Abstract
Purpose:
To compare the IOP-lowering efficacy of the fixed combination of travoprost 0.004%/timolol 0.5% dosed once daily in the morning with the concomitant administration of travoprost 0.004% dosed once daily in the evening and timolol 0.5% dosed once daily in the morning.
Methods:
This was an analysis of pooled data from two similarly designed prospective, randomized, controlled clinical trials comparing the fixed combination and concomitant therapy.
Results:
Mean IOP ranged from 15.7 to 16.8 mmHg for the fixed combination group, and from 15.1 to 16.4 mmHg for the concomitant group. Mean IOP reductions were up to 9.0 mmHg in the fixed combination group, and up to 8.8 mmHg in the concomitant group. The differences in mean IOP change between treatment groups ranged from −0.2 to +0.9 mmHg across visits and time points. The safety profile was generally similar between groups. An exception was the incidence of ocular hyperemia, which was 13.7% with the fixed combination and 20.8% with concomitant therapy (p = 0.02).
Conclusion:
The fixed combination of travoprost 0.004% and timolol 0.5% provides IOP-lowering efficacy that is similar to concomitant administration of travoprost 0.004% dosed once daily in the evening and timolol 0.5% dosed once daily in the morning.
Introduction
Travoprost is a member of the prostaglandin analogue class of intraocular pressure (IOP)-lowering drugs employed to slow the progression of glaucoma. The prostaglandin analogue class has gained first-line therapy status with prescribers in recent years, largely due to unrivaled IOP-lowering efficacy and favorable ocular and systemic safety. Travoprost’s IOP-lowering efficacy and safety have been reported previously by CitationNetland et al (2001); CitationGoldberg et al (2001); CitationFellman et al (2002); and others (CitationParrish et al 2003).
Despite the ever-improving efficacy and safety of new IOP-lowering drugs and classes, initial monotherapy is inadequate to control IOP in a substantial proportion of glaucoma patients. In the Ocular Hypertension Treatment Study, 40% of patients in the treatment arm required more than one medication to achieve a 20% IOP reduction (CitationKass et al 2002). Retrospective data demonstrate that more than 50% of patients prescribed initial monotherapy will undergo a treatment change within 2 years (CitationKobelt-Nguyen et al 1998). And recent pharmacy services data demonstrate that at best, 30%–40% of patients prescribed monotherapy with a given IOP-lowering agent will still be actively refilling prescriptions for that agent a year after the initiation of therapy (either due to patient non-adherence or a physician-directed change in therapy) (CitationReardon et al 2004).
These investigations have taken place since the availability of prostaglandin analogues, demonstrating that even with this new class of agents, adjunctive therapy is often required for long-term IOP control. Topical beta blockers are commonly used as adjunctive therapy with prostaglandins in clinical practice. Orengo-Nania and colleagues reported the safety and efficacy of concomitant therapy with travoprost 0.004% and timolol 0.5% in patients with inadequate IOP control on timolol 0.5% alone (CitationOrengo-Nania et al 2001). When travoprost 0.004% was added concomitantly to timolol 0.5% in this population of patients, additional mean IOP reductions ranging from 5.7 to 7.2 mmHg were observed, with no unexpected side effects following concomitant administration of the two drugs.
Subsequently, a fixed combination of travoprost and timolol has been developed. The efficacy and safety of travoprost 0.004% and timolol 0.5% in fixed combination have recently been reported from two prospective, randomized, controlled clinical trials (CitationHughes et al 2005; CitationSchuman et al 2005). Given the similarity of study designs, a pooled analysis is appropriate to further characterize the efficacy of the fixed combination compared to concomitant administration of its components.
Methods
Data from two clinical trials were pooled for the current analysis. Each of these trials has been reported separately (CitationHughes et al 2005; CitationSchuman et al 2005). The protocol for each trial was approved by Institutional Review Boards for participating sites and the studies were conducted in accordance with the Declaration of Helsinki and relevant ICH guidelines. All participating patients gave written informed consent.
In the first trial, patients with open-angle glaucoma or ocular hypertension were randomized to receive either travoprost 0.004%/timolol 0.5% fixed combination once daily in the morning or concomitant administration of travoprost 0.004% dosed once daily in the evening and timolol 0.5% dosed once daily in the morning (CitationHughes et al 2005). The same two treatment groups were included in the second trial. In addition, a timolol 0.5% arm dosed twice daily was included in the second trial for internal validation (CitationSchuman et al 2005). (Data from the timolol-only arm are not included in this pooled analysis since it was in only one of the two studies.) In both studies, the primary efficacy parameter was mean IOP at the 8 AM, 10 AM, and 4 PM time points at Week 2, Week 6, and Month 3. For each patient, the eye with the higher IOP at baseline was selected for analysis. The primary statistical objective for each study was to demonstrate non-inferiority of the fixed combination to concomitant therapy. Primary efficacy was pre-specified in the protocol and analysis plan to be based upon IOP assessments collected through the Month 3 visit. In addition, patients remained on assigned masked treatment for an additional three months primarily for safety follow-up (providing up to 6 months total on masked therapy), although the first 3 months remained the basis for primary efficacy. The pooled analysis followed the same a priori statistical design set forth in the protocols for the individual studies.
Hypothesis tests were performed using repeated measures analysis of variance. Since primary efficacy was a test of non-inferiority, the analysis was based on the per protocol data set and confirmed with intent-to-treat. All patients who received study medications and completed at least one study visit were included in the intent-to-treat data set. Last-observation-carried-forward was used to impute missing values in the intent-to-treat data set. Further to the criteria for inclusion in the intent-to-treat analysis, patients were excluded from the per protocol data set if they did not satisfy pre-randomization inclusion/exclusion criteria. Also, data points potentially affected by protocol violations (eg, dosing non-adherence) were excluded from the per protocol analysis. Analysis of both data sets was planned to demonstrate robustness of study findings. For the test of non-inferiority, a two-sided 95% confidence interval for the treatment group difference was constructed at each visit and time point based on the analysis of variance. In order to demonstrate non-inferiority, all of the upper confidence limits must have been less than or equal to +1.5 mmHg. The 1.5 mmHg non-inferiority margin is widely used, reported, and accepted as an appropriate margin for comparison of IOP-lowering medications.
Safety evaluation included adverse event reports, ocular parameters (logMAR visual acuity; ocular signs consisting of cornea, iris/anterior chamber, lens, aqueous flare, and inflammatory cells; ocular hyperemia; dilated fundus examination consisting of vitreous, retina/macula/choroid, optic nerve, and cup/disc ratio; visual fields; iris and eyelash photography), and non-ocular parameters (pulse rate, systolic blood pressure, and diastolic blood pressure). Conjunctival hyperemia was assessed by study investigators on a scale ranging from 0 (none) to 3 (severe), in increments of 0.5, using a set of photographic standards of ocular hyperemia provided by Alcon Laboratories. Ocular photographs were taken using a Sony CD Mavica digital camera to assess iris color and eyelash changes from baseline. Other aspects of the two protocols, including inclusion and exclusion criteria, were similar and are described elsewhere (CitationHughes et al 2005; CitationSchuman et al 2005).
Results
In total, 635 patients (322 travoprost 0.004%/timolol 0.5% and 313 concomitant therapy) are in the pooled analysis and were included in the evaluation of safety. Of these, 622 patients (317 travoprost 0.004%/timolol 0.5% and 305 concomitant therapy) were included in the intent-to-treat analysis and 599 patients (306 travoprost 0.004%/timolol 0.5% and 293 concomitant therapy) were included in the per protocol analysis.
Mean IOP in the per protocol analysis at each visit and time point is given in . Baseline IOP ranged from 23.1 to 25.4 mmHg in the fixed combination group, and from 23.0 to 25.0 in the concomitant group. The difference of 0.4 mmHg in mean baseline IOP at 8 AM is not clinically relevant, although statistically significant due to the pooled sample size. Mean on-therapy IOP ranged from 15.7 to 16.8 mmHg in the fixed combination group, and from 15.1 to 16.4 mmHg in the concomitant group. Differences in mean IOP between groups (fixed combination minus concomitant) ranged from 0.2 to 1.0 mmHg across visits and time points, with upper 95% confidence limits for these differences ranging from 0.7 to 1.5 mmHg, thereby satisfying the criterion for non-inferiority (less than or equal to 1.5 mmHg at all 9 time points). Mean IOP is shown graphically in .
Figure 1 Comparison of mean IOP for travoprost 0.004%/timolol 0.5% fixed combinaiton and concomitant travoprost 0.004% and timolol 0.5%.
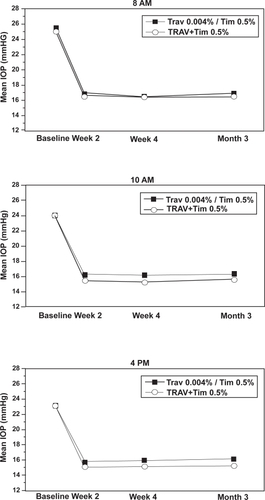
Table 1 Mean IOP comparison for test on non-inferiority
Mean IOP change from baseline at each visit and time point is given in . Mean IOP reductions were up to 9.0 mmHg in the fixed combination group, and up to 8.8 mmHg in the concomitant group. Treatment-group differences ranged from −0.2 to +0.9 mmHg, with upper 95% limits ranging from 0.3 to 1.4 mmHg, again satisfying the criterion for non-inferiority.
Table 2 Mean IOP change from baseline comparison for test of non-inferiority
The per protocol results were confirmed in the intent-to-treat analysis (data not shown). The upper 95% confidence limits for treatment-group differences ranged from 0.6 to 1.4 mmHg in the analysis of IOP, and from 0.2 to 1.3 mmHg in the analysis of IOP change from baseline. The concordance of the per protocol and intent-to-treat results demonstrate the robustness of findings in the per protocol analysis with respect to missing data and truncated observations.
Adverse events in the overall population were predominately nonserious, generally mild to moderate in intensity, usually resolved with or without treatment, and generally did not interrupt continuing patient participation in the studies (see ). Adverse events in the travoprost 0.004%/timolol 0.5% group were similar in type and frequency to those seen in the group exposed to concomitant therapy of the individual components. Ocular hyperemia was the most frequent adverse event in those patients with exposure to travoprost 0.004%/timolol 0.5% and in those with concomitant therapy of the individual components (13.7% and 20.8%, respectively, p = 0.02). In addition to the review of adverse events, no safety concerns were identified based upon an analysis of ocular and nonocular parameters. Overall, travoprost 0.004%/timolol 0.5% ophthalmic solution administered once daily is safe and well tolerated with a similar safety profile observed when compared with concomitant therapy with the individual components.
Table 3 Overall frequency and incidence of adverse events occurring at rates greater than or equal to 1%
Discussion
This pooled analysis of two prospective, randomized, controlled clinical trials demonstrates that the fixed combination of travoprost and timolol offers comparable IOP reduction and a similar safety profile to concomitant therapy with travoprost and timolol. Both mean IOP reduction and mean IOP were statistically similar between the two groups, with upper 95% confidence limits for the between-group differences falling at or below 1.5 mmHg at all visits and time points.
Prostaglandin analogues and beta blockers are the most widely used IOP-lowering medications in the United States today (CitationFechtner and Realini 2004). This pattern of clinical drug usage is important in the context of the history of fixed combinations of IOP-lowering drugs in the US. Several combinations of a miotic (pilocarpine) with an adrenergic agonist (epinephrine) were already in use at the time of the 1962 Harrison-Kefauver amendments to the Food, Drug, and Cosmetic Act and were “grandfathered”. These combinations were in ordinary clinical practice at a time when beta blockers, topical carbonic anhydrase inhibitors (CAI), and prostaglandin analogues did not exist for glaucoma management. Only two fixed combinations actually have achieved FDA regulatory approval, and both contained drugs which were widely used for concomitant dosing at the time that they were approved – i) a beta blocker (betaxolol) and a miotic (pilocarpine), which was not marketed and ii) a beta blocker (timolol) and a CAI (dorzolamide), which is still in use (CitationStrohmaier et al 1998).
Data suggest that prostaglandin analogues may offer slightly more IOP reduction when dosed in the evening compared to the morning (CitationAlm and Stjernschantz 1995) but beta blockers offer better aqueous suppression when dosed in the morning rather than the evening (CitationTopper and Brubaker 1985). Thus, the concomitantly treated arm in this pooled analysis exhibited a measurable, statistically significant (only at 10 AM and 4 PM) but clinically irrelevant IOP benefit by administering each component at its optimal time of day. The IOP-lowering efficacy between travoprost/timolol fixed combination and the concomitantly treated arm is similar at the 8 AM time point which approximates the peak of the diurnal IOP curve and which is 24 hours following the dose of travoprost/timolol fixed combination.
This small and clinically irrelevant IOP difference observed with the travoprost/timolol fixed combination versus concomitant therapy may be outweighed by the benefits of fixed combination therapy. Benefits of the fixed combination for patients include fewer bottles and fewer drops per day, which may reduce confusion and enhance adherence. For patients with prescription drug benefits, the fixed combination permits the purchase of two drugs with one co-payment. Safety may also be enhanced, as fixed combination therapy reduces long-term exposure to preservatives, which may over time lead to chronic conjunctival inflammation (CitationBaun et al 1995; CitationBroadway et al 1994a) and reduced surgical success (CitationBroadway et al 1994b; CitationLavin et al 1990) if trabeculectomy is ultimately required. There may also be efficacy advantages, not only from improved affordability and improved adherence, but also by reducing the washout effect that arises when patients on multiple topical IOP-lowering medications instill successive medications too closely together, washing out medications before full therapeutic benefits are achieved (Serle JB, ARVO Abstract 971, 2004).
Importantly, the safety profile of the travoprost/timolol fixed combination was similar to the safety profile seen with concomitant use. The side effects of the fixed combination were the side effects expected from the two constituent drugs except that the reported hyperemia was significantly (p = 0.02) less with the fixed combination (13.7%) compared with the concomitant dosing (20.8%).
In summary, the fixed combination of travoprost 0.004%/timolol 0.5% dosed once daily in the morning provides statistically equivalent IOP reduction to concomitant administration of travoprost 0.004% dosed once daily in the evening and timolol 0.5% dosed once daily in the morning. Mean IOP reductions on the travoprost/timolol fixed combination were as much as 9.0 mmHg. Therapy with fixed combinations may offer many potential benefits to patients, including convenience, safety, cost, and efficacy.
Acknowledgements
This study was supported by Alcon Research, Ltd.
References
- AlmAStjernschantzJ1995Effects on intraocular pressure and side effects of 0.005% latanoprost applied once daily, evening or morning. A comparison with timolol. Scandinavian latanoprost study groupOphthalmology1021743529098273
- BaunOHeegaardSKessingSV1995The morphology of conjunctiva after long-term topical anti-glaucoma treatment. A quantitative analysisActa Ophthalmol Scand7324257493236
- BroadwayDCGriersonIO’BrienC1994aAdverse effects of topical antiglaucoma medication. I. The conjunctival cell profileArch Ophthalmol1121437457980133
- BroadwayDCGriersonIO’BrienC1994bAdverse effects of topical antiglaucoma medication. II. The outcome of filtration surgeryArch Ophthalmol1121446547980134
- FechtnerRDRealiniT2004Fixed combinations of topical glaucoma medicationsCurr Opin Ophthalmol15132515021225
- FellmanRLSullivanEKRatliffM2002Comparison of travoprost 0.0015% and 0.004% with timolol 0.5% in patients with elevated intraocular pressure: a 6-month, masked, multicenter trialOphthalmology109998100811986110
- GoldbergICunha-VazJJakobsenJE2001Comparison of topical travoprost eye drops given once daily and timolol 0.5% given twice daily in patients with open-angle glaucoma or ocular hypertensionJ Glaucoma104142211711841
- HughesBABacharachJCravenER2005A three-month, multicenter, double-masked study of the safety and efficacy of travoprost 0.004%/timolol 0.5% ophthalmic solution compared to travoprost 0.004% ophthalmic solution and timolol 0.5% dosed concomitantly in subjects with open angle glaucoma or ocular hypertensionJ Glaucoma14392916148589
- KassMAHeuerDKHigginbothamEJ2002The ocular hypertension treatment study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucomaArch Ophthalmol1207011312049574
- Kobelt-NguyenGGerdthamUGAlmA1998Costs of treating primary open-angle glaucoma and ocular hypertension: a retrospective, observational two-year chart review of newly diagnosed patients in Sweden and the United StatesJ Glaucoma7951049559495
- LavinMJWormaldRPMigdalCS1990The influence of prior therapy on the success of trabeculectomyArch Ophthalmol108154382244836
- NetlandPALandryTSullivanEK2001Travoprost compared with latanoprost and timolol in patients with open-angle glaucoma or ocular hypertensionAm J Ophthalmol1324728411589866
- Orengo-NaniaSLandryTVon TressM2001Evaluation of travoprost as adjunctive therapy in patients with uncontrolled intraocular pressure while using timolol 0.5%Am J Ophthalmol132860811730649
- ParrishRKPalmbergPSheuWP2003A comparison of latanoprost, bimatoprost, and travoprost in patients with elevated intraocular pressure: a 12-week, randomized, masked-evaluator multicenter studyAm J Ophthalmol13568870312719078
- ReardonGSchwartzGFMozaffariE2004Patient persistency with topical ocular hypotensive therapy in a managed care populationAm J Ophthalmol137S31214697909
- SchumanJSKatzGJLewisRA2005Efficacy and safety of a fixed combination of travoprost 0.004%/timolol 0.5% ophthalmic solution once daily for open-angle glaucoma or ocular hypertensionAm J Ophthalmol1402425016086946
- StrohmaierKSnyderEDuBinerH1998The efficacy and safety of the dorzolamide-timolol combination versus the concomitant administration of its components. Dorzolamide-Timolol Study GroupOphthalmology1051936449787367
- TopperJEBrubakerRF1985Effects of timolol, epinephrine, and acetazolamide on aqueous flow during sleepInvest Ophthalmol Vis Sci26131594044159