Abstract
Two women with breast carcinoma developed clinical evidence of a sphenocavernous syndrome with magnetic resonance (MRI) that suggested a basal skull meningioma but in whom biopsy of the lesion revealed metastatic breast carcinoma. The difficulties in distinguishing between the two lesions on the basis of standard MRI are discussed, as are newer imaging techniques that may reduce or eliminate these problems.
Although meningiomas have well-known clinical and imaging features, metastases from a variety of different neoplasms such as prostate (CitationFink 1979; CitationLippman et al 1986; CitationFranco et al 1999), breast (CitationShapiro and Janzen 1959), lung (CitationKhalfallah et al 1999), kidney (CitationKillebrew et al 1983), gall bladder (CitationKawamata et al 1999), colon (CitationNeroni et al 1999), and adenoid cystic carcinoma (CitationMorioka et al 1993), as well as leiomyosarcoma (CitationBuff et al 1991), can simulate them. In particular, although it often involves bone, metastatic breast adenocarcinoma can present as an isolated dural lesion without apparent involvement of adjacent bone (CitationJohnson et al 2002). Indeed, CitationTsukada and colleagues (1983) found solitary metastases to the dura in breast cancer in approximately 8% of autopsied patients. We describe two patients with pathologically proven adenocarcinoma of the breast, one of whom developed a cavernous sinus syndrome and the other, an orbital apex syndrome, in whom standard magnetic resonance imaging (MRI) showed a lesion of the skull base consistent with a basal skull meningioma. In each case, the correct diagnosis of a soft-tissue, skull-base metastasis was not made until the lesion was biopsied.
Cases
Case 1
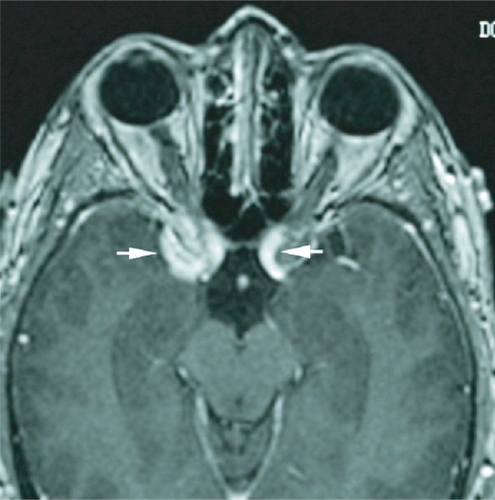
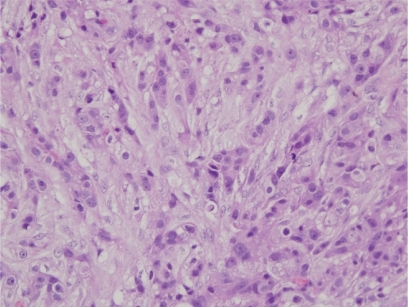
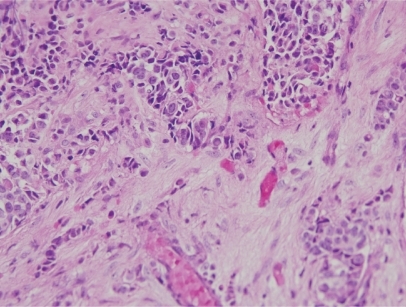
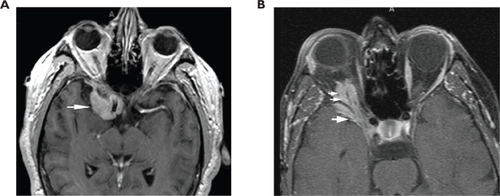
A 48-year-old woman developed adenocarcinoma of the breast treated with lumpectomy and radiation therapy. Three years later, she developed painless vertical diplopia and was found to have a right trochlear nerve paresis. MRI revealed an enhancing, right paraclinoid lesion and a smaller, similar lesion on the left (). An evaluation revealed no evidence of metastases, and it was thought that the patient had a basal skull meningioma; however, she then developed increasing oblique diplopia and right ptosis and was found to have a right oculomotor nerve paresis. Repeat MRI showed an increase in the size and extent of the right-sided lesion with no change in the appearance of the lesion on the left. The patient underwent biopsy of the lesion that revealed findings consistent with metastatic carcinoma of the breast (). She subsequently underwent whole-brain fractionated radiation therapy combined with stereotactic radiosurgery to the anterior skull base in the region of the lesion, followed by chemotherapy. Over the next several months, the trochlear nerve paresis resolved, and the oculomotor nerve paresis improved but did not resolve. The patient died 22-months after diagnosis from the effects of metastatic disease.
Case 2
A 56-year-old woman was found to have adenocarcinoma of the breast at which time she was also found to have changes on computed tomographic scanning consistent with bony metastases to the vertebrae. She underwent a radical mastectomy and then began chemotherapy, but shortly thereafter, she developed headache and blurred vision in the inferior field of the right eye. Neuro-ophthalmologic examination showed evidence of an isolated, right retrobulbar optic neuropathy characterized by visual acuity of 20/40 in the eye associated with color vision of 3/10 using Hardy-Rand-Rittler pseudoisochromatic plates, an inferior field defect, and a relative afferent pupillary defect. Ophthalmoscopic examination of the ocular fundi revealed no abnormalities.
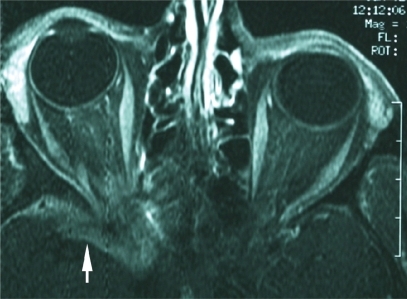
The patient underwent MRI that showed an enhancing soft-tissue lesion at the apex of the right orbit extending through the superior orbital fissure onto the medial sphenoid wing and around the temporal lobe tip (). There was no involvement of adjacent bone. The differential diagnosis was metastatic carcinoma versus sphenoid wing meningioma. The patient refused biopsy of the lesion but agreed to undergo radiation therapy. The lesion was subsequently treated with conventional fractionated radiation totaling 30 Gy.
Figure 3 Case 2. Initial T1-weighted, axial MRI after intravenous injection of paramagnetic contrast material shows an enhancing soft-tissue lesion at the apex of the right orbit extending through the superior orbital fissure onto the medial sphenoid wing and around the temporal lobe tip (arrowhead).
Shortly after completing radiation therapy, the patient experienced improvement in visual sensory function, and this was taken as evidence that the lesion was a meningioma, particularly as it did not decrease in size or disappear on MRI as might be expected if it were a metastasis. Three months later, however, she developed recurrent, progressive loss of vision in the right eye and a right oculomotor nerve paresis. After repeat MRI showed an increase in the size of the mass, the patient underwent biopsy and subtotal resection of the mass, histopathologic examination of which was found to be consistent with metastatic adenocarcinoma (). She was treated with further radiation therapy as well as chemotherapy but died 6-months later.
Discussion
Many patients with orbital or central nervous system (CNS) metastases from adenocarcinoma of the breast either have or soon develop other manifestations of disseminated disease. The diagnosis of CNS metastasis usually is made by a variety of tests, including serum assay for carcinoembryonic antigen (CEA); bone scan; positron emission tomographic (PET) scanning; MRI of the brain, spine, or both; lumbar puncture; and computed tomographic (CT) scanning or MRI of the chest, abdomen, and pelvis. When evidence of disseminated disease is absent, as in our first patient, the likelihood of a solitary, enhancing, soft-tissue lesion of the skull base being a meningioma is much greater than the likelihood that it is an isolated metastasis; however, even when evidence of disseminated disease is present, as in our second patient, it may be impossible to differentiate a metastasis from a meningioma using conventional MRI (CitationJohnson et al 2002).
Meningiomas have typical imaging features. They generally appear as an extra-axial mass with a broad attachment to the dura that is hyperdense on CT scanning and that enhances after intravenous injection of iodinated contrast material. On MRI, the lesion usually is isointense with gray matter on T1-weighted images, more variable on T2-weighted images, and homogeneously enhancing on T1-weighted images following intravenous administration of gadolinium-DTPA (CitationBuetow et al 1991; CitationSheporaitis et al 1992) (). Dural metastases generally present in one of two ways: as a subdural hematoma or as a tumor mass. It is the latter presentation that can be difficult to differentiate from a meningioma (CitationRumana et al 1998; CitationKhalfallah et al 1999). Twenty-nine cases of dural metastases interpreted as meningioma have previously been reported (CitationTagle et al 2002). Typically, these tumors produce MR images characterized by an increased signal on T2-weighted images and an enhancing dural tail on T1-weighted images after intravenous injection of gadolinium (CitationQuint and McGillicuddy 1994). The enhancement of the meninges adjacent to a meningioma on MRI, also seen with CT scanning, was once considered specific for meningioma (CitationGoldsher et al 1990; CitationNakau et al 1997); however, it is clear that this sign also can be present in patients with meningeal metastases (CitationWilms et al 1991; CitationQuint and McGillicuddy 1994).
Figure 5 T1-weighted, axial MRIs after intravenous injection of paramagnetic contrast material in two cases of biopsy-proven sphenoid wing meningiomas. (A) Note similarity of enhancing lesion (arrowhead) that surrounds the right anterior clinoid process to the appearance of the lesion in Case 1. (B) Note similarity of enhancing lesion that involves the right orbit (small double arrowheads) as well as the medial sphenoid wing around the temporal lobe tip (large single arrowhead) to our Case 2.
Although standard MRI may be unable to differentiate between intracranial meningiomas and metastases, it appears that dural metastases and meningiomas have different perfusion-sensitive characteristics when assessed using dynamic perfusion MRI (DPMRI) (CitationKremer et al 2004), a technique that provides information about a tumor’s vascularity that is not obtained using conventional MRI. DPMRI has been used not only to differentiate dural metastases from meningiomas (CitationKremer et al 2004) but also to differentiate high-grade from low-grade gliomas (CitationProvenzale et al 2002), lymphomas from high-grade gliomas (CitationKremer et al 2002; CitationHartmann et al 2003), and meningiomas from neuromas (CitationMaeda et al 1994). The technique is based on the measurement of the MR signal intensity using a T2-weighted sequence during the first pass of a bolus of a paramagnetic contrast agent. This signal intensity is transiently reduced by susceptibility effects due to the first pass of paramagnetic agent through the vasculature (CitationBarbier et al 2001). It provides a map of cerebral blood volume (CBV) and allows the calculation of the relative cerebral blood volume (rCBV), defined as the ratio between the CBV in the tumor and the CBV in the white matter. In contrast to the high rCBV typical of meningiomas, dural metastases show a low rCBV (CitationKremer et al 2004). Thus, this technique appears to be able to distinguish between these two lesions. Had this technique been available for our patients, we might have been able to spare them a craniotomy.
Conclusion
Although metastatic breast carcinoma was considered in the differential diagnosis of these two cases, it was not until exploratory surgery and histopathological study that the correct diagnosis could be established. It can be difficult to distinguish dural metastases from meningiomas using standard neuroimaging techniques, but new tools such as dynamic susceptibility contrast MR imaging may help make this a less difficult problem in the future.
Disclosure
Presented as a poster at the 2006 Meeting of the North American Neuro-Ophthalmology Society.
References
- BarbierELamalleLDecorpsM2001Methodology of brain perfusion imagingJ Magn Reson Imaging1349652011276094
- BuetowMPBuetowPCSmirniotopoulosJG1991Typical, atypical, and misleading features in meningiomaRadiographics1110871061749851
- BuffBLJrSchickRMNorregaardT1991Meningeal metastasis of leiomyosarcoma mimicking meningioma: CT and MRI findingsJ Comput Assist Tomogr1516671987192
- FinkLH1979Metastasis of prostatic adenocarcinoma simulating a falx meningiomaSurg Neurol122537515927
- FrancoEGil-NecigaECanoG1999Sphenoid metastasis mimicking a meningioma as the initial feature of adenocarcinoma of the prostateRev Neurol299293210637842
- GoldsherDLittAWPintoRS1990Dural “tail” associated with meningiomas on Gd-DTPA-enhanced MR images: characteristics, differential diagnostic value, and possible implications for treatmentRadiology176447502367659
- HartmannMHeilandSHartingI2003Distinguishing of primary cerebral lymphoma from high grade glioma with perfusion weighted magnetic resonance imagingNeuroscience Lett33811922
- JohnsonMDPowellSZBoyerPJ2002Dural lesions mimicking meningiomasHuman Pathol3312112612514791
- KawamataTKawamuraHKuboO1999Central nervous system metastasis from gallbladder carcinoma mimicking a meningioma. Case illustrationJ Neurosurg91105910584859
- KhalfallahMRochePHFigarrela-BrangerD1999Isolated dural metastasis mimicking meningioma. A case reportNeurochirurgie45250410567968
- KillebrewKKrigmanMMahaleyMSJr1983Metastatic renal carcinoma mimicking a meningiomaNeurosurgery1343046633836
- KremerSGrandSRemyC2002Cerebral blood volume mapping by MR imaging in the initial evaluation of brain tumorsJ Neuroradiol291051312297732
- KremerSGrandSRemyC2004Contribution of dynamic contrast MR imaging to the differentiation between dural metastasis and meningiomaNeuroradiology46642815232661
- LippmanSMBuzaidACIaconoRP1986Cranial metastase from prostate cancer simulating meningioma: report of two cases and review of the literatureNeurosurgery1982033785633
- MaedaMItohSKimuraH1994Vascularity of meningiomas and neurinomas: assessment with dynamic susceptibility contrast MR imagingAJR Am J Roentgenol16318168010210
- MoriokaTMatsushimaTIkezakiK1993Intracranial adenoid cystic carcinoma mimicking meningioma: report of two casesNeuroradiology3546258397344
- NakauHMiyazawaTTamaiS1997Pathologic significance of meningeal enhancement (“flare sign”) of meningiomas on MRISurg Neurol48584919400640
- NeroniMArticoMPastoreFS1999Diaphragma sellae metastasis from colon carcinoma mimicking a meningioma. A case reportNeurochirurgie45160310448659
- ProvenzaleJMWangGRBrennerT2002Comparison of permeability in high-grade and low-grade brain tumors using dynamic susceptibility contrast MR imagingAJR Am J Roentgenol178711611856703
- QuintDMcGillicuddyJ1994Meningeal metastasis of the cerebellopontine angle demonstrating “dural tail” signCan Assoc Radiol J454038118713
- RumanaCSHessKRShiWM1998Metastatic brain tumors with dural extensionJ Neurosurg8955289761048
- ShapiroRJanzenAH1959Osteoblastic metastases to floor of skull simulating meningioma en plaqueAm J Roentgenol Radium Ther Nucl Med819646
- SheporaitisLOsbornASmirniotopoulosJ1992Radiologicpathologic correlation: intracranial meningiomaAJNR Am J Neuroradiol1329371595462
- TaglePVillanuevaPTorrealbaG2002Intracranial metastasis or meningioma? An uncommon clinical diagnostic dilemmaSurg Neurol58241512480230
- TsukadaYFouadAPickrenJW1983Central nervous system metastasis from breast carcinoma. Autopsy studyCancer522349546640506
- WilmsGLammensMMarchalG1991Prominent dural enhancement adjacent to nonmeningiomatous malignant lesions on contrastenhanced MR imagesAJNR Am J Neuroradiol1276141882761