Abstract
Optic neuropathy is a frequent cause of vision loss encountered by ophthalmologist. The diagnosis is made on clinical grounds. The history often points to the possible etiology of the optic neuropathy. A rapid onset is typical of demyelinating, inflammatory, ischemic and traumatic causes. A gradual course points to compressive, toxic/nutritional and hereditary causes. The classic clinical signs of optic neuropathy are visual field defect, dyschromatopsia, and abnormal papillary response. There are ancillary investigations that can support the diagnosis of optic neuropathy. Visual field testing by either manual kinetic or automated static perimetry is critical in the diagnosis. Neuro-imaging of the brain and orbit is essential in many optic neuropathies including demyelinating and compressive. Newer technologies in the evaluation of optic neuropathies include multifocal visual evoked potentials and optic coherence tomography.
Keywords:
- optic neuropathy
- optic neuritis
- non-arteritic anterior ischemic optic neuropathy (NAION)
- arteritic anterior ischemic optic neuropathy (AION)
- traumatic optic neuropathy
- Leber’s optic neuropathy
- dominant optic atrophy
- recessive optic atrophy
- radiation optic neuropathy
- optical coherence tomography
- multiple sclerosis
Diagnosis
History
The mode of onset of visual loss is an important clue to the etiology of the optic neuropathy. Rapid onset is characteristic of optic neuritis, ischemic optic neuropathy, inflammatory (non-demyelinating) and traumatic optic neuropathy. A gradual onset over months is typical of compressive toxic/nutritional optic neuropathy. A more protracted history over years is seen in compressive and hereditary optic neuropathies. The ophthalmologist should make an effort in eliciting history of associated symptoms that can help refine the differential diagnosis. In a young patient, history of eye pain associated with eye movement, prior history of neurological symptoms such as paresthesia, limb weakness, and ataxia is suggestive of demyelinating optic neuritis. These neurological complaints may have been misdiagnosed or dismissed by other physicians. For example, chronic arm paresthesia is usually initially misdiagnosed as “carpal tunnel syndrome”. Diminution of vision following a rise in body temperature such as following exercise or a hot shower is known as Uhthoff’s phenomenon. Although this symptom is usually associated with demyelinating optic neuropathy, it has been reported in a variety of other optic neuropathies including Leber’s hereditary optic neuropathy, and optic neuropathy in sarcoidosis (CitationRiordan-Eva et al 1995; CitationHaupert and Newman 1997).
In an elderly with signs of optic neuropathy, the presence of preceding transient visual loss, diplopia, temporal pain, jaw claudications, fatigue, weight loss and myalgias is strongly suggestive of arteritic ischemic optic neuropathy (AION) due to giant cell arteritis (GCA). In children, history of recent flu-like illness or vaccination days or weeks before vision loss points to a para-infectious or post-vaccinial optic neuritis, respectively.
Symptoms such as diplopia and facial pain are suggestive of multiple cranial neuropathies seen in inflammatory or neoplastic lesions of the posterior orbit or parasellar region. Transient visual obscurations (periods of vision blackouts lasting seconds caused by change in body position), transient diplopia and headache should raise the suspicion of increased intra-cranial pressure.
The use of any medications should be carefully noted since some are either directly or indirectly toxic to the optic nerve. These include drugs as ethambutol, amiodarone, alcohol, and immunosuppressive medications such as methotrexate and cyclosporine.
History of diabetes, hypertension and hypercholesterolemia is common in patients with non-arteritic ischemic optic neuropathy (NAION). Patients who are being treated for or have history of malignancy may have infiltrative or para-neoplastic optic neuropathy.
Inquiry into the patient’s general health, eating and social habits (drinking, smoking) is important in suspected toxic/nutritional optic neuropathy. A detailed family history is important in diagnosing hereditary autosomal and mitochondrial optic neuropathies.
Neuro-ophthalmic examination
Acute or chronic vision loss can be caused by any diseases of the anterior visual pathway including the retinal diseases. In many cases, the signs of retinopathy are subtle and it is difficult to determine if the cause of vision loss is a retinopathy or optic neuropathy. Therefore, it is important to establish the classic signs of optic neuropathy; visual field defect, decreased color vision, and the presence of relative afferent papillary defect (RAPD).
Visual acuity can be normal or impaired depending on whether the central visual field is affected. In many cases, visual acuity is normal, yet the patient as a large visual field defect that spares the central field. In the optic neuritis treatment trial, 11% of patients had a visual acuity of 20/20 or better (CitationBeck et al 2003). Color vision can be assessed by using the Ishihara color plates or the American Optical Hardy-Ritter-Rand (AOHRR) pseudoisochromatic color plates. Most patients with acquired optic neuropathy will have dyschromatopsia. However, some patients, especially those with ischemic optic neuropathies may have normal color vision at least in their intact visual field areas. When assessing color vision, it is important to not only assess the number of plates recognized by each eye, but also the speed with which they are recognized and the patients’ subjective comparison between each eye. Dyschromatopsia can also occur in macular disease but in this case the visual acuity tends to be profoundly affected. A mild to moderate visual acuity loss in association with dyschromatopsia is a sensitive indicator of optic neuropathy.
A RAPD can be detected by performing the swinging light-pupil test. In the presence of bilateral symmetric optic neuropathy, a RAPD may be absent and the briskness of pupillary constriction to light will reflect the degree of optic nerve dysfunction. When checking for RAPD, it is important to use a very bright light in a dark room to assess the full amplitude of pupillary response. A RAPD can be estimated subjectively by asking the patient about the difference in brightness of light presented in front of each eye.
Patients with optic neuropathy often have red color desaturation and a red-capped bottle can be presented to each eye and the patient can be asked about the difference in brightness of the red color. A positive response would be that the red color looks “faded”, “pink” or “washed out”.
Fundus examination may show normal, swollen, pale or anamolous optic disc. The optic disc is usually swollen in NAION and inflammatory (non-demyelinating) optic neuritis. In demyelinating optic neuritis, however, the optic disc is normal in 65% of cases (retrobulbar neuritis). If disc swelling is present in demyelinating optic neuritis, it is usually mild and diffuse in nature. Severe disc swelling with the presence of hemorrhages and exudates is very atypical of demyelinating optic neuritis and should point to an alternative etiology such as NAION, inflammatory, or infiltrative optic neuropathy. In NAION, disc swelling can be sectoral or diffuse and typically associated with peripaillary hemorrhages. A small cup-to-disc ratio (also called disc at risk or congenitally anamolous) is seen in the other eye. Diffuse pallid or “chalky white” swelling with cotton wool spots is usually seen in AION due to GCA. Temporal pallor is seen in conditions that selectively affect the papillo-macular bundle such as toxic/nutritional and hereditary optic neuropathies.
The ophthalmologist should always look for evidence of uveitis such as cells in the anterior vitreous or signs of posterior uveitis such as retinal vasculitis, retinitis, and choroiditis. This would indicate that the disc swelling is secondary to a uveitic process.
A pale optic disc indicates long-standing optic neuropathy such as compressive, hereditary, toxic/nutritional optic neuropathies. This can also occur as a sequel of an acute inflammatory or ischemic optic neuropathy. Sectoral pallor with retinal arteriole attenuation should point to a previous NAION. When optic atrophy is present, it is possible to detect slit-like or wedge-shaped defects in the peripapillary nerve fiber layer. The red-free ophthalmoscope is most useful to detect these subtle changes.
Diffuse cupping with pallor occurs in the end stage following AION (CitationDanesh-Meyer et al 2001). Occasionally, optic disc cupping is seen and the disc appearance may resemble glaucomatous disc cupping. Pallor of the neuro-retinal rim is a specific but insensitive sign of non-glaucomatous cupping. Cupping of the optic nerve head has been described in congenital optic disc anamolies, compressive optic neuropathy from large intra-cranial aneurysms or masses, hereditary optic neuropathy (dominant optic atrophy, Leber’s mitochondrial optic neuropathy), radiation optic neuropathy and methanol poisoning (CitationTrobe et al 1980a, Citation1980b; CitationBianchi-Marzoli et al 1995) The ophthalmologist should carefully consider these underlying causes before resorting to the diagnosis of “low tension glaucoma”.
An anamolous optic nerve indicates a congenital anamoly such as optic nerve hypopolasia, optic nerve coloboma, morning-glory disc anamoly and optic nerve drusen. These entities will not be discussed further and the discussion will be limited to acquired optic neuropathies.
Investigations
Visual field testing
Visual filed testing is an integral component of the neuro-ophthalmic examination and is critical in the diagnosis of optic neuropathy. Both manual kinetic or automated static perimetry can be used. The visual field defects in optic neuropathies can take several patterns including central, diffuse, arcuate, and altitudinal defect. The pattern of visual filed defect is not specific of any etiology and almost any type of field defect can occur with any optic neuropathy. However, altitudinal defects are more common in ischemic optic neuropathies and central, or cecocentral defects frequently accompany toxic/nutritional and hereditary optic neuropathies. A cental scotoma affects central fixation and is due to a lesion in the fovea or papillomacular bundle. A cecocentral sctoma extends from fixation towards the blind-spot and is due to invelvement of the papillomacular bundle arising from the fovea towards the optic disc. A hemianopic defect respecting the vertical midline indicates a lesion at or posterior the chiasm. A junctional scotoma, defined as ipsilateral central field defect and contralateral superotemporal field defect indicates a compressive lesion at the junction of the optic nerve and the chiasm.
Contrast sensitivity
Contrast sensitivity is usually reduced in patients with optic neuropathy. Testing charts such as the perri-robson charts can be useful in patients with normal snellen visual acuity. The sensitivity and specificity of this tool, however, is yet to be determined.
Electrophysiological tests
Visual evoked potential (VEP) can be abnormal in any optic neuropathy but it is no substitute to a careful clinical examination. VEPs measure the cortical activity in response to flash or pattern stimulus. They are abnormal in the presence of any lesion along the anterior visual pathway. Although VEP is not necessary in the diagnosis of optic neuropathy, it can be useful in patients with early or sub-clinical optic neuropathy who may have normal pupillary responses and no discernible optic disc changes on clinical examination. VEPs are used commonly in patients with demyelinating disease to detect occult optic nerve dysfunction and to identify a second site of involvement as part of the neurological assessment. Pettern ERG (PERG) can be abnormal in dysfunction of the macula or retinal ganglion cells, which do not contribute significantly to the full field ERG. Therefore, PERG can be useful in a patient with abnormal VEP to identify a macular lesion. A more recent technology, multifocal VEP, has been shown to be of useful values in detecting optic neuritis patients at high-risk patients to develop clinically definite multiple sclerosis (CitationFraser et al 2006). Panfield eletroretinography can be useful to rule out diffuse retinal diseases such as retinitis pigmentosa and multifocal electroretinography can be useful to distinguish macular disease from optic neuropathy.
Optical coherence tomography (OCT)
OCT is a new technology that uses low coherence light to penetrate tissue and a camera to analyze the reflected image. By performing circular scans around the optic nerve head, the peripapillary nerve fiber layer can be analyzed. This has been useful in the follow up of patients with optic neuritis, traumatic optic neuropathy, and Leber’s hereditary optic neuropathy (CitationMedeiros et al 2003; CitationBarboni et al 2005; CitationLim et al 2005; CitationCostello et al 2006; CitationPro et al 2006; CitationTrip et al 2006). OCT can also be used to document peripaillary nerve fiber layer thickening in subtle NAION cases and to follow up resolution of disc edema (CitationSavini et al 2006).
Specific diagnosis
Acute demyelinating optic neuritis
Because optic neuritis can be the initial manifestation of multiple sclerosis (MS), its recognition is of important prognostic value. Optic neuritis is more common in females with a peak age of onset between 30–40 years (CitationBeck et al 2003). Magnetic resonance imaging (MRI) of the brain is needed to detect the presence of white matter lesions. The risk of developing clinically definite multiple sclerosis over 10 years is 56% if one or more baseline lesion 3 mm diameter is found, whereas the risk in normal MRI is 22% (). The risk is low (5%) in males with severe disc edema with peripaillary hemorrhages and normal MRI (CitationBeck et al 2003). Initiating treatment with beta-1a interferon should be strongly considered in high-risk patients since this has been found to decrease the risk of conversion to clinically definite multiple sclerosis by 50% (CitationBeck et al 2002).
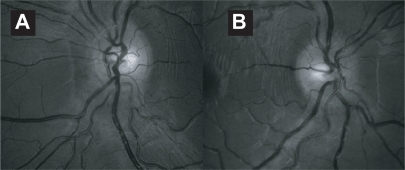
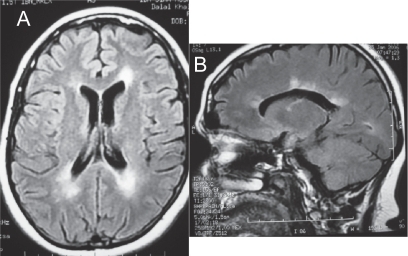
Figure 1 (A) Axial MRI of the brain (FLAIR sequence) showing the classic periventricular white matter lesions seen in MS. (B) Sagittal MRI showing peri-collosal white matter lesions also known as “Dawson’s fingers”.
Optic neuritis improves in 90% of cases over several weeks to near normal visual acuity (CitationBeck 1995). The use of intravenous steroids was found to hasten the visual recovery but not the final visual outcome (CitationBeck 1995). However, the early use of steroids may be warranted to minimize axonal loss of the optic nerve, which has been found to occur early in the disease (CitationArnold 2005; CitationTrip et al 2005; CitationCostello et al 2006). Lack of improvement after about 4–6 weeks with or without steroids is atypical of optic neuritis and should prompt searching for an alternative causes such as inflammatory (sarcoid, vasculitis), infiltrative and compressive. In such cases, the recommended additional investigations include spinal tap, complete blood count, antinuclear antibodies (ANA), chest radiographs, angiotensin converting enzyme levels (ACE), and anti-neutrophil cytoplasmic antibodies (ANCA) (CitationLee et al 2000).
Optic neuritis in children is classically thought to differ from adult optic neuritis by frequent bilateral involvement and disc swelling, and more severe initial vision loss. A history of viral infection or vaccination is common (). Other neurological manifestations may occur if there is concurrent acute disseminated encephalomyelitis (CitationBangsgaard et al 2006). MRI of the brain and orbit is recommended to rule out demyelination, and meningeal enhancement seen in infectious or non-infectious meningitis. Contrary to the common notion that childhood optic neuritis is less likely to be associated with multiple sclerosis (CitationLucchinetti et al 1997), a recent report found an association with MS in 36% of patients with optic neuritis, especially with bilateral involvement (CitationWilejto et al 2006).
Neuromyelitis optica (Devic’s disease)
When optic neuritis is preceded or followed by transverse or ascending myelopathy often causing paraplegia, the condition is referred to as neuromyelitis optica (NMO). This condition is more common in young patients with a predilection for Asians and Africans (CitationCabre et al 2001; CitationNakashima et al 2006). The visual loss is often severe, bilateral and may precede or follow the myelopathy. The interval between myelopathy and optic neuritis can be months to years. MRI of the spine will show demyelination extending over 3 or more vertebral segments. There is a debate about whether NMO is simply a sub-type of MS (CitationRubiera et al 2006). Although, lesions outside the optic nerve and spinal cord were considered incompatible with NMO, the criteria of diagnosis have been revised recently to allow for the presence of MRI brain lesion “not compatible with MS” (CitationPittock et al 2006; CitationWingerchuk et al 2006). A serum marker, NMO Ig-G, has been found useful to distinguish NMO from other related disorders such as MS (CitationLennon et al 2004).
Non-arteritic ischemic optic neuropathy (NAION)
NAION can occasionally be confused with acute demyelinating optic neuritis. There are some features that may help distinguishing the two conditions. The presence of severe disc edema with hemorrhages is characteristic of NAION and atypical of optic neuritis (). Patients with NAION are usually over 50 years old and have systemic vascular risk factors such as diabetes, hypertension, and smoking (CitationTsai et al 1998; CitationMcCulley et al 2005). Nevertheless, NAION can occur in young patients (below 45 years) and patients who lack any vascular risk factors. In young patients, it has been associated with hypercholesterolemia and hyperhomocysteinemia (CitationPianka et al 2000; CitationDeramo et al 2003). Visual acuity can be normal or severely affected. The visual field defect is usually inferonasal arcuate or altitudinal. The optic nerve head in the other eye is often has small cup-to-disc ratio (0.1 or less) (CitationBurde 1993; CitationFeldon 1999). This optic nerve head configuration seems to play a more important role than the systemic risk factors (CitationTsai et al 1998). Recent reports have linked NAION to the sleep apnea syndrome (CitationMojon et al 2002; CitationPalombi et al 2006), but it is unknown whether treatment of sleep apnea can prevent NAION (CitationBehbehani, Mathews et al 2005).
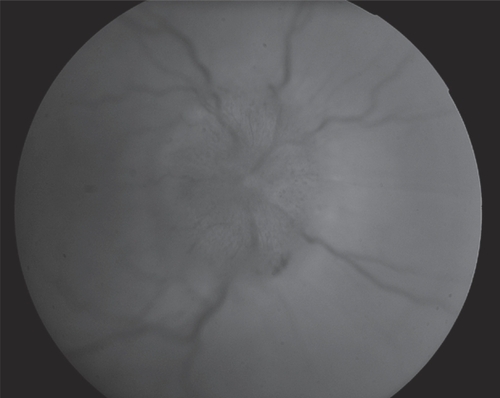
Figure 3 A 57 year old patient with history of hypertension, diabetes and hypercholesterolemia and NAION in his right eye. There is pallid swelling of the right disc with hemorrhage superiorly. The left optic disc has a cup to disc ratio of 0.1 (not shown).
Visual acuity usually remains static or improves slightly in the vast majority of patients with NAION (CitationArnold and Hepler 1994). In a small subset of patients, visual acuity may actually worsen over the first few weeks (progressive NAION) (CitationSergott et al 1989; CitationSpoor et al 1993; CitationArnold and Hepler 1994). This progressive phase may cause a diagnostic difficulty but it rarely lasts more than 3–4 weeks and is then followed by stabilization of visual function. Bilateral simultaneous involvement is very unusual of NAION and but can occur during a hypotensive episode such as blood loss or over-dosing of anti-hypertensive medications (CitationHayreh 1999). Recurrence in the same eye is uncommon with a risk of 5% (CitationHayreh et al 2001). The risk of later involvement in the follow eye within 5 years is 15% (CitationNewman et al 2002).
In posterior ischemic optic neuropathy (PION) there is no disk swelling. PION can occur in severe blood loss, hypotension, hemodialysis, anemia, and after long surgeries, such as spinal surgeries, in which the patient is positioned prone (CitationBuono et al 2003; CitationMurphy 2003; CitationHayreh 2004). The visual loss is often bilateral but can be asymmetrical. Pupillary responses to light will be sluggish, which helps distinguish PION from a cortical cause of vision loss.
Arteritic ischemic optic neuropathy (AION)
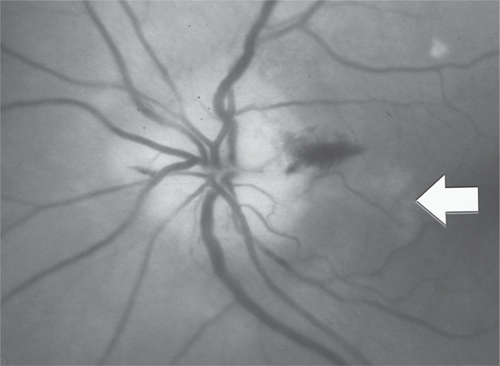
In patients over 60 year old with features of ischemic optic neuropathy, the ophthalmologist should strongly consider the possibility of giant cell arteritis (GCA). Careful medical history should be obtained, inquiring about temporal pain, jaw claudications, transient visual or diplopia, fever, weight loss, myalgias and fatigue (CitationHayreh et al 1998b). Some patients may not have the constitutional symptoms and have only visual symptoms (occult GCA) (CitationLiu et al 1994; CitationHayreh et al 1998a). Other clinical features that may help distinguishing patients with AION from NAION include cup-to-disk ratio of greater than 0.2 in the other eye, early massive or bilateral simultaneous visual loss, markedly pallid disk edema often described as “chalky-white” swelling in 68.7% of cases, and choroidal infarcts () (CitationHayreh et al 1998a, Citation1998b). Rarely, the optic disc can look normal (PION) (CitationSadda et al 2001). End-stage optic disc appearance of patients with AION is characterized by marked cupping with pallor and should not be confused with glaucomatous cupping (CitationDanesh-Meyer et al 2001). The superficial temporal arteries should be palpated and the pulse felt. Patients with GCA have a “cord-like” feel with reduced pulses.
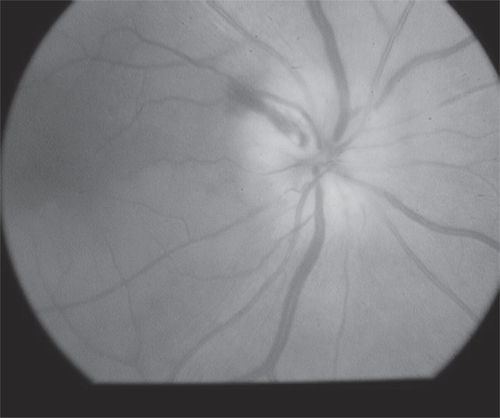
Figure 4 Disc swelling in GCA. Note pallid disc swelling with hemorrhages and an adjacent area of choroidal infarction (arrow) (courtesy of Peter J Savino, MD).
Laboratory evaluation should include complete blood count, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP). ESR is elevated in GCA but 2–30% of patients with biopsy-proven GCA will have normal ESR (CitationBrittain et al 1991; CitationLiu, Glaser et al 1994; CitationHayreh et al 1997; CitationSalvarani and Hunder 2001). Elevated CRP was found to be more sensitive (97.5%) than ESR (76%–86%) for the detection of GCA. ESR and CRP combined give the best specificity (97.0%) and sensitivity (99%) for diagnosis (CitationHayreh et al 1997; CitationParikh et al 2006). Thrombocytosis is another useful feature that can complement ESR and CRP (CitationLincoff et al 2000; CitationCostello et al 2004).
Steroid treatment should be instituted in patients who are considered at high risk to have GCA based on the clinical and laboratory features. Untreated GCA will cause visual loss in the fellow eye in about a third of cases with the risk highest in the first 4 weeks. Either oral (80–120 mg prednisone) or intravenous (1 gram methyleprdnisolone) for 3–5 days followed by oral steroids can be used. There is no evidence that either route of administration is more effective in preventing possible early visual deterioration (CitationHayreh and Zimmerman 2003). There are reports of the successful use of heparin if visual deterioration occurs despite being on steroid treatment (CitationBuono et al 2004).
Definitive diagnosis of AION is established by temporal artery biopsy and histopathological confirmation. Although the inflammation can be seen histopathologically in the vessels for weeks despite steroid therapy, temporal artery biopsy should be performed as soon as the patient is started on steroids to avoid equivocal results. Jaw claudications and neck pain are significant indicators of a positive temporal artery biopsy independent of the ESR and CRP (CitationHayreh et al 1997) The odds ratio for a positive temporal artery biopsy was 9.0 times greater with jaw claudications, 3.3 greater with neck pain, and 3.2 greater with an elevated ESR and CRP (CitationHayreh et al 1997). Biopsy can be performed on one side and if it is negative and the index of suspicion of GCA is high, a biopsy is performed the contra-lateral side. Some authors advocate bilateral temporal artery biopsies at the onset because an overall chance of discordance of 4%–5% (CitationDanesh-Meyer et al 2000; CitationPless et al 2000).
Inflammatory (non-demyelinating) optic neuropathy
This category comprises many entities in which the optic nerve is involved by either an ocular or systemic inflammatory process. Optic disc swelling frequently occurs with posterior uveitis and retinitis. Therefore, when evaluating optic disk swelling, it is useful to look for evidence of anterior or posterior segment inflammation. Optic neuropathy can also occur in the context orbital inflammatory disease (orbital pseudotumor). MRI of the orbit will show inflammation of the optic nerve sheath (optic perineuritis) (CitationFay et al 1997).
The optic nerve can be involved in a variety of systemic auto-immune and infectious disorders such as sarcoidosis, systemic lupus erythematosus, Behcet’s disease inflammatory bowel disease, Sjogren’s syndrome Wegener’s granulomatosis, syphilis, Lyme disease and cat-scratch disease (CitationJabs et al 1986; CitationWise and Agudelo 1988; CitationKansu et al 1989; CitationBelden et al 1993; CitationHan et al 2006).
Auto-immune optic neuropathy is a recurrent, steroid-responsive optic neuropathy. The anti-nuclear antibody (ANA) and anticardiolipin antibody are frequently positive (CitationKupersmith et al 1988; CitationFrohman et al 2003). The diagnosis is made by performing a skin biopsy, which often shows evidence of vasculitis when studied with immunoflorescence.
Chronic relapsing inflammatory optic neuropathy is another entity characterized by recurrences and steroid-responsiveness. The syndrome can behave as granulomatous optic neuropathy and may require long-term immunosuppressive therapy (CitationKidd et al 2003).
Some of the features that should raise the suspicion of an inflammatory optic neuritis include lack of spontaneous improvement of visual function after 30 days, or exquisite steroid-responsiveness and steroid-dependency. In such cases, a spinal tap and additional laboratory studies directed by the history and neuro-ophthalmic examination are indicated (see discussion under demyelinating optic neuritis).
Infiltrative optic neuropathies
The optic nerve can be infiltrated in systemic malignancies such as lymphoma, leukemia, multiple myeloma, and carcinoma (CitationBrown et al 1981; CitationBrazis et al 1990; CitationBehbehani, Vacarezza et al 2005; CitationShimada et al 2006). The optic disc can be swollen or normal in appearance (). MRI of the brain and orbit may show meningeal and optic nerve enhancement. Spinal tap is recommended in cases of suspected CNS malignancy but more than one spinal tap may be needed to detect malignant cells (Citationvan Oostenbrugge and Twijnstra 1999) In case of localized optic nerve infiltration with no evidence of systemic disease, histopathological diagnosis may require direct optic nerve sheath biopsy (CitationBehbehani, Vacarezza et al 2005).
Compressive optic neuropathy
In compressive optic neuropathy, visual loss is usually gradual and progressive. Common causes include orbital and intracranial meningiomas, pituitary adenomas, intracranial aneurysms, craniopharyngiomas, and gliomas of the anterior visual pathway (). Vision loss, however, can be fast and dramatic in pituitary apoplexy, or ruptured aneurysm. Visual field testing aids in the localization of the lesion and neuro-imaging with MRI of the brain and orbit is essential.
Figure 6 An axial contrast-enhanced MRI of the orbit showing enhancement of the intra-orbital and intra-canalicular optic nerve in a lady with optic nerve sheath meningioma in the right eye.
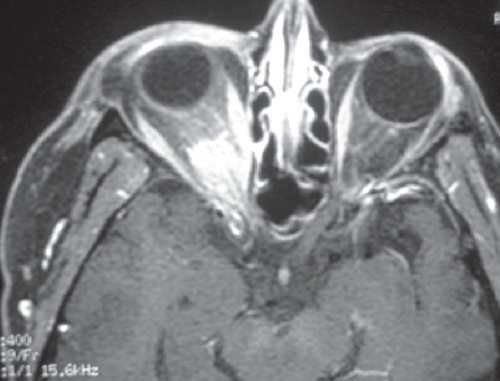
Compressive optic neuropathy can also occur in thyroid eye disease and can present as asymmetric progressive visual loss. This will require prompt therapy (orbital radiation, orbital decompression, high-dose systemic steroids).
Hereditary optic neuropathy
The hereditary optic neuropathies are a broad category including autosomally inherited diseases (dominant, recessive, X-linked) and diseases caused by inheritance of defective mitochondrial genome. Mitochondrial dysfunction may be a final common pathway among these disorders (CitationNewman 2005; CitationAtkins et al 2006). Hereditary optic neuropathies can be isolated or associated with other neurological signs and symptoms.
Patients with dominant optic neuropathy (Kjers’ type) often present in the first decade of life with bilateral symmetric visual loss. Visual acuity can range from 20/20 to 20/400 (CitationHoyt 1980; CitationDas et al 2006). Visual field testing frequently reveals bilateral central or cecocentral scotomas. In some cases, a central hemianopic bitemporal visual field defect is found, which in the absence of positive family history may erroneously lead to a search for a chiasmal lesion (CitationManchester and Calhoun 1958). Patients will have color vision deficit along the tritan (blue-yellow) axis. The optic disc will show temporal pallor and in some cases severe excavation and cupping. There various responsible genetic mutations occur in the OPA1 gene located on the chromosome 3 q region (CitationVotruba 2004).
Recessive optic neuropathy is rare and tends to present in the first year of life (CitationFrancois 1976). It can be associated with diabetes mellitus, diabetes inspidus, and deafness (Wolfram syndrome) (CitationAjlouni et al 2002). An X-linked optic atrophy has also been described and the genetic mutation has been localized to Xp11.4–Xp11.2 (OPA2) (CitationKatz et al 2006). Optic neuropathy can also occur in neurological conditions such as spinocerebellar degeneration, Friedriech’s ataxia, and olivo-ponto-cerebellar atrophy.
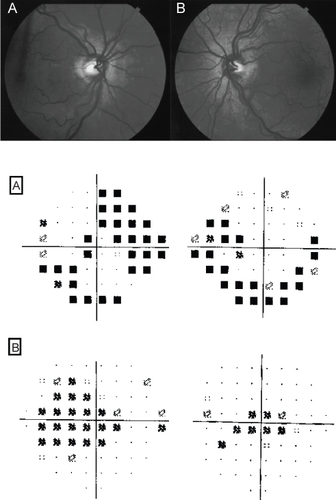
Leber’s hereditary mitochondrial optic neuropathy (LHON) classically presents with acute unilateral, painless, visual loss. However, some cases may stay asymptomatic or have a chronic course (CitationKerrison 2005). Sequential bilateral involvement may occur weeks or months later. Visual filed defects tend to be central or cecocentral as the papillo-macular bundle is first and most severely affected (CitationBarboni et al 2005). Fundoscopy may show disk swelling, thickening of the peripapillary retinal nerve fiber layer and peripapillary retinal telangectatic vessels which do not leak on flourescin angiography (). Occasionally, optic nerve pallor can be seen initially. Because of the wide age range (6–80 years old) at which LHON may present, it is frequently misdiagnosed (CitationAjax and Kardon 1998). Young patients are often diagnosed as optic neuritis and older patients as ischemic or infiltrative optic neuropathy. MRI may show optic nerve enhancement and white matter lesions, which may add to the diagnostic difficulties (CitationPaulus et al 1993; CitationVaphiades and Newman 1999). Therefore, a high index of suspicion is essential. Some patients may spontaneously improve to normal or near normal visual acuity (CitationNakamura and Yamamoto 2000).
Figure 7 A 55 year old lady carrier of the 3460 G LHON mitochondrial mutation, with bilateral disc swelling (top figure). Automated 24–2 perimetry (middle figure) shows bilateral arcuate defects and 10–2 perimetry shows bilateral central scotomas (bottom figure). The patient is a carrier the 3460 G LHON mitochondrial mutation.
LHON has 4 primary mitochondrial genome mutations; G11778A, G3460A and T14484C and T10663C. The disease affects males more than females with a ratio of 2.5:1 for the G11778A and G3460A mutation and 6:1 ratio for the T14484C mutation (CitationRiordan-Eva et al 1995). The frequencies of mutation may vary across different countries and newer mutations have been described worldwide (CitationZhadanov et al 2005; CitationHoushmand et al 2006). Some patients may demonstrate neurological manifestations such as peripheral neuropathy, ataxia, dystonia and cardiac conduction defects (CitationBower et al 1992; CitationMurakami et al 1996; CitationWatanabe et al 2006). These patients fall in the spectrum mitochondrial encephalomyelopathies and their clinical manifestations may vary considerably (CitationGropman et al 2004; CitationBlakely et al 2005).
Toxic-nutritional optic neuropathies
Optic nerve dysfunction can be caused by various drugs, toxins and nutritional deficiencies. The most common offenders are ethambutol, amiodarone, methanol, ethanol and tobacco (CitationDeVita et al 1987; CitationPurvin et al 2006). Similar to hereditary optic neuropathies, toxic-nutritional optic neuropathies are also characterized by selective maculo-papillar bundle involvement, leading to central or cecocentral scotomas. Ethambutol causes optic neuropathy in 1% of patients using the anti-tuberculous medication. Although the dosage of 25 mg/kg/day for 2 months followed by 15 mg/kg/day maintenance doses is considered safe, toxicity has been reported at below this dosage (CitationTsai and Lee 1997). Patients with low zinc levels were found to be more vulnerable to develop optic neuropathy (CitationDe Palma et al 1989). The visual symptoms usually start 2–8 months after the drug is started. Pupillary abnormalities can be subtle, and visual evoked potential may be needed to confirm the diagnosis. The diagnosis is often made late when optic nerve pallor has already set in and vision is severely affected. The drug should be stopped as soon as the diagnosis is made, although in advanced cases vision continues to deteriorate even following drug cessation.
The anti-arrhythmic drug amiodarone can cause bilateral optic neuropathy. Because patients using this drug have systemic vascular risk factors, there is still controversy whether patients affected may simply have NAION. Some of the clinical features that may help distinguish this entity from NAION are the bilateral simultaneous onset, the insidious nature of vision loss, and the protracted disc edema which can last for months (CitationMurphy and Murphy 2005; CitationPurvin et al 2006). The disc edema in NAION usually resolves within 6–8 weeks.
Other medications which can cause toxic optic neuropathy include methotrexate, cyclosporine, vincristine, cisplatin and chloramphenicol (CitationGodel et al 1980; CitationWalter et al 2000; CitationWang et al 2000; CitationClare et al 2005; CitationWeisfeld-Adams et al 2005).
Tobacco-alcohol amblyopia is a term used for a condition that may be due both the toxic effect of the tobacco and a nutritional deficiency state. Because many patients who abuse alcohol and tobacco do not get optic neuropathy, the mechanism is unlikely to be a direct insult to the optic nerve. A more plausible mechanism is that genetic and nutritional deficiency factors increase the susceptibility for optic neuropathy for the effects of certain toxins. Deficiency of thiamine (B1), riboflavin (B2), folate, B12 and B6 have all been associated with optic neuropathy (CitationRizzo and Lessell 1993; CitationGolnik and Schaible 1994; CitationSadun et al 1994; CitationHsu et al 2002). Patients who follow high protein, ketogenic and low carbohydrate diet are at risk for developing thiamin deficiency. Similarly, patients who have undergone gastrointestinal surgery may develop optic neuropathy due interference with the absorption of vitamin B12.
Radiation optic neuropathy (RON)
Patients with RON can present with vision loss, months or years following history of radiation exposure to the brain or orbit. The risk increases in patients who had also received chemotherapy (CitationKline et al 1985). The mechanism of RON is ischemia caused by endothelial cell injury from radiation. The optic disc is usually normal but can be swollen. MRI of the orbit may show optic nerve enhancement with gadolinium (CitationGuy et al 1990; CitationLessell 2004). Patients may also have radiation retinopathy with retinal hemorrhages, cotton wool spots, exudates and macular edema. Because many of those patients were treated for brain or orbital tumors, recurrent tumor is initially the main consideration when patients become symptomatic. There is no treatment of proven efficacy for RON. Hyperbaric oxygen, steroids, antiplatelet drugs, and anticoagulants have all been used with limited success (CitationBarbosa et al 1999; CitationDanesh-Meyer et al 2004; CitationBoschetti et al 2006). The visual prognosis is poor with 45% of eyes ending with no light perception visual acuity (CitationLessell 2004).
Traumatic optic neuropathy
Traumatic optic neuropathy presents with typical signs and symptoms. Patients usually had suffered craniofacial trauma but occasionally mild orbital or eye injury (CitationHou and Murphy 2004; CitationNishi et al 2006). A RAPD is the main clue to the diagnosis. CT scan of the orbit is recommended to detect any bony fractures, fractures of the optic canal, and acute orbital hemorrhages. High-dose steroid therapy was adopted as a treatment because of their beneficial effect in studies on spinal cord injuries. Subsequently, however, it was shown that high-dose steroids may be harmful to the optic nerve if started 8 hours after the injury (CitationSteinsapir 2006). In a non-randomized trial, no clear benefit was found with either steroids or surgical decompression of the optic canal (CitationLevin et al 1999). There are anecdotal reports of beneficial outcome of surgery in cases of optic canal fracture and optic nerve sheath hematoma (CitationJiang et al 2001; CitationWohlrab et al 2002; CitationRajiniganth et al 2003).
Paraneoplastic optic neuropathy
Optic neuropathy can occur as a remote effect of both small cell and non-small cell lung carcinoma of the lung (CitationAsproudis et al 2005; CitationSheorajpanday et al 2006). Some patients may have a concurrent paraneoplastic cerebellar syndrome of dysarthria and ataxia (CitationLuiz et al 1998; CitationThambisetty et al 2001). Patients often have bilateral disc swelling and progressive visual loss before the diagnosis of the systemic malignancy is made. The collapsing response-mediating protein (CRMP-5) has been found recently to be a useful marker in the diagnosis of this condition in patients with lung carcinoma (CitationYu et al 2001; CitationCalvert 2006).
Conclusion
Optic neuropathy can be caused by demyelination, inflammation, ischemia, infiltration, compression, and hereditary and toxic/nutritional causes. Careful clinical evaluation is essential to rule in the diagnosis of optic neuropathy. Recognition of same entities can not only alter the visual prognosis but also the neurological prognosis. Some additional tests particularly visual field testing and neuro-imaging are very useful in the clinical evaluation. The ophthalmologist should be familiar with the various entities that can cause optic neuropathy. With careful clinical evaluation and appropriate investigations, a specific diagnosis can be made in most cases.
The author has no relevant financial interest in this article.
References
- AjaxETKardonR1998Late-onset Leber’s hereditary optic neuropathyJ Neuroophthalmol183019532536
- AjlouniKJarrahN2002Wolfram syndrome: identification of a phenotypic and genotypic variant from JordanAm J Med Genet11561512116178
- ArnoldAC2005Evolving management of optic neuritis and multiple sclerosisAm J Ophthalmol1391101815953446
- ArnoldACHeplerRS1994Natural history of nonarteritic anterior ischemic optic neuropathyJ Neuroophthalmol146697951929
- AsproudisICNikasAN2005Paraneoplastic optic neuropathy in a patient with a non-small cell lung carcinoma: a case reportEur J Ophthalmol15420315945016
- AtkinsEJBiousseV2006The natural historyof optic neuritisRev Neurol Dis3455616819420
- BangsgaardRLarsenVA2006Isolated bilateral optic neuritis in acute disseminated encephalomyelitisActa Ophthalmol Scand84815717083545
- BarboniPSaviniG2005Retinal nerve fiber layer evaluation by optical coherence tomography in Leber’s hereditary optic neuropathyOphthalmology112120615629831
- BarbosaAPCarvalhoD1999Inefficiency of the anticoagulant therapy in the regression of the radiation-induced optic neuropathy in Cushing’s diseaseJ Endocrinol Invest22301510342365
- BeckRW1995The optic neuritis treatment trial: three-year follow-up resultsArch Ophthalmol11313677864737
- BeckRWChandlerDL2002Interferon beta-1a for early multiple sclerosis: CHAMPS trial subgroup analysesAnn Neurol514819011921054
- BeckRWTrobeJD2003High- and low-risk profiles for the development of multiple sclerosis within 10 years after optic neuritis: experience of the optic neuritis treatment trialArch Ophthalmol121944912860795
- BehbehaniRMathewsMK2005Nonarteritic anterior ischemic optic neuropathy in patients with sleep apnea while being treated with continuous positive airway pressureAm J Ophthalmol1395182115767063
- BehbehaniRSVacarezzaN2005Isolated optic nerve lymphoma diagnosed by optic nerve biopsyAm J Ophthalmol13911283015953457
- BeldenCJHamedLM1993Bilateral isolated retrobulbar optic neuropathy in limited Wegener’s granulomatosisJ Clin Neuroophthalmol13119238340477
- Bianchi-MarzoliSRizzoJF3rd1995Quantitative analysis of optic disc cupping in compressive optic neuropathyOphthalmology102436407891982
- BlakelyELde SilvaR2005LHON/MELAS overlap syndrome associated with a mitochondrial MTND1 gene mutationEur J Hum Genet13623715657614
- BoschettiMDe LucchiM2006Partial visual recovery from radiation-induced optic neuropathy after hyperbaric oxygen therapy in a patient with Cushing diseaseEur J Endocrinol154813816728540
- BowerSPHawleyI1992Cardiac arrhythmia and Leber’s hereditary optic neuropathyLancet339142781350847
- BrazisPWLiesegangTJ1990When do optic disc edema and peripheral neuropathy constitute poetry?Surv Ophthalmol35219252177227
- BrittainGPMcIlwaineGG1991Plasma viscosity or erythrocyte sedimentation rate in the diagnosis of giant cell arteritis?Br J Ophthalmol7565691751458
- BrownGCShieldsJA1981Leukemic optic neuropathyInt Ophthalmol3111167014488
- BuonoLMForoozanR2004Heparin therapy in giant cell arteritisBr J Ophthalmol8829830114736795
- BuonoLMForoozanR2003Posterior ischemic optic neuropathy after hemodialysisOphthalmology1101216812799249
- BurdeRM1993Optic disk risk factors for nonarteritic anterior ischemic optic neuropathyAm J Ophthalmol116759648250081
- CabrePHeinzlefO2001MS and neuromyelitis optica in Martinique (French West Indies)Neurology565071411222796
- CalvertPC2006A CR(I)MP in the optic nerve: recognition and implications of paraneoplastic optic neuropathyJ Neuroophthalmol26165716966931
- ClareGColleyS2005Reversible optic neuropathy associated with low-dose methotrexate therapyJ Neuroophthalmol251091215937433
- CostelloFCouplandS2006Quantifying axonal loss after optic neuritis with optical coherence tomographyAnn Neurol59963916718705
- CostelloFZimmermanMB2004Role of thrombocytosis in diagnosis of giant cell arteritis and differentiation of arteritic from non-arteritic anterior ischemic optic neuropathyEur J Ophthalmol142455715206651
- Danesh-MeyerHVSavinoPJ2000Low diagnostic yield with second biopsies in suspected giant cell arteritisJ Neuroophthalmol20213511001197
- Danesh-MeyerHVSavinoPJ2001The prevalence of cupping in end-stage arteritic and nonarteritic anterior ischemic optic neuropathyOphthalmology108593811237915
- Danesh-MeyerHVSavinoPJ2004Visual loss despite anticoagulation in radiation-induced optic neuropathyClin Experiment Ophthalmol32333515180850
- DasSBendokBR2006Return of vision after transarterial coiling of a carotid cavernous sinus fistula: case reportSurg Neurol66825discussion 85.16793452
- De PalmaPFrancoF1989The incidence of optic neuropathy in 84 patients treated with ethambutolMetab Pediatr Syst Ophthalmol128022770528
- DeramoVASergottRC2003Ischemic optic neuropathy as the first manifestation of elevated cholesterol levels in young patientsOphthalmology11010416discussion 1046.12750110
- DeVitaEGMiaoM1987Optic neuropathy in ethambutol-treated renal tuberculosisJ Clin Neuroophthalmol777862956288
- FayAMKaneSA1997Magnetic resonance imaging of optic perineuritisJ Neuroophthalmol1724799427176
- FeldonSE1999Anterior ischemic optic neuropathy: trouble waiting to happenOphthalmology106651210201582
- FrancoisJ1976[Hereditary optic atrophies]J Genet Hum241832001003172
- FraserCLKlistornerA2006Multifocal visual evoked potential analysis of inflammatory or demyelinating optic neuritisOphthalmology113323 e1323 e216406544
- FrohmanLTurbinR2003Autoimmune optic neuropathy with anticardiolipin antibody mimicking multiple sclerosis in a childAm J Ophthalmol1363586012888064
- GodelVNemetP1980Chloramphenicol optic neuropathyArch Ophthalmol981417217417077
- GolnikKCSchaibleER1994Folate-responsive optic neuropathyJ Neuroophthalmol1416397804421
- GropmanAChenTJ2004Variable clinical manifestation of homoplasmic G14459A mitochondrial DNA mutationAm J Med Genet A1243778214735585
- GuyJMancusoA1990Gadolinium-DTPA-enhanced magnetic resonance imaging in optic neuropathiesOphthalmology975929discussion 599–600.2342804
- HanSHLeeOY2006[A case of optic neuritis associated with Crohn’s disease]Korean J Gastroenterol4842516861881
- HaupertCLNewmanNJ1997Prolonged Uhthoff phenomenon in sarcoidosisAm J Ophthalmol12456469323955
- HayrehSS1999Role of nocturnal arterial hypotension in the development of ocular manifestations of systemic arterial hypertensionCurr Opin Ophthalmol104748210662254
- HayrehSS2004Posterior ischaemic optic neuropathy: clinical features, pathogenesis, and managementEye18118820615534605
- HayrehSSPodhajskyPA1997Giant cell arteritis: validity and reliability of various diagnostic criteriaAm J Ophthalmol123285969063237
- HayrehSSPodhajskyPA1998aOccult giant cell arteritis: ocular manifestationsAm J Ophthalmol12552169559738
- HayrehSSPodhajskyPA1998bOcular manifestations of giant cell arteritisAm J Ophthalmol125509209559737
- HayrehSSPodhajskyPA2001Ipsilateral recurrence of non-arteritic anterior ischemic optic neuropathyAm J Ophthalmol1327344211704035
- HayrehSSZimmermanB2003Visual deterioration in giant cell arteritis patients while on high doses of corticosteroid therapyOphthalmology11012041512799248
- HouLCMurphyMA2004Traumatic optic neuropathy caused by a merchandise display hookJ Pediatr Ophthalmol Strabismus412495015305540
- HoushmandMMahmoudiT2006Identification of a new human mtDNA polymorphism (A14290G) in the NADH dehydrogenase subunit 6 geneBraz J Med Biol Res397253016751977
- HoytCS1980Autosomal dominant optic atrophy. A spectrum of disabilityOphthalmology87245517422264
- HsuCTMillerNR2002Optic neuropathy from folic acid deficiency without alcohol abuseOphthalmologica21665711901292
- JabsDAMillerNR1986Optic neuropathy in systemic lupus erythematosusArch Ophthalmol10456483954662
- JiangRSHsuCY2001Endoscopic optic nerve decompression for the treatment of traumatic optic neuropathyRhinology3971411486441
- KansuTKirkaliP1989Optic neuropathy in Behcet’s diseaseJ Clin Neuroophthalmol9277802531168
- KatzBJZhaoY2006A family with X-linked optic atrophy linked to the OPA2 locus Xp11.4–Xp11.2Am J Med Genet A14022071116969871
- KerrisonJB2005Latent, acute, and chronic Leber’s hereditary optic neuropathyOphthalmology1121215629812
- KiddDBurtonB2003Chronic relapsing inflammatory optic neuropathy (CRION)Brain1262768412538397
- KlineLBKimJY1985Radiation optic neuropathyOphthalmology9281118264047605
- KupersmithMJBurdeRM1988Autoimmune optic neuropathy: evaluation and treatmentJ Neurol Neurosurg Psychiatry51138163266235
- LeeAGLinDJ2000Atypical features prompting neuroimaging in acute optic neuropathy in adultsCan J Ophthalmol353253011091914
- LennonVAWingerchukDM2004A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosisLancet36421061215589308
- LessellS2004Friendly fire: neurogenic visual loss from radiation therapyJ Neuroophthalmol242435015348995
- LevinLABeckRW1999The treatment of traumatic optic neuropathy: the International Optic Nerve Trauma StudyOphthalmology10612687710406604
- LimETSellebjergF2005Acute axonal damage predicts clinical outcome in patients with multiple sclerosisMult Scler11532616193890
- LincoffNSErlichPD2000Thrombocytosis in temporal arteritis rising platelet counts: a red flag for giant cell arteritisJ Neuroophthalmol20677210870916
- LiuGTGlaserJS1994Visual morbidity in giant cell arteritis. Clinical characteristics and prognosis for visionOphthalmology1011779857800356
- LucchinettiCFKiersL1997Risk factors for developing multiple sclerosis after childhood optic neuritisNeurology49141389371931
- LuizJELeeAG1998Paraneoplastic optic neuropathy and autoantibody production in small-cell carcinoma of the lungJ Neuroophthalmol18178819736201
- ManchesterPTJrCalhounFPJr1958Dominant hereditary optic atrophy with bitemporal field defectsAMA Arch Ophthalmol604798413570776
- McCulleyTJLamBL2005A comparison of risk factors for postoperative and spontaneous nonarteritic anterior ischemic optic neuropathyJ Neuroophthalmol2522415756128
- MedeirosFAMouraFC2003Axonal loss after traumatic optic neuropathy documented by optical coherence tomographyAm J Ophthalmol135406812614771
- MojonDSHedgesTR3rd2002Association between sleep apnea syndrome and nonarteritic anterior ischemic optic neuropathyArch Ophthalmol120601512003609
- MurakamiTMitaS1996Hereditary cerebellar ataxia with Leber’s hereditary optic neuropathy mitochondrial DNA 11778 mutationJ Neurol Sci14211138902729
- MurphyMA2003Bilateral posterior ischemic optic neuropathy after lumbar spine surgeryOphthalmology1101454712867409
- MurphyMAMurphyJF2005Amiodarone and optic neuropathy: the heart of the matterJ Neuroophthalmol25232616148635
- NakamuraMYamamotoM2000Variable pattern of visual recovery of Leber’s hereditary optic neuropathyBr J Ophthalmol84534510781521
- NakashimaIFujiharaK2006Clinical and MRI features of Japanese patients with multiple sclerosis positive for NMO-IgGJ Neurol Neurosurg Psychiatry771073516505005
- NewmanNJ2005Hereditary optic neuropathies: from the mitochondria to the optic nerveAm J Ophthalmol1405172316083845
- NewmanNJSchererR2002The fellow eye in NAION: report from the ischemic optic neuropathy decompression trial follow-up studyAm J Ophthalmol1343172812208242
- NishiTUedaT2006Traumatic optic neuropathy caused by blunt injury to the inferior orbital rimJ Neuroophthalmol2644616518166
- PalombiKRenardE2006Non-arteritic anterior ischaemic optic neuropathy is nearly systematically associated with obstructive sleep apnoeaBr J Ophthalmol908798216556620
- ParikhMMillerNR2006Prevalence of a normal C-reactive protein with an elevated erythrocyte sedimentation rate in biopsy-proven giant cell arteritisOphthalmology1131842516884778
- PaulusWStraubeA1993Central nervous system involvement in Leber’s optic neuropathyJ Neurol24025138496715
- PiankaPAlmogY2000Hyperhomocystinemia in patients with nonarteritic anterior ischemic optic neuropathy, central retinal artery occlusion, and central retinal vein occlusionOphthalmology10715889210919914
- PittockSJLennonVA2006Brain abnormalities in neuromyelitis opticaArch Neurol63390616533966
- PlessMRizzoJF3rd2000Concordance of bilateral temporal artery biopsy in giant cell arteritisJ Neuroophthalmol20216811001198
- ProMJPonsME2006Imaging of the optic disc and retinal nerve fiber layer in acute optic neuritisJ Neurol Sci250114917027854
- PurvinVKawasakiA2006Optic neuropathy in patients using amiodaroneArch Ophthalmol12469670116682592
- RajiniganthMGGuptaAK2003Traumatic optic neuropathy: visual outcome following combined therapy protocolArch Otolaryngol Head Neck Surg1291203614623751
- Riordan-EvaPSandersMD1995The clinical features of Leber’s hereditary optic neuropathy defined by the presence of a pathogenic mitochondrial DNA mutationBrain118319377735876
- RizzoJF3rdLessellS1993Tobacco amblyopiaAm J Ophthalmol1168478328548
- RubieraMRioJ2006Neuromyelitis optica diagnosis in clinically isolated syndromes suggestive of multiple sclerosisNeurology6615687016717222
- SaddaSRNeeM2001Clinical spectrum of posterior ischemic optic neuropathyAm J Ophthalmol1327435011704036
- SadunAAMartoneJF1994Epidemic optic neuropathy in Cuba. Eye findingsArch Ophthalmol11269198185530
- SalvaraniCHunderGG2001Giant cell arteritis with low erythrocyte sedimentation rate: frequency of occurence in a population-based studyArthritis Rheum45140511324777
- SaviniGBellusciC2006Detection and quantification of retinal nerve fiber layer thickness in optic disc edema using stratus OCTArch Ophthalmol1241111716908813
- SergottRCCohenMS1989Optic nerve decompression may improve the progressive form of nonarteritic ischemic optic neuropathyArch Ophthalmol1071743542597065
- SheorajpandayRSlabbynckH2006Small cell lung carcinoma presenting as collapsin response-mediating protein (CRMP)-5 paraneoplastic optic neuropathyJ Neuroophthalmol261687216966932
- ShimadaYShibuyaM2006Bilateral optic neuropathy associated with multiple myelomaJ Neuroophthalmol261172016845312
- SpoorTCMcHenryJG1993Progressive and static nonarteritic ischemic optic neuropathy treated by optic nerve sheath decompressionOphthalmology100306118459997
- SteinsapirKD2006Treatment of traumatic optic neuropathy with high-dose corticosteroidJ Neuroophthalmol2665716518171
- ThambisettyMRScherzerCR2001Paraneoplastic optic neuropathy and cerebellar ataxia with small cell carcinoma of the lungJ Neuroophthalmol21164711725180
- TripSASchlottmannPG2005Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritisAnn Neurol583839116075460
- TripSASchlottmannPG2006Optic nerve atrophy and retinal nerve fibre layer thinning following optic neuritis: evidence that axonal loss is a substrate of MRI-detected atrophyNeuroimage312869316446103
- TrobeJDGlaserJS1980aNonglaucomatous excavation of the optic discArch Ophthalmol981046507387507
- TrobeJDGlaserJS1980bOptic atrophy. Differential diagnosis by fundus observation aloneArch Ophthalmol98104057387506
- TsaiRKLeeYH1997Reversibility of ethambutol optic neuropathyJ Ocul Pharmacol Ther1347379326729
- TsaiRKLiuYT1998Risk factors of non-arteritic anterior ischemic optic neuropathy (NAION): ocular or systemicKaohsiung J Med Sci1422159589616
- van OostenbruggeRJTwijnstraA1999Presenting features and value of diagnostic procedures in leptomeningeal metastasesNeurology53382510430430
- VaphiadesMSNewmanNJ1999Optic nerve enhancement on orbital magnetic resonance imaging in Leber’s hereditary optic neuropathyJ Neuroophthalmol19238910608675
- VotrubaM2004Molecular genetic basis of primary inherited optic neuropathiesEye1811263215534598
- WalterSHBertzH2000Bilateral optic neuropathy after bone marrow transplantation and cyclosporin A therapyGraefes Arch Clin Exp Ophthalmol238472610943669
- WangMYArnoldAC2000Bilateral blindness and lumbosacral myelopathy associated with high-dose carmustine and cisplatin therapyAm J Ophthalmol130367811020424
- WatanabeMMitaS2006Leber’s hereditary optic neuropathy with dystonia in a Japanese familyJ Neurol Sci24331416380132
- Weisfeld-AdamsJDDuttonGN2005Vincristine sulfate as a possible cause of optic neuropathyPediatr Blood Cancer
- WilejtoMShroffM2006The clinical features, MRI findings, and outcome of optic neuritis in childrenNeurology672586216864818
- WingerchukDMLennonVA2006Revised diagnostic criteria for neuromyelitis opticaNeurology661485916717206
- WiseCMAgudeloCA1988Optic neuropathy as an initial manifestation of Sjogren’s syndromeJ Rheumatol157998023262752
- WohlrabTMMaasS2002Surgical decompression in traumatic optic neuropathyActa Ophthalmol Scand802879312059868
- YuZKryzerTJ2001CRMP-5 neuronal autoantibody: marker of lung cancer and thymoma-related autoimmunityAnn Neurol491465411220734
- ZhadanovSIAtamanovVV2005A novel mtDNA ND6 gene mutation associated with LHON in a Caucasian familyBiochem Biophys Res Commun332411152115922297