Abstract
Ocular motor disorders are a well recognized feature of multiple sclerosis (MS). Clinical abnormalities of eye movements, early in the disease course, are associated with generalized disability, probably because the burden of disease in affected patients falls on the brainstem and cerebellar pathways, which are important for gait and balance. Measurement of eye movements, especially when used to detect internuclear ophthalmoplegia (INO), may aid diagnosis of MS. Measurement of the ocular following response to moving sinusoidal gratings of specified spatial frequency and contrast can be used as an experimental tool to better understand persistent visual complaints in patients who have suffered optic neuritis. Patients with MS who develop acquired pendular nystagmus often benefit from treatment with gabapentin or memantine.
Introduction
No evaluation of a patient either suspected or diagnosed with MS is complete without a systematic examination of their vision and eye movements (CitationLeigh and Zee 2006). Although disturbances of vision and eye movements are recognized as important for making the diagnosis of MS, the neuro-ophthalmologic examination also provides insights into the nature of the disorder in any patient and even in estimating prognosis (CitationFrohman et al 2005). Here we review four aspects of the role of eye movements in MS: (1) the value of clinical examination of eye movements in making the diagnosis and estimating the prognosis in MS; (2) how measurement of eye movements may contribute to the diagnosis of MS; (3) how eye movements can be used as an experimental tool to investigate the visual disturbance due to optic neuritis; and (4) current concepts of treatment of acquired forms of nystagmus due to MS and their visual consequences.
Clinical examination of eye movements
A systematic examination of eye movements in patients with MS shows a range of disorders (). The most common eye movement abnormalities are saccadic dysmetria, internuclear ophthalmoplegia (INO), disorders of the vestibulo-ocular reflex (VOR), and gaze-evoked nystagmus (CitationDowney et al 2002; CitationSerra et al 2003; CitationDerwenskus et al 2005). Saccades are the rapid, brief eye movements by which we shift the point of fixation from one feature of interest to the next. Normal subjects show mild undershoots (hypometria), especially for larger saccades; however, with repetitive saccades, the eye lands on the visual target. Patients with MS may show persistent dysmetria, including overshoots (hypermetria) and directional dysmetria (eg, an inappropriate vertical component during a horizontal saccade, requiring a vertical corrective movement). In normal subjects, saccades of each eye are conjugate vertically, but are mildly disjunctive horizontally, with the abducting eye initially leading (producing transient divergence) and the adducting eye eventually catching up (causing transient convergence) (CitationCollewijn et al 1988). This minor, transient disconjugacy of horizontal saccades in normal subjects cannot be discerned at the bedside. However, in INO, the adducting movement is visibly slowed (adduction lag) compared with the abducting movement. Slowing of adduction occurs because the pulse of innervation that causes rapid contraction of the medial rectus muscle is deficient, since the demyelinated axons of the medial longitudinal fasciculus (MLF) cannot conduct the high-frequency discharge (CitationLeigh and Zee 2006). In classic INO, the findings include restricted adduction and adduction lag in the eye ipsilateral to the MLF lesion and dissociated abducting “nystagmus” of the contralateral eye (which is discussed in the last section). Milder forms of INO cause adduction lag but no restriction of adduction or abducting “nystagmus” (CitationCrane et al 1983; CitationMüri and Meienberg 1985). This adduction lag is most easily visualized with rapid eye movements; thus, INO is best detected by testing saccades, and may be missed if only pursuit movements are examined (“follow my finger”).
Table 1 Summary of common disorders of ocular motility in MS (CitationDowney et al 2002)
The VOR is most conveniently tested at the bedside using the head impulse test: a small but rapid head turn is evoked by the examiner while the patient views a stationary far visual target (CitationHalmagyi and Curthoys 1988). An inadequate vestibular response is evidenced by a corrective saccade at the end of the head rotation. In patients with INO, the horizontal VOR may be impaired in the adducting eye. With vertical head rotations, an asymmetry of upward versus downward head rotations may be evident since the VOR to upward head rotations depends more on the MLF than in response to downward head rotations. Patients with acute INO may also develop a skew deviation, the eye being higher on the side of the lesion. Furthermore, some MS patients show deviation of the subjective visual vertical, assessed with a simple laser pointer fitted with inexpensive line-generating optics (CitationSerra et al 2003), or a Maddox rod.
Carefully conducted clinical studies have established that patients who have abnormal eye movements are more disabled than those with normal motility, and that this difference is sustained after two years (CitationDowney et al 2002; CitationSerra et al 2003; CitationDerwenskus et al 2005). The reason for the increased disability in MS patients with abnormal eye movements is less due to their impaired vision than to their disturbance of gait. In such patients, MRI scans confirm that both abnormal eye movements and gait disturbance are caused by prominent involvement of the brainstem and cerebellar pathways.
Measuring eye movements to aid diagnosis of MS
Clinical detection of INO and saccadic abnormalities in MS may be challenging (CitationFrohman TC et al 2003), and measurement of eye movements may help confirm the diagnosis during early stages of the disease. A representative example is shown in .
Figure 1 Comparison of a rightward saccade made to a 22 degree target jump by a normal subject (A) and a patient with MS who has INO (B). Note how the normal subject’s eyes move together, with right eye (abduction) velocity being slightly bigger than that of the left eye. In contrast, the MS patient’s adducting left eye lags behind the abducting right, and right eye velocity greatly exceeds that of the left. Positive values indicate rightward movements. Note the different axes for position and velocity.
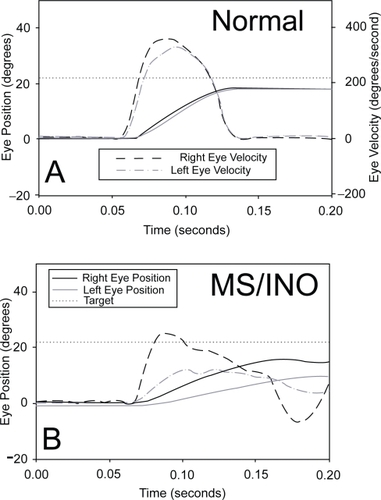
Detection of a saccadic abnormality may be better when targets are presented randomly, so that neither their time of onset nor their location can be predicted. Large saccades (20 degrees or greater) are more likely to show changes in velocity than are small saccades. In making paired comparison of the peak velocity of abducting and adducting saccades, it is important to take account of the fact that normal subjects show somewhat greater peak velocities in the abducting eye, depending on the methodology being used (CitationCollewijn et al 1988). A more reliable approach is to measure the ratio of abducting/adducting movements during horizontal saccades, and compare the patient’s value with that of group of normal subjects recorded in the same laboratory (CitationFlipse et al 1996; CitationFrohman et al 2002; CitationFrohman EM et al 2003). Patients with INO have abduction/adduction ratios for peak velocity or peak acceleration that lie outside the 95% prediction intervals for normal subjects. Another approach is to plot eye velocity versus eye displacement (position) for each eye – phase plane plots. Normal subjects show very similar initial phase-plane plots for each eye whereas in INO, the plots of each eye deviate at the start of the movement.
Other saccadic abnormalities in multiple sclerosis include increased reaction time (latency), inaccuracy (dysmetria), and decreased velocity (CitationReulen et al 1983; CitationBrigell et al 1988; CitationMeienberg et al 1986). Some patients with MS show saccadic oscillations or unwanted saccades that intrude on steady fixation or smooth pursuit (CitationHerishanu and Sharpe 1983; CitationAshe et al 1991; CitationSchon et al 2001).
Smooth-pursuit tracking may be impaired (reduced gain) (CitationSharpe et al 1981; CitationReulen et al 1983), and there may be impaired cancellation of the horizontal vestibulo-ocular reflex (VOR) when patients attend to a head-fixed target during rotation in a chair (CitationSharpe et al 1981). Unilateral internuclear ophthalmoplegia may impair asymmetrically the vertical vestibulo-ocular reflex during stimulation of the contralateral posterior semicircular canal, which depends on the medial longitudinal fasciculus for normal responses (CitationCremer et al 1999). Bilateral internuclear ophthalmoplegia impairs the vertical vestibulo-ocular reflex, gaze holding, smooth pursuit, and eye-head tracking – all functions that also depend on the medial longitudinal fasciculus (CitationRanalli and Sharpe 1988). Patients with head tremor and an impaired vestibulo-ocular reflex may report oscillopsia – illusory motion of the visual world (CitationProudlock et al 2002).
Less commonly, MS may cause horizontal and vertical gaze palsies (CitationWall and Wray 1983; CitationMartyn and Kean 1988; CitationTan and Kansu 1990; CitationMilea et al 2001), or individual oculomotor, trochlear or abducens palsies due to brainstem plaques (CitationLeigh and Zee 2006), upbeat and downbeat nystagmus (CitationFisher et al 1983; CitationMasucci and Kurtzke 1988; CitationBaloh and Yee 1989), and a range of vestibular and optokinetic abnormalities (CitationKatsarkas 1982; CitationHuygen et al 1990; CitationTodd et al 2001). MRI often aids identification of brainstem or cerebellar lesions responsible for such abnormalities; thin cuts and proton density images may be required.
Diagnosis of multiple sclerosis depends on demonstration of lesions disseminated throughout the nervous system (CitationFrohman et al 2005). Early diagnosis has become more important because prompt initiation of immunotherapy has the best chance of arresting the disease. Although detection of subtle deficits of ocular motility, such as INO, may be useful in making a diagnosis, some caution is required since these tests are not specific for multiple sclerosis. Thus, the clinician must weigh the results of ocular motor studies with other clinical or laboratory findings before making a diagnosis in the context of current criteria (CitationDalton et al 2002). Routine examination of eye movements during follow-up visits to the MS clinic (along with careful evaluation of vision) may provide a useful method of evaluating progress and prognosis. As noted above, patients who are more generally disabled are more likely to develop abnormalities of eye movements, probably because the brunt of the disease is affecting brainstem and cerebellar connections (CitationDowney et al 2002; CitationSerra et al 2003; CitationDerwenskus et al 2005).
Application of eye movements to investigate visual disorders in MS
The signature disturbance of vision in MS is optic neuritis, which typically presents as monocular loss of central vision, accompanied by discomfort with eye movements (CitationFrohman et al 2005). Thanks to the longitudinal Optic Neuritis Treatment study, we now have a more detailed knowledge of the clinical characteristics and course of the syndrome, in which over 95% of patients recover 20/40 or better vision by 12 months after the onset of symptoms (CitationBeck and Cleary 1993; CitationAtkins et al 2006).
Some patients who suffer optic neuritis make a good recovery of vision as tested by high-contrast Snellen optotypes but show residual visual impairment, particularly with contrast sensitivity and with low-contrast optotypes such as Sloan letters (CitationBalcer et al 2003). Color vision may also be asymmetrically impaired in such patients.
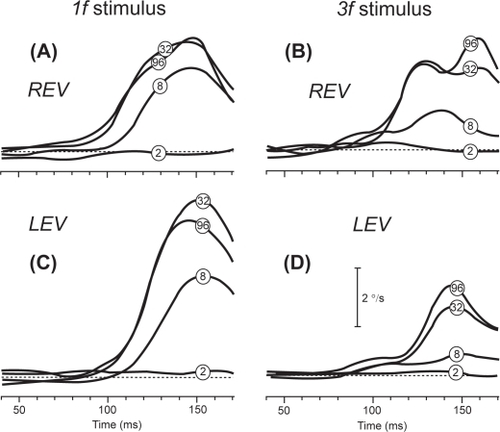
We recently investigated whether motion vision could remain compromised after recovery from optic neuritis in a 42-year-old man with MS (CitationRucker et al 2006). Three years after left optic neuritis had reduced his vision to counting fingers, his visual acuity had improved to 20/40 OS (20/20 OD) with Snellen letters but remained at 20/200 OS (20/32 OD) with Sloan letters at 10% contrast. Color vision was normal OD but essentially absent OS. Despite this improvement, he reported subjective visual difficulties OS, which he noted while he was in motion. Rather than perform a subjective test of motion vision, we measured his ocular following response (OFR). The OFR is a measure of first-order, luminance driven motion vision which consists of a sudden conjugate eye movement elicited at ultra-short latencies by rapid shift of a large visual stimulus (CitationSheliga et al 2005). One advantage of studying motion vision by measuring the short-latency OFR is that this eye-tracking response is rather automatic and not substantially influenced by the level of attentiveness of the subject. The stimuli we applied consisted of ¼-cycle shifts of a sine-wave grating with a spatial frequency of either 0.153 or 0.458 cycles/degree. We found that his responses to the higher spatial frequency stimulus were impaired in the affected left eye, suggesting predominant post-optic neuritis involvement of parvocellular visual function (). Responses to the low spatial frequency stimulus were similar between the two eyes and to controls, suggesting preservation of the magnocellular visual function, which may function as a visual field-holding reflex (CitationMiles 1998). Although OFR has been applied to patients with cortical disturbances of vision, this preliminary study suggests that OFR may be a valuable, objective means of further study of visual dysfunction following optic neuritis.
Figure 2 Representative mean right-left velocity profiles of the ocular following responses (OFR) versus time elicited in a patient with MS who had partially recovered from a left optic neuritis. The patient responded to successive ¼-wavelength shifts applied to 1f stimuli (left column) and 3f stimuli (right column) of different contrast (contrast of the pattern is shown by encircled numbers superimposed on traces). A and B: right-eye-viewing sessions (REV). C and D: left-eye-viewing sessions (LEV). Note the smaller LEV responses to the 3f stimuli versus 1f stimuli. Adapted from (CitationRucker et al 2006).
Acquired nystagmus in MS and treatment of its visual consequences
Several forms of nystagmus occur in MS. So-called dissociated nystagmus with INO is more correctly classified as a disorder of saccadic metrics: the adducting eye fails to get on target, and the brain then programs a series of corrective saccades that are not evident in the paretic, adducting eye, but are observed in the abducting eye (CitationLeigh and Zee 2006). Patching the affected eye in unilateral INO for several days reduces “dissociated nystagmus” while patching the normal eye increases it, indicating that plastic-adaptive mechanisms contribute to the nystagmus (CitationZee et al 1987).
Although upbeat, downbeat, and seesaw nystagmus all may occur in MS, it is acquired pendular nystagmus (APN) that is most visually disabling. APN is also a feature of other disorders of central myelin, including Pelizaeus-Merzbacher disease (CitationTrobe et al 1991), peroxisomal assembly disorders (CitationKori et al 1998), and in toluene abuse (CitationMaas et al 1991). Since patients with MS who have pendular nystagmus often also have evidence of optic nerve demyelination, it follows that prolonged response time of the visual processing might be responsible for the ocular oscillations. Support for this hypothesis comes from the observation that oscillations are usually larger in the eye with evidence of more severe optic nerve demyelination (CitationBarton and Cox 1993). However, the nystagmus often remains unchanged in darkness (when visual inputs have no influence on eye movements). Furthermore, spontaneous ocular oscillations can be induced in normal subjects by experimentally delaying the latency of visual feedback during fixation; however, the frequency of these induced oscillations is less than 2 Hz, which is lower than in most patients with pendular nystagmus due to multiple sclerosis (CitationAverbuch-Heller et al 1995). Furthermore, when this experimental technique was applied to patients with acquired pendular nystagmus, it did not change the characteristics of the nystagmus, but instead superimposed lower-frequency oscillations similar to those induced in normal subjects (CitationAverbuch-Heller et al 1995). Thus, disturbance of visual fixation due to visual delays cannot wholly account for the high-frequency oscillations that often characterize acquired pendular nystagmus.
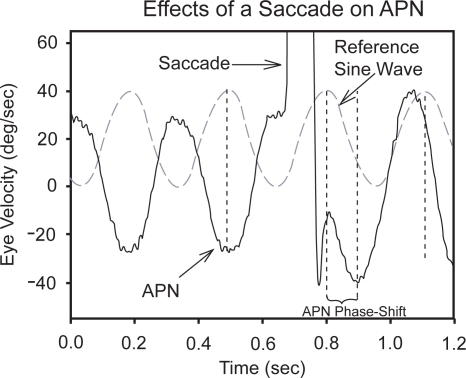
An important observation concerning acquired pendular nystagmus in multiple sclerosis is that some patients show transient suppression of their oscillations following a saccade (). Systematic comparison of the oscillations prior to, and following, a saccade has demonstrated that the oscillations are phase-shifted (reset) leading to the hypothesis that the oscillations of APN in patients with multiple sclerosis arise in the neural integrator for eye movements, and that large saccades affect the timing of the oscillations by “resetting” the integrator with the large pulse of neural activity (CitationDas et al 2000). The nucleus prepositus hypoglossi and medial vestibular nucleus are important components of the neural integrator, which is probably affected by feedback of signals via cell groups of the paramedian tracts (PMT) to the cerebellar flocculus (CitationLeigh and Zee 2006). Patients with APN show a preponderance of multiple sclerosis plaques in their paramedian pons in the region of the PMT cell groups (CitationLopez et al 1995). Thus, it is postulated that the neural integrator loses normal feedback, becomes unstable, and begins to oscillate, causing acquired pendular nystagmus. The neurotransmitters used by neurons that contribute to normal neural integration of ocular motor signals are know to include glutamate and gabapentin (CitationStraube et al 1991; CitationArnold et al 1999). This has led to clinical trials that have demonstrated gabapentin (CitationAverbuch-Heller et al 1997; CitationBandini et al 2001), and memantine (CitationStarck et al 1997) may ameliorate the visually disabling acquired pendular nystagmus that occurs in multiple sclerosis. Our personal experience is that most patients with APN due to MS visually benefit from either gabapentin or memantine. Both drugs are usually well tolerated, although gabapentin sometimes increases ataxia.
Figure 3 An example of how a large rightward horizontal saccade could reset the phase of acquired pendular nystagmus (APN) in a patient with multiple sclerosis. A sine wave at a similar frequency to the oscillations is shown in dashed gray for reference. Note that before the saccade, the horizontal component of APN and sine wave are about 180 degrees out of phase; after the saccade, this phase difference is greatly reduced (compare positions of vertical dashed lines). The single-position traces are offset to aid clarity of display; positive deflections indicate rightward eye rotations.
Summary
In the past twenty years, eye movements have moved beyond the bounds of neuro-ophthalmology and are now widely used by neuroscientists, visual scientists, and psychologists, as well as clinicians in several specialties. The popularity of eye movements as an experimental tool arises, in part, because they can be conveniently and accurately measured and analyzed, but also because much is known concerning their neural substrate. It is therefore, no surprise, that eye movements have been commonly applied to better understand the visual and motor disorders in patients with MS. In this review we draw attention to how eye movements can aid diagnosis of MS at the bedside and in the laboratory. In addition, eye movements can be used as an experimental took to better understand the disorders of vision that occur in MS. Finally, better knowledge concerning neuropharmacology has led to effective drug treatments for abnormal eye movements that degrade vision in patients with demyelinating disease.
References
- ArnoldDBRobinsonDALeighRJ1999Nystagmus induced by pharmacological inactivation of the brainstem ocular motor integrator in monkeyVision Res3942869510755164
- AsheJHainTCZeeDS1991Microsaccadic flutterBrain114461722004251
- AtkinsEJBiousseVNewmanNJ2006The natural history of optic neuritisRev Neurol Dis3455616819420
- Averbuch-HellerLTusaRJFuhryL1997A double-blind controlled study of gabapentin and baclofen as treatment for acquired nystagmusAnn Neurol41818259189045
- Averbuch-HellerLZivotofskyAZDasVE1995Investigations of the pathogenesis of acquired pendular nystagmusBrain188369787735879
- BalcerLJBaierMLCohenJA2003Contrast letter acuity as a visual component for the Multiple Sclerosis Functional CompositeNeurology6113677314638957
- BalohRWYeeRD1989Spontaneous vertical nystagmusRev Neurol (Paris)145527322682931
- BandiniFCastelloEMazzellaL2001Gabapentin but not vigabatrin is effective in the treatment of acquired nystagmus in multiple sclerosis:. How valid is the GABAergic hypothesis?J Neurol Neurosurg Psychiatry711071011413274
- BartonJJSCoxTA1993Acquired pendular nystagmus in multiple sclerosis – Clinical observations and the role of optic neuropathyJ Neurol Neurosurg Psychiatry5626278459242
- BeckRWClearyPA1993Optic Neuritis Study Group. Optic neuritis treatment trial: one year follow-up resultsArch Ophthalmol11177358512477
- BrigellMGGoodwinJALoranceR1988Saccadic latency as a measure of afferent visual conductionInvest Ophthalmol Vis Sci29133183417417
- CollewijnHErkelensCJSteinmanRM1988Binocular co-ordination of human horizontal saccadic eye movementsJ Physiol (Lond)404157823253429
- CraneTBYeeRDBalohRW1983Analysis of characteristic eye movement abnormalities in internuclear ophthalmoplegiaArch Ophthalmol101206106824462
- CremerPDMigliaccioAAHalmagyiGM1999Vestibulo-ocular reflex pathways in internuclear ophthalmoplegiaAnn Neurol455293310211481
- DaltonCMBrexPAMiszkielKA2002Application of the new McDonald criteria to patients with clinically isolated syndromes suggestive of multiple sclerosisAnn Neurol52475312112046
- DasVEOrugantiPKramerPD2000Experimental tests of a neural-network model for ocular oscillations caused by disease of central myelinExp Brain Res1331899710968219
- DerwenskusJRuckerJCSerraA2005Abnormal eye movements predict disability in MS: two-year follow-upAnn N Y Acad Sci1039521315827013
- DowneyDLStahlJSBhidayasiriR2002Saccadic and vestibular abnormalities in multiple sclerosis: sensitive clinical signs of brainstem and cerebellar involvementAnn N Y Acad Sci9564384011960834
- FisherAGrestyMChambersB1983Primary position upbeating nystagmus: A variety of central positional nystagmusBrain106949646606479
- FlipseJPStraathofCSVan der SteenJ1996Binocular saccadic acceleration in multiple sclerosisNeuro-ophthalmology16436
- FrohmanEMFrohmanTCO’SuilleabhainP2002Quantitative oculographic characterisation of internuclear ophthalmoparesis in multiple sclerosis: the versional dysconjugacy index Z scoreJ Neurol Neurosurg Psychiatry7351512082045
- FrohmanEMFrohmanTCZeeDS2005The neuro-ophthalmology of multiple sclerosisLancet Neurol41112115664543
- FrohmanEMO’SuilleabhainPDeweyRBJr2003A new measure of dysconjugacy in INO: the first-pass amplitudeJ Neurol Sci210657112736091
- FrohmanTCFrohmanEMO’SuilleabhainP2003Accuracy of clinical detection of INO in MS: corroboration with quantitative infrared oculographyNeurology618485014504338
- HalmagyiGMCurthoysIS1988A clinical sign of canal paresisArch Neurol4573793390028
- HerishanuYOSharpeJA1983Saccadic intrusions in internuclear ophthalmoplegiaAnn Neurol1467726614872
- HuygenPLMVerhagenWIMHommesOR1990Short vestibulo-ocular reflex time constants associated with oculomotor pathology in multiple sclerosisActa Otolaryngol (Stockh)10925332309556
- KatsarkasA1982Positional nystagmus of the “central type” as an early sign of multiple sclerosisJ Otolaryngol119137077734
- KoriAARobinNHJacobsJB1998Pendular nystagmus in a peroxisomal assembly disorderArch Neurol5555489561985
- LeighRJZeeDS2006The Neurology of Eye Movements (Book/DVD)4th edNew YorkOxford University Press
- LopezLIGrestyMABronsteinAM1995Acquired pendular nystagmus: Oculomotor and MRI findingsActa Oto – Laryngologica2857
- MaasEFAsheJSpiegelP1991Acquired pendular nystagmus in toluene addictionNeurology4128251992377
- MartynCNKeanD1988The one-and-a-half syndrome Clinical correlations with a pontine lesion demonstrated by nuclear magnetic resonance imaging in a case of multiple sclerosisBr J Ophthalmol72515173415943
- MasucciEFKurtzkeJF1988Downbeat nystagmus secondary to multiple sclerosisAnn Ophthalmol2034783190116
- MeienbergOMüriRRabineauPA1986Clinical and oculographic examinations of saccadic eye movements in the diagnosis of multiple sclerosisArch Neurol43438433964108
- MileaDNapolitanoMDechyH2001Complete bilateral horizontal gaze paralysis disclosing multiple sclerosisJ Neurol Neurosurg Psychiatry70252511160480
- MilesFA1998The neural processing of 3-D visual information: evidence from eye movementsEur J Neurosci10811229753150
- MüriRMMeienbergO1985The clinical spectrum of internuclear ophthalmoplegia in multiple sclerosisArch Neurol4285154026628
- ProudlockFAGottlobIConstantinescuCS2002Oscillopsia without nystagmus caused by head titubation in a patient with multiple sclerosisJ Neuroophthalmol22889112131465
- RanalliPJSharpeJA1988Vertical vestibulo-ocular reflex, smooth pursuit and eye-head tracking dysfunction in internuclear ophthalmoplegiaBrain11112993173208059
- ReulenJPHSanderEACMJogenhuisLAH1983Eye movement abnormalities in multiple sclerosis and optic neuritisBrain106121406831194
- RuckerJCSheligaBMFitzGibbonEJ2006Contrast sensitivity, first-order motion and Initial ocular following in demyelinating optic neuropathyJ Neurol2531203916649097
- SchonFHodgsonTLMortD2001Ocular flutter associated with a localized lesion in the paramedian pontine reticular formationAnn Neurol504131611558800
- SerraADerwenskusJDowneyDL2003Role of eye movement examination and subjective visual vertical in clinical evaluation of multiple sclerosisJ Neurol2505697512736736
- SharpeJAGoldbergHJLoAW1981Visual-vestibular interaction in multiple sclerosisNeurology31427336971417
- SheligaBMChenKJFitzGibbonEJ2005Short-latency disparity vergence in humans: evidence for early spatial filteringAnn N Y Acad Sci1039252915826979
- StarckMAlbrechtHStraubeA1997Drug therapy for acquired pendular nystagmus in multiple sclerosisJ Neurology244916
- StraubeAKurzanRBüttnerU1991Differential effects of bicuculline and muscimol microinjections into the vestibular nuclei on simian eye movementsExp Brain Res86347581756810
- TanEKansuT1990Bilateral horizontal gaze palsy in multiple sclerosisJ Clin Neuro-ophthalmol101246
- ToddLKingJDarlingtonCL2001Optokinetic reflex dysfunction in multiple sclerosisNeuroreport12139940211388418
- TrobeJDSharpeJAHirshDK1991Nystagmus of Pelizaeus-Merzbacher diseaseArch Neurol4887911986731
- WallMWraySH1983The one-and-a-half syndrome – a unilateral disorder of the pontine tegmentum: a study of 20 cases and review of the literatureNeurology33971806683820
- ZeeDSHainTCCarlJR1987Abduction nystagmus in internuclear ophthalmoplegiaAnn Neurol2138383579224