoprost (Lumigan® 0.03%; Allergan, Irvine, CA, USA) is a synthetic PG. But unlike the prostaglandin F2α analogs latanoprost, travoprost, and unoprostone, bimatoprost has an ethyl amide rather than an isopropyl ester at the C-1 carbon of the alpha chain. Bimatoprost has been proposed to be pharmacologically similar to a newly discovered class of fatty acid amides otherwise known as “prostamides” (CitationWoodward et al 2001, Citation2003). There is some evidence suggesting that long-term therapy with bimatoprost increases both pressure-dependent trabecular outflow and pressure-independent uveoscleral outflow via remodeling of extracellular matrix in the trabecular meshwork and ciliary muscle, respectively (CitationBrubaker 2001; CitationBrubaker et al 2001). It has been proposed that bimatoprost does not require hydrolysis to a free fatty acid to stimulate prostaglandin FP receptors and the pharmacological activities of bimatoprost are postulated to be mediated by a novel receptor that is different from prostaglandin receptors (CitationWoodward et al 2001). However, such a receptor has not yet been identified. Whether bimatoprost is a pro-drug that is hydrolyzed to an active fatty acid for its hypotensive activity remains controversial. Although there are experiments demonstrating bimatoprost hydrolysis occurs in human ocular tissue, the rate of hydrolysis is either very slow or occurs in the presence of high concentrations of bimatoprost (CitationMaxey et al 2002; CitationDavies et al 2003; CitationHellberg et al 2003). In a masked vehicle-controlled study to determine the level of bimatoprost free acid in eyes treated with bimatoprost 0.03% before cataract surgery, aqueous concentrations of the free acid of bimatoprost were 22.0 ± 7.0 nmol and 7.0 ± 4.6 nmol at 2 and 12 hours after the last dose of bimatoprost, respectively (CitationCamras et al 2004). This concentration is about one-fifth the concentration of the free acid of latanoprost in the aqueous humor, but the free acid of bimatoprost has been shown to be 3–10 times more potent at the FP receptor than the free acid of latanoprost (CitationSharif et al 2002).
Efficacy
Dose-response and dose-frequency studies in patients with elevated IOP have demonstrated that the most effective regimen is a once-daily dosage of bimatoprost 0.03% (CitationLaibovitz et al 2001). Twice-daily administration was found to confer no additional efficacy and in some studies was less effective than once-daily administration (CitationBrandt et al 2001; CitationLaibovitz et al 2001; Sherwood et al 2003). Mean reductions in IOP 12 hours after administration of bimatoptost 0.03% ranged from 6.8 to 9.2 mmHg in randomized clinical trials, with reductions in IOP maintained throughout the 24-hour dosage interval and maintained for up to 1 year (CitationBrandt et al 2001; CitationDubiner et al 2001; CitationGandolfi et al 2001; CitationLaibovitz et al 2001; Sherwood et al 2003). Bimatoprost 0.3% once-daily administration produced significantly greater reductions in IOP of about 2–4 mmHg than timolol 0.5% given twice daily, and a significantly higher percentage of patients achieved a target IOP of ≤17 mmHg with bimatoprost than with timolol treatment (CitationBrandt et al 2001; CitationLaibovitz et al 2001; Sherwood et al 2003). The combination of dorzolamide 2%/timolol 0.5% was compared with bimatoprost 0.03% in a 3-month randomized, single-blinded trial involving 177 patients with ocular hypertension or glaucoma inadequately controlled with beta-blocker therapy. Treatment with bimatoprost 0.03% produced significantly greater mean reductions in IOP at 8 AM than treatment with dorzolamide 2%/timolol 0.5% at every visit throughout the study. Furthermore, approximately twice as many patients had an IOP of ≤16 mmHg after 3 months’ treatment with bimatoprost than with dorzolamide/timolol (31% vs 14%) (CitationColeman et al 2003).
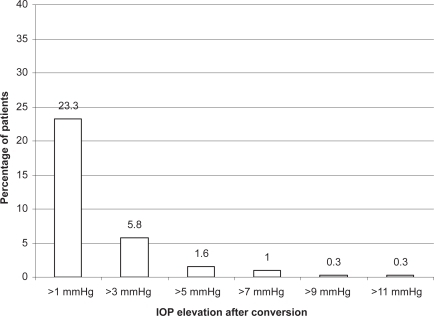
The clinical efficacy of bimatoprost 0.03% has also been compared with other topical PGs. In a meta-analysis of randomized clinical trials to estimate the IOP reduction achieved by the most frequently prescribed glaucoma drugs, including latanoprost, travoprost, and bimatoprost, IOP was reduced from baseline by 31%–33% for the peak and 28%–29% for the trough with topical PGs, and bimatoprost achieved the highest reduction of IOP at peak (Citationvan der Valk et al 2005). In a 6-month randomized clinical trial comparing bimatoprost and latanoprost in patients with ocular hypertension or glaucoma, the mean change from baseline IOP was significantly greater for bimatoprost patients than for latanoprost patients, the percentage of patients achieving a ≥20% IOP decrease was higher with bimatoprost than with latanoprost, and more bimatoprost patients achieved a lower pressure range (CitationNoecker et al 2003). However, similar differences of IOP response between the two agents was not repeated in other studies. In a small, double-blinded, phase II study (n = 64) and a 3-month investigator-masked trial, no significant difference in the IOP-lowering efficacy of the drugs was observed 12 hours post-dose at the end of the treatment periods; however, diurnal control of IOP was more consistent with bimatoprost (CitationDubiner et al 2001; CitationGandolfi et al 2001). Although the percentage of patients achieving a target IOP of ≤17 mmHg was not significantly different between treatment groups, a significantly greater proportion of the bimatoptost-treated patients reached a target IOP of ≤15 mmHg (29% vs 14%). In a 12-week comparison of the three PGs, latanoprost, bimatoprost, and travoprost, no statistical differences were observed between the three agents in mean IOP (CitationParrish et al 2003). The conflicting findings are likely due to the similarity in efficacy between the three agents. In a recent published randomized, double-blinded, crossover comparison of bimatoprost and latanoprost, the 24-hour diurnal IOP is statistically lower with bimatoprost than latanoprost. However, the difference was small (0.5 mmHg) and may not be clinically meaningful (CitationKonstas et al 2005). In a retrospective review of more than 300 patients in a health management organization that had switched from latanoprost to bimatoprost, a statistically significant mean IOP difference of 0.5 mmHg was observed favoring bimatoprost (CitationLaw et al 2005). This study also showed that approximately 13% of patients had a further IOP reduction of >3 mmHg after switching from latanoprost to bimatoprost compared with 5% of patients had a >3 mmHg reduction while using latanoprost before the switch ( and ). It is likely that bimatoprost can achieve a small but statistically greater mean IOP reduction than latanoprost, but the clinical significance is uncertain. In addition, individual responses to the different agents can be variable.
Figure 1 Cumulative percentage of patients with intraocular pressure (IOP) reduction after switching from latanoprost to bimatoprost. Reproduced with permission from Law SK, Song J, Fang E, et al. 2005. Feasibility and efficacy of a mass switch from latanoprost to bimatoprost in glaucoma patients in a pre-paid health maintenance organization. Ophthalmology, 112:2123–30. Copyright © Elsevier.
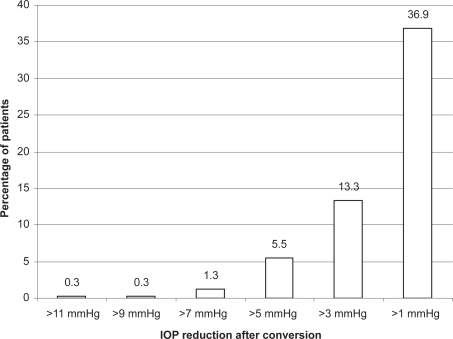
Figure 2 Cumulative percentage of patients with intraocular pressure (IOP) elevation after switching from latanoprost to bimatoprost. Reproduced with permission from Law SK, Song J, Fang E, et al. 2005. Feasibility and efficacy of a mass switch from latanoprost to bimatoprost in glaucoma patients in a pre-paid health maintenance organization. Ophthalmology, 112:2123–30. Copyright © Elsevier.
Additivity
Because of its similarity with other prostaglandin agents such as latanoprost and travoprost, bimatoprost is expected to be additive to other antiglaucoma agents with different mechanisms of action, including β-blockers, selective α-agonists, carbonic anhydrase inhibitors, and parasympathomimetics. However, the additivity among the three agents (latanoprost, travoprost, bimatoprost) is inconsistent. In a study with monkey eyes with laser-induced unilateral glaucoma, the IOP effects of the bimatoprost 0.03% or travoprost 0.004% were additive to that of latanoprost 0.005%, with bimatoprost showing a greater additive response than travoprost (CitationGagliuso et al 2004). However, no similar finding was observed in human studies. A paradoxical IOP elevation was reported after combined therapy with latanoprost and bimatoprost (CitationHerndon et al 2002). In an open label clinical trial, bimatoprost 0.03% was combined with latanoprost 0.005% in one randomly assigned eye (case eye) of each patient in phase 1, and in phase 2, bimatoprost was discontinued from the case eyes while bimatoprost was substituted for latanoprost in the fellow eye (control eye). When bimatoprost and latanoprost were used together, the mean IOP increased by 1.8 mmHg, and returned to previous values after discontinuation of bimatoprost; no mean IOP change was observed throughout the study in the control eyes (CitationDoi et al 2005).
Systemic adverse reactions
Systemic adverse events reported after treatment with bimatoprost 0.03% have included colds and upper respiratory tract infections occurring in approximately 10% of patients, and headaches, abnormal liver function tests, asthenia, and hirsutism. Bimatoprost 0.03% did not have any clinically significant effect on heart rate or blood pressure in patients with glaucoma or ocular hypertension in clinical trials (CitationBrandt et al 2001; CitationDubiner et al 2001; CitationLaibovitz et al 2001).
Since bimatoprost is similar in structure and effects with the other topical PGs, it is important to be aware of the side effects reported with the other PGs. There is a case report of abdomincal cramps associated with travoprost confirmed by dechallenge and rechallenge procedures (CitationLee 2005). Other systemic events, each with an incidence of 1% or 2%, included chest pain/angina, muscle/joint/back pain, and rash/allergic skin reaction. Angina (CitationMitra et al 2001), arterial hypertension (CitationPeak and Sutton 1998), and tachycardia have been anecdotally reported following latanoprost use. In a randomized study, headache was more frequent in patients receiving latanoprost than in those receiving bimatoprost, although this difference did not reach statistical significance (CitationGandolfi et al 2001). Patients with no prior history of migraine and/or headache have reported migraine after receiving latanoprost treatment (CitationWeston 2001).
Bimatoprost is classified as category C according to the use-in-pregnancy ratings of the US Food and Drug Administration (FDA). Experience of bimatoprost use during pregnancy is limited. However, in an observation study of 10 pregnant women exposed to latanoprost during the first trimester, 9 women delivered normal fetuses with no malformations. One pregnancy was complicated by miscarriage, which occurred 2 weeks after treatment was ended in a 46-year-old woman, primi-gravida, who had increased reproductive risk related to her advanced age (CitationDe Santis et al 2004).
Local adverse reactions
The most common adverse event associated with bimatoprost 0.03% treatment was conjunctival hyperemia, which occurred in 42%–46% of patients (CitationBrandt et al 2001; CitationGandolfi et al 2001; CitationSherwood et al 2001). Approximately 1%–4% of patients discontinued treatment with bimatoprost 0.03% because of conjunctival hyperemia (CitationBrandt et al 2001; CitationSherwood et al 2001). Bimatoprost 0.03% has been reported to cause changes in the pigmentation of tissues, including pigmentation of the periocular skin and iris, and eyelash darkening. Eyelash growth has also been reported in 12.6% to 35.7% of patients during clinical trials of bimatoprost 0.03% (CitationBrandt et al 2001; CitationGandolfi et al 2001; CitationSherwood et al 2001). Significantly more conjunctival hyperemia and increased eyelash growth were reported with bimatoprost than with latanoporst, whereas headache was more frequently seen in latanoprost than bimatoprost recipients (CitationGandolfi et al 2001; CitationNoecker et al 2003; CitationParrish et al 2003; CitationStewart et al 2003). Cystoid macular edema in patients at high risk can occur when treated with bimatoprost. There is a case report of cystoid macular edema, which developed in a patient switched from latanoprost to bimatoprost 9 months after cataract surgery (CitationCarrillo et al 2004).
Anterior uveitis has also been observed in approximately 1% of patients receiving latanoprost which resolves with corticosteroid therapy (CitationSmith et al 1999). Association of latanoprost and uveitis has been confirmed with a dechallenge and rechallenge method in two studies (Fechter et al 1998; CitationWarwar et al 1998). Use of other PGs, including bimatoprost should be cautioned in patients with known tendency of ocular inflammatory reaction. In fact, administration of latanoprost to patients with active uveitis at the time of treatment does not appear to lower IOP (CitationSmith et al 1999; CitationSacca et al 2001). The association of PGs and ocular infection of herpes simplex virus has been documented with latanoprost. Latanoprost has been shown to worsen acute herpetic keratitis in the rabbit eye (New Zealand white (NZW) rabbit) and increase the risk of recurrences in latently infected animals (CitationKaufman et al 1999). However, in the Induced Reactivation and Spontaneous Shedding HSV-1/NZW rabbit latency models, latanoprost was not found to promote ocular shedding of HSV-1 (CitationGordon et al 2003). There are case reports that herpes simplex keratitis developed after initiation of latanoprost therapy with dechallenge and rechallenge method (CitationWand et al 1999). In one case report, 2 patients developed HSV dermatitis of the periocular skin after using latanoprost (CitationMorales et al 2001). Use of any topical PGs in patients with history of ocular herpetic infection may better be avoided.
Indications
Bimatoprost is effective in reduction of elevated IOP in patients with open-angle glaucoma or ocular hypertension. It is similar in efficacy and safety profiles to another older PG, latanoprost, which has gained wider experience and demonstrated effectiveness in several glaucoma subtypes, including chronic angle closure glaucoma (CitationAung et al 2000; CitationHung et al 2000; CitationChew et al 2004; CitationSihota et al 2004; CitationKook et al 2005), pigmentary glaucoma (CitationMastropasqua et al 1999), pseudoexfoliation glaucoma (CitationKonstas et al 2003, Citation2004), and low tension glaucoma (CitationRulo et al 1996; CitationMcKibbin et al 1999; CitationAng et al 2004). Because of its similarity with latanoprost, bimatoprost is expected to be effective in IOP reduction in these glaucoma subtypes. However, its effectiveness in IOP reduction in glaucoma associated with uveitis or neovascularization is probably questionable in addition to the possibility of aggravation of inflammation. Although PGs are shown to be effective in IOP reduction for chronic angle closure glaucoma, its effectiveness is unreliable if a quick reduction of IOP is desirable such as in acute angle closure attack, because of its relatively slow onset of action.
Position of bimatoprost in the management of glaucoma
Therapeutic use of topical PGs in management of glaucoma has grown rapidly over the past decade, and it now surpasses nonselective β-blockers as the first choice of glaucoma medical therapy due to the excellent efficacy and systemic safety profiles as well as improved compliance with once-a-day usage. Bimatoprost is currently approved by the FDA and the EC for first-line therapy for the reduction of elevated IOP in patients with open-angle glaucoma or ocular hypertension.
There is subtle yet important distinction between the concepts of first-line versus first-choice medical therapy. Topouzis clarified that first-line treatment could be defined as a treatment that has been approved by an official regulatory body as an initial therapy to control IOP, whereas a first-choice treatment is one that a physician prefers to use as an initial IOP-lowering therapy (CitationTopouzis 2006). In the healthcare system of certain countries, a designation of first-line therapy may be a result of nonmedical decisions including the cost of medication. While first choice therapy is the result of the physician’s medical judgment based on experience and medical evidence and the patient’s decision (CitationTopouzis 2006). The influence of first-line concept on first-choice of medical therapy often cannot be ignored. In an ophthalmic practice environment that physicians have the freedom to choose the best topical glaucoma therapy possible for their patients, the factors of first-choice consideration include the ability of the drug to lower IOP, contra-indication and adverse effects, and treatment convenience and cost. Based on these criteria for evaluation, bimatoprost is highly effective for IOP reduction, has excellent systemic safety profiles, and is easy to use with once-daily schedule. In terms of local adverse profile, it is associated with a higher rate of conjunctival hyperemia, pigmentation of the periocular skin and iris, and eyelash darkening compared with other PGs. However, in a study of assessing the feasibility of an automatic switch of a large number of patients from latanoprost to bimatoprost in a prepaid health maintenance organization, a large majority of patients (87% based on computerized ophthalmic medication dispensing record, and 89.3% based on clinical data of one clinical facility) stay with bimatoprost after the switch (CitationLaw et al 2005). Being a newer class of antiglaucoma medications without available generic substitutes, brimatoprost is generally more expensive than other classes of antiglaucoma medications.
Decision of first-choice medical therapy should be based on an individualized approach. A good choice of medication does not necessarily equate to appropriate use of medication. Some general principles of medication use apply to bimatoprost as well. It is well recognized that responses to medical therapy vary among individual patients. Therefore, enough time should be allowed for the clinician to determine whether the patient has responded to the medications or not. Once the patient has started a new medicine, he should be monitored for adverse reactions. Commonly, some patients will experience mild side effects, such as ocular irritation or blurred vision, when first using a topical medication. Thus, a useful follow-up time interval is about 2–4 weeks to determine the clinical effectiveness of therapy and the patient’s ability to tolerate the medication. Lack of compliance to medical therapy is a major hurdle for glaucoma control. In the follow-up evaluations, the patient’s compliance, the correct method of instillation of the drops, the proper storage of medications, and the appropriate treatment schedule should be reviewed with the patient and family or caregiver, if present (CitationLaw and Coleman 2000). Overall, a proactive approach taken by the prescribing physician may translate into a long-lasting patient–physician relationship and better treatment outcome.
Summary
In summary, the goal of treating patients with glaucoma is to minimize any adverse impact of the disease and treatment on the patient’s life. Bimatoprost, based on the efficacy, safety and ease of use, has been approved as a first-line treatment for glaucoma, and well positioned among the first-choices in glaucoma medical therapy.
Disclosures
The author has no proprietary interest in the development or marketing of any of the products or devices mentioned in the study.
References
- AndersonDR1989Glaucoma: The damage caused by pressure. XLVI Edward Jackson Memorial LectureAm J Ophthalmol108485952683792
- AngAReddyMAShepstoneL2004Long term effect of latanoprost on intraocular pressure in normal tension glaucomaBr J Ophthalmol88630415090413
- ArmalyMFKruegerDEMaunderLR1980Biostatistical analysis of the collaborative glaucoma study. I. Summary report of the risk factors for glaucomatous visual-field defectsArch Ophthalmol982163717447768
- AungTWongHTYipCC2000Comparison of the intraocular pressure-lowering effect of latanoprost and timolol in patients with chronic angle closure glaucoma: a preliminary studyOphthalmology10711788310857840
- BrandtJDVanDenburghAMChenK2001Bimatoprost Study Group. Comparison of once- or twice-daily bimatoprost with twice-daily timolol in patients with elevated IOP: a 3-month clinical trialOphthalmology10810233111382623
- BrubakerRF2001Mechanism of action of bimatoprost (Lumigan)Surv Ophthalmol45Suppl 4S3475111434937
- BrubakerRFSchoffEONauCB2001Effects of AGN 192024, a new ocular hypotensive agent, on aqueous dynamicsAm J Ophthalmol131192411162974
- CamrasCBTorisCBSjoquistB2004Detection of the free acid of bimatoprost in aqueous humor samples from human eyes treated with bimatoprost before cataract surgeryOphthalmology1112193815582073
- CarrilloMMNicolelaMT2004Cystoid macular edema in a low-risk patient after switching from latanoprost to bimatoprostAm J Ophthalmol137966815126179
- ChewPTAungTAquinoMVEXACT Study Group2004IOP reducing effects and safety of latanoprost versus timolol in patients with chronic angle-closure glaucomaOphthalmology1114273415019314
- ColemanALLernerFBernsteinP2003A 3-month randomized controlled trial of bimatoprost (LUMIGAN) versus combined timolol and dorzolamide (Cosopt) in patients with glaucoma or ocular hypertensionOphthalmology1102362814644719
- ColemanRASmithWLNarumiyaS1994International union of pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypesPharmacol Rev4620597938166
- DavidRLivingstonDGLuntzMH1977Ocular hypertension: A long-term follow-up of treated and untreated patientsBr J Ophthalmol6166874588521
- DaviesSSJuWKNeufeldAH2003Hydrolysis of bimatoprost (Lumigan) to its free acid by ocular tissue in vitroJ Ocul Pharmacol Ther19455412648303
- De SantisMLuccheseACarducciB2004Latanoprost exposure in pregnancyAm J Ophthalmol138305615289149
- DoiLMMeloLAPrataJA2005Effects of the combination olf bimatoprost and latanoprost on intraocular pressure in primary open angle glaucoma: a randomized clinical trialBr J Ophthalmol89547915834081
- DubinerHCookeDDirksM2001Efficacy and safety of bimatoprost in patients with elevated intraocular pressure: a 30-day comparison with latanoprostSur ophthalmol45Suppl 4S35360
- FechtnerRDKhouriASZimmermanTJ1998Anterior uveitis associated with latanoprostAm J Ophthalmol12637419683147
- GagliusoDJWangRFMittagTW2004Additivity of bimatoprost or travaprost to latanoprost in glaucomatous monkey eyesArch Ophthalmol1221342715364714
- GandolfiSSimmonsSTSturmR2001Three-month comparison of bimatoprost and latanoprost in patients with glaucoma and ocular hypertensionAdv Ther181102111571823
- GiuffreG1985The effects of prostaglandin F2α in the human eyeGraefes Arch Clin Exp Ophthalmol222139413856545
- GordonMOBeiserJABrandtJD2002The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucomaArch Ophthalmol1207142012049575
- GordonYJYatesKAMahFS2003The effects of Xalatan on the recovery of ocular herpes simplex virus type 1 (HSV-1) in the induced reactivation and spontaneous shedding rabbit modelsJ Ocul Pharmacol Ther192334512828841
- HellbergMRKeTLHaggardK2003The hydrolysis of the prostaglandin analog prodrug bimatoprost to 17-phenyl-trinor PGF2alpha by human and rabbit ocular tissueJ Ocul Pharmacol Ther199710312804054
- HerndonLWAsraniSGWilliamsGH2002Paradoxical intraocular pressure elevation after combined therapy with latanoprost and bimatoprostArch Ophthalmol120847912049597
- HollowsFCGrahamPA1966Intraocular pressure, glaucoma, and glaucoma suspects in a defined populationBr J Ophthalmol50570865954089
- HungPTHsiehJWChenYF2000Efficacy of latanoprost as an adjunct to medical therapy for residual angle-closure glaucoma after iridectomyJ Ocul Pharmacol Ther1643710673130
- KaufmanHEVarnellEDThompsonHW1999Latanprost increases the severity and recurrence of herpetic keratitis in the rabbitAm J Ophthalmol127531610334345
- KolkerAE1972Glaucoma family study: ten-year follow-up (preliminary report)Isr J Med Sci81357614647787
- KonstasAGKatsimbrisJMLallosN2005Latanoprost 0.005% versus bimatoprost 0.03% in primary open-angle glaucoma patientsOphthalmology11226226615691561
- KonstasAGKozobolisVPTersisI2003The efficacy and safety of the timolol/dorzolamide fixed combination vs latanoprost in exfoliation glaucomaEye1741612579169
- KonstasAGMylopoulosNKarabatsasCH2004Diurnal intraocular pressure reduction with latanoprost 0.005% compared to timolol maleate 0.5% as monotherapy in subjects with exfoliation glaucomaEye18893915002024
- KookMSChoHSYangSJ2005Efficacy of latanprost in patients with chronic angle closure glaucoma and no visible ciliary body face: a preliminary studyJ Ocul Pharmacol Ther21758415718831
- LaibovitzRAVanDenburghAMFelixC2001Comparison of the ocular hypotensive lipid AGN 192024 with timolol: dosing, efficacy, and safety evaluation of a novel compound for glaucoma managementArch Ophthalmol119994100011448321
- LawSKCaprioliChapter 56: Medical therapy of glaucomaTasmanWJaegerEADuane’s Clinical OphthalmologyBaltimoreLippincott Williams and Wilkins
- LawSKColemanAL2000Following the progress of patients with glaucomaComp Ophthalmol Update1319
- LawSKSongJFangE2005Feasibility and efficacy of a mass switch from latanoprost to bimatoprost in glaucoma patients in a pre-paid health maintenance organizationOphthalmology11221233016225924
- LeeYC2005Abdominal cramp as an adverse effect of travoprostAm J Ophthalmol139202315652856
- LindseyJDToHDWeinrebRN1994Induction of c-fos by prostaglandin F2α in human ciliary smooth muscle cellsInvest Ophthalmol Vis Sci35242508300352
- McKibbinMMenageMJ1999The effect of once-daily latanoprost on intraocular pressure and pulsatile ocular blood flow in normal tension glaucomaEye1331410396380
- MastropasquaLCarpinetoPCiancagliniM1999A 12-month, randomized, double-masked study comparing latanoprost with timolol in pigmentary glaucomaOphthalmology106550510080213
- MaxeyKMJohnsonJLLaBrecqueJ2002The hydrolysis of bimatoprost in corneal tissue generates a potent prostanoid FP receptor agonistSurv Ophthalmol47Suppl 1S344012204699
- MitraMChangBJamesT2001Drug points. Exacerbation of angina associated with latanoprostBMJ32378311588081
- MoralesJShihabZMBrownSM2001Herpes simplex virus dermatitis in patients using latanoprostAm J Ophthalmol132114611438068
- NoeckerRSDirksMSChoplinNT2003Bimatoprost/Latanoprost Study Group. A six-month randomized clinical trial comparing the intraocular pressure-lowering efficacy of bimatoprost and lantanoprost in patients with ocular hypertension or glaucomaAm J Ophthalmol135556312504698
- ParrishRKPalmbergPSheuWPXLT Study Group2003A comparison of lantaoprost, bimatoprost, and travaprost in patients with elevated intraocular pressure: a 12-week, randomized, masked-evaluator multicenter studyAm J Ophthalmol13568870312719078
- PeakASSuttonBM1998Systemic adverse effects associated with topically applied latanoprostAnn Pharmacother3250459562149
- RichterMKraussAHWoodwardDF2003Morphological changes in the anterior eye segment after long-term treatment with different receptor selective prostaglandin agonists and a prostamideInvest Ophthalmol Vis Sci4444192614507888
- RuloAHGreveELGeijssenHC1996Reduction of intraocular pressure with treatment of latanoprost once daily in patients with normal-pressure glaucomaOphthalmology1031276828764799
- SaccaSPascottoASiniscalchiC2001Ocular complications of latanoprost in uveitic glaucoma: three case reportsJ Ocul Pharmacol Ther171071311324978
- SchachtschabelULindseyJDWeinrebRN2000The mechanism of action of prostaglandins on uveoscleral outflowCurr Opin Ophthalmol11112510848216
- SharifNAKellyCRCriderJY2002Agonist activity of bimatoprost, travoprost, latanoprost, unoprostone isopropyl ester and other prostaglandin analogs at the cloned human ciliary body FP prostaglandin receptorJ Ocul Pharmacol Ther183132412222762
- SherwoodMBrandtJBimatoprost Study Groups 1 and 22001Six-month comparison of bimatoprost once-daily and twice-daily with timolol twice-daily in patients with elevated intraocular pressureSurv Ophthalmol45Suppl 4S361811434939
- SihotaRSaxenaRAgarwalHC2004Crossover comparison of timolol and latanoprost in chronic primary angle-closure glaucomaArch Ophthalmol122185914769594
- SmithSLPruittCASineCS1999Latanoprost 0.005% and anterior segment uveitisActa Ophthalmol Scand776687210634560
- SommerA1989Intraocular pressure and glaucomaAm J Ophthalmol10718682913813
- SommerATielschJMKatzJ1991Relationship between intra-ocular pressure and primary open angle glaucoma in black and white Americans: The Baltimore Eye StudyArch Ophthalmol109109051867550
- StewartWCKolkerAEStewartJA2003Conjunctical hyperemia in healthy subjects after short-term dosing with latanoprost, bimatoprost, and travoprostAm J Ophthalmol1353142012614748
- The Eye Diseases Prevalence Research Group2004Prevalence of open-angle glaucoma among adults in the United StatesArch ophthalmol12253253815078671
- TielschJMSommerAKatzJ1991Racial variations in the prevalence of primary open angle glaucoma. The Baltimore Eye SurveyJAMA266369742056646
- TopouzisF2006First line versus first choiceInternational glaucoma review supplement735
- TorisCBCamrasCBYablonskiME1997Effects of exogenous prostaglandins on aqueous humor dynamics and blood-aqueous barrier functionSurv Ophthalmol41Suppl 2S69759154279
- van der ValkRWebersCABSchoutenJSAG2005Intraocular pressure-lowering effects of all commonly used glaucoma drugsOphthalmology11211778515921747
- WandMGilbertCMLiesegangTJ1999Latanoprost and herpes simplex keratitisAm J Ophthalmol127602410334356
- WarwarREBullockJDBallalD1998Cystoid macular edema and anterior uveitis associated with latanoprost use. Experience and incidence in a retrospective review of 94 patientsOphthalmology10526389479285
- WestonBC2001Migraine headache associated with latanoprostArch Ophthalmol119300111176999
- WoodwardDFKraussAHChenJ2001The pharmacology of bimatoprost (Lumigan)Surv Ophthalmol45Suppl 4S3374511434936
- WoodwardDFKraussAHChenJ2003Pharmacological characterization of a novel antiglaucoma agent, Bimatoprost (AGN 192024)J Pharmacol Exp Ther3057728512606640
- ZhanGLCamrasCBOpereC1998Effect of prostaglandins on cyclic AMP production in cultured human ciliary muscle cellsJ Ocul Pharmacol Ther1445559493782